Heinrich J.G., Aldinger F. (Eds.) Ceramic Materials and Components for Engines
Подождите немного. Документ загружается.

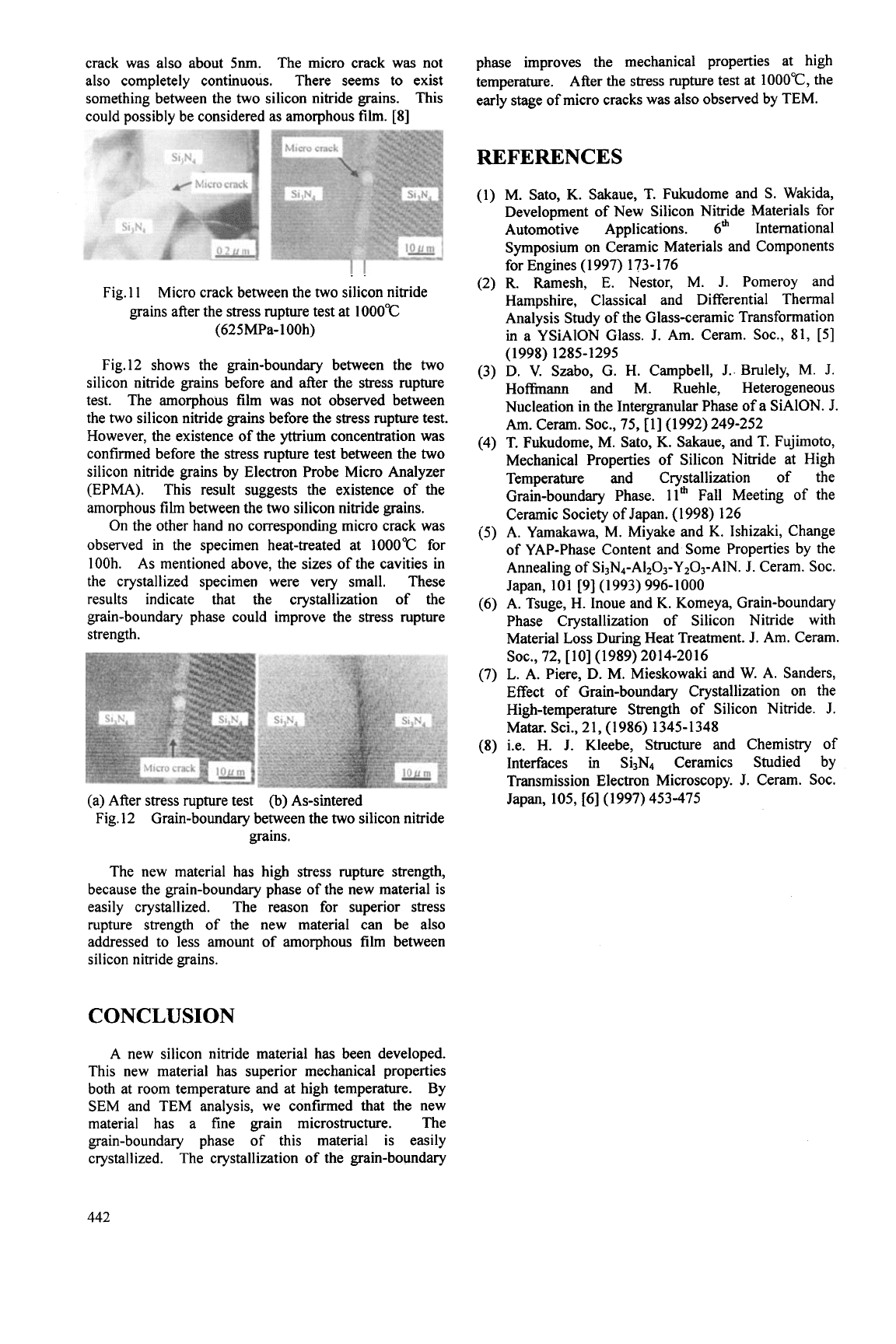
crack was also about
5nm.
The micro crack was not
also completely continuous. There seems to exist
something between the two silicon nitride grains. This
could possibly be considered as amorphous film. [8]
phase improves the mechanical properties at high
temperature. After the stress rupture test at lOOO'C, the
early stage of micro cracks was also observed by TEM.
II
Fig.
11
Micro crack between the two silicon nitride
grains after the stress rupture test at
1000°C
(625MPa-
1
OOh)
Fig.12 shows the grain-boundary between the two
silicon nitride grains before and after the stress rupture
test. The amorphous film was not observed between
the two silicon nitride grains before the stress rupture test.
However, the existence of the yttrium concentration was
confirmed before the stress rupture test between the two
silicon nitride grains by Electron Probe Micro Analyzer
(EPMA). This result suggests the existence of the
amorphous
film
between the two silicon nitride
grains.
On the other hand no corresponding micro crack was
observed
in
the specimen heat-treated at
1000°C
for
100h. As mentioned above, the sizes of the cavities in
the crystallized specimen were very small. These
results indicate that the crystallization of the
grain-boundary phase could improve the stress rupture
strength.
REFERENCES
(1)
M. Sato,
K.
Sakaue, T. Fukudome and
S.
Wakida,
Development of New Silicon Nitride Materials for
Automotive Applications. 6" International
Symposium on Ceramic Materials and Components
for Engines
(
1997) 173- 176
(2)
R.
Ramesh, E. Nestor, M.
J.
Pomeroy and
Hampshire, Classical and Differential Thermal
Analysis Study of the Glass-ceramic Transformation
in a YSiAlON Glass. J. Am. Ceram. SOC., 81,
[5]
(3) D.
V.
Szabo, G. H. Campbell, J. Brulely, M. J.
Hof&nann and M. Ruehle, Heterogeneous
Nucleation in the Intergranular Phase of a SiAlON.
J.
Am.
Ceram. SOC., 75, [I] (1992) 249-252
(4) T. Fukudome, M. Sato,
K.
Sakaue, and T. Fujimoto,
Mechanical Properties of Silicon Nitride at High
Temperature and Crystallization of the
Grain-boundary Phase. ll* Fall Meeting of the
Ceramic Society of Japan.
(1
998) 126
(5)
A. Yamakawa, M. Miyake and
K.
Ishizaki, Change
of YAP-Phase Content and Some Properties by the
Annealing of Si3N4-AI2O3-Y2O3-A1N. J. Ceram. SOC.
Japan, 101
[9]
(1993) 996-1000
(6) A. Tsuge, H. Inoue and
K.
Komeya, Grain-boundary
Phase Crystallization of Silicon Nitride with
Material
Loss
During
Heat Treatment. J. Am. Ceram.
(1998) 1285-1295
(a) After stress rupture test
(b)
As-sintered
Japan,
105,
[6] (1997) 453-475
Fig. 12
Grain-boundary between the two silicon nitride
grains.
The new material has high stress rupture strength,
because the grain-boundary phase
of
the new material is
easily crystallized. The reason for superior stress
rupture strength of the new material can be also
addressed to less amount
of
amorphous film between
silicon nitride grains.
CONCLUSION
A new silicon nitride material has been developed.
This new material has superior mechanical properties
both at room temperature and at high temperature. By
SEM and TEM analysis, we confirmed that the new
material has a fine grain microstructure. The
grain-boundary phase of this material is easily
crystallized. The crystallization
of
the grain-boundary
442

SINTERING AND MICROSTRUCTURE
OF
SILICON NITRIDE
WITH MAGNESIA AND CERIUM ADDITIVES
Haitao Yang, Ling Gao and Runzhang Yuan
State Key Lab
of
Advanced Technology for Materials Synthesis and Processing,
Wuhan University of Technology,
Wuhan
430070,
P. R. China
ABSTRACT
This paper deals with the pressure less sintering and
microstructure of Si3N4 -MgO-Ce02 ceramics. The
sintered Si3N4-MgO-Ce02 ceramics achieved a relative
density of 98,5% and a bendino strength of 950 Mpa.
XRD, TEM and EDAX analysis indicated that the main
composition of the glassy phase in the sintered Si3N4-
MgO-Ce02 ceramics was cerium silicate and hardly
contained any MgO. Abnormal grain growth occurred at
1850 "C, which led to micro cracks and dislocation.
Some whiskers made of silicon were growing in fiactal
patterns during TEM observation, which provided a
good experimental example for further study
of
fiactal
growth.
Keywords: Silicon, Nitride, Sintering, Microstructure
The TEM specimens were prepared in the usual was by
cutting, grinding and finally ion beam thinning.
RESULTS AND
DISCUSSION
Effect
of
additive compositions
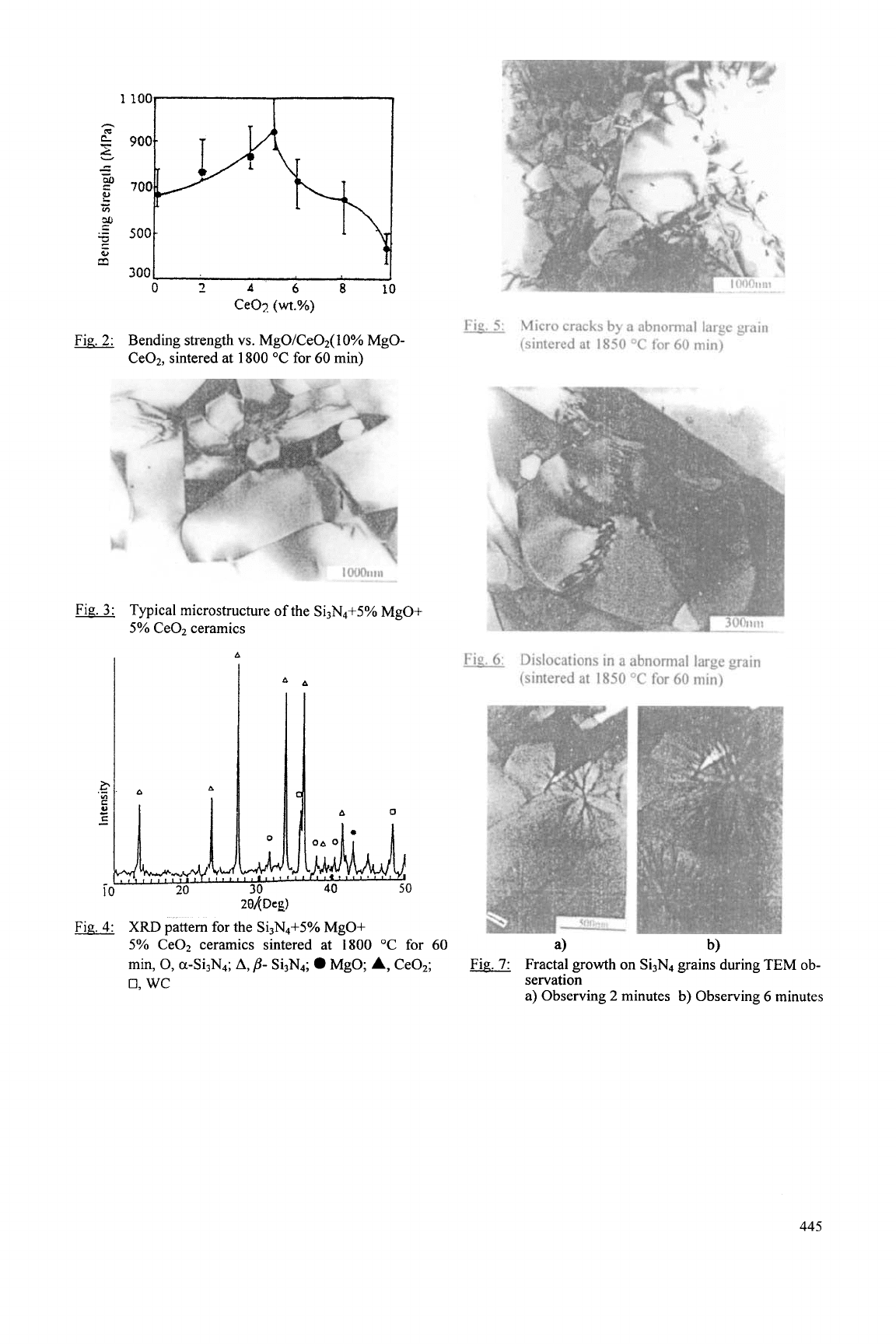
The selected compositions are listed in Table
2.
Figure
1
shows the relationship of MgOICeO, as a
sintering aid for Si3N4. The relative density of the sintered
Si3N4 with
10%
MgO is 97%, in comparison with the result
of only 91% for that with 10% CeO,. But the combination
of MgO and CeOz is better than either one. A highest
relative density of 98.5% is obtained at a MgO/CeO,
weight ratio of 5/5.
Bending Strength
INTRODUCTION
It is difficult to densify pure silicon nitride into
useful high strength ceramics due to its covalent nature
of bonding. Metal oxides such as Mg0['221, A1203[3*41,
and rare-earth oxides[5971 have been found to be effective
sintering and microstructure of silicon nitride with a
combination if ceria and magnesia additives
-
although
the effect of these
two
cations on sintering have both
been extensively studied separately in combination with
silica.
EXPERIMENTAL
Starting materials and selected compositions
The characteristics of the Si3N4 powder used in the
present study are listed
in
Table
1.
The selected
compositions are listed in Table
2.
Experiment
The selected compositions were mixed and ball
milled in alcohol for 24
h
with WC-~YOCO cemented
carbide medium. The powder mixtures were dry-pressed
into bars in a steel die at
120
Mpa. The compacts were
embedded within a Si3N4+50wt. %BN mixed-powder
bed in a molybdenum crucible and pressure less sintered
in a 1 atm N2 atmosphere. The bulk density of the
sintered specimens was measured using Archimede's
Principle. Without being grinding, the as-sintered bar
specimens, 5 x 5 x
30
mm
in size, were used for 3-point
bending strength measurements. Phase identification
was made by X-ray diffraction using CuKa radiation.
An
H-800
transmission electron Microscope fitted with
an EDAX was used for TEM work.
The as- sintered bars were used directly for 3-point
bending strength vs. MgO/Ce02 weight ratio. The strength
is very poor if MgO or Ce02 is added alone. But the
strength is excellent if MgO and Ce02 are added in optimal
ratio. The highest strength of 950 Mpa for the composition
with a MgO/Ce02 weight ration of 5/5 indicates that the
combination of MgO and Ce02 sintering aids is very
effective.
Microstructures
Glassy phase: Fig.
3
shows a typical microstructure
of
the Si3N4+5% Ce02 ceramics sintered at 1800 "C for 60
min. The glassy phase remains at multigrain junctions as
well as
p
-
p
Si3N4 grain boundaries. The glassy phase can
be confirmed directly by its electron diffraction, which
appears to be an ambiguous circle because of the absence of
Bragg diffraction. The result of the EDAX analysis of a
glassy phase shows that Si, Ce are rich in the glassy phase
but the amount
of
Mg is very poor (Mg: 0.64 at%, Si: 65.34
at%, Ce: 9.23 at%, Ce: 9.23 at%, Cu: 21.31 at%, W: 3.48
at% and Cu was introduced by specimen holder). This
reveals that after sintering at
1800
"C, the main
composition of the glassy phase in the sintered
Si3N4+5%MgO+5%Ce02 ceramics is cerium silicate and
hardly contains any MgO. This result is confirmed by X-ray
diffraction
(XRD)
analysis. Fig.
4
shows the XRD pattern
for the Si3N4+5%MgO+5%Ce02 ceramics sintered at
1800
"C for
60
min. There are no traces of Ce02, but MgO is
found.
This
suggests that Ce02 has entered the glassy
phase, and most of the MgO did not enter the glassy phase.
443
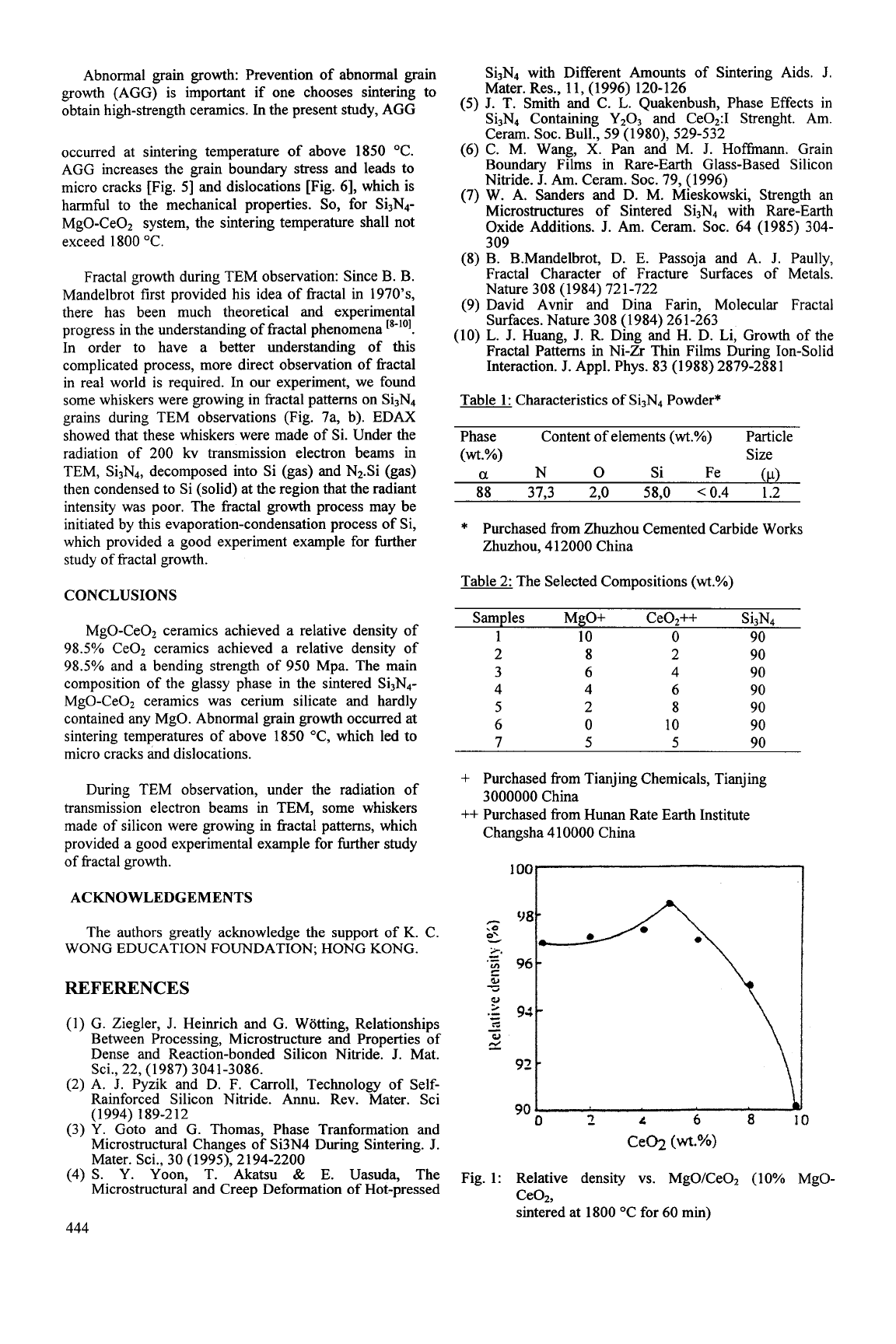
Abnormal grain growth: Prevention of abnormal grain
growth (AGG) is important if one chooses sintering to
obtain high-strength ceramics. In the present study, AGG
occurred at sintering temperature of above
1850
"C.
AGG increases the grain boundary stress and leads to
micro cracks [Fig.
51
and dislocations [Fig.
61,
which is
harmhl to the mechanical properties.
So,
for Si3N4-
MgO-CeOZ system, the sintering temperature shall not
exceed
1800
"C.
Fractal growth during TEM observation: Since B. B.
Mandelbrot first provided his idea of fractal in
1970's,
there has been much theoretical and experimental
progress in the understanding of fractal phenomena
[8-101.
In order to have a better understanding of this
complicated process, more direct observation of fractal
in real world is required. In our experiment, we found
some whiskers were growing in fractal patterns on Si3N4
grains during TEM observations (Fig.
7a,
b). EDAX
showed that these whiskers were made of Si. Under the
radiation of
200
kv transmission electron beams in
TEM, Si3N4, decomposed into Si (gas) and N2.Si (gas)
then condensed to Si (solid) at the region that the radiant
intensity was poor. The fractal growth process may be
initiated by this evaporation-condensation process of Si,
which provided a good experiment example for further
study of fractal growth.
CONCLUSIONS
MgO-Ce02 ceramics achieved a relative density of
98.5%
CeOz ceramics achieved a relative density of
98.5%
and a bending strength of
950
Mpa. The main
composition of the glassy phase in the sintered Si3N4-
MgO-CeOz ceramics was cerium silicate and hardly
contained any MgO. Abnormal grain growth occurred at
sintering temperatures of above
1850
"C, which led to
micro cracks and dislocations.
During TEM observation, under the radiation of
transmission electron beams in TEM, some whiskers
made of silicon were growing in fractal patterns, which
provided a good experimental example for further study
of fractal growth.
ACKNOWLEDGEMENTS
The authors greatly acknowledge the support of
K.
C.
WONG
EDUCATION
FOUNDATION;
HONG KONG.
REFERENCES
(1)
G. Ziegler,
J.
Heinrich and G. Wotting, Relationships
Between Processing, Microstructure and Properties of
Dense and Reaction-bonded Silicon Nitride.
J.
Mat.
Sci.,
22, (1987) 3041-3086.
(2)
A.
J.
Pyzik and D. F. Carroll, Technology of Self-
Rainforced Silicon Nitride. Annu. Rev. Mater. Sci
(3)
Y. Goto and G. Thomas, Phase Tranformation and
Microstructural Changes of
Si3N4
During Sintering.
J.
Mater. Sci.,
30 (1995), 2194-2200
(4)
S.
Y. Yoon, T. Akatsu
&
E. Uasuda, The
Microstructural and Creep Deformation of Hot-pressed
(1994) 189-212
Si3N4 with Different Amounts of Sintering Aids.
J.
Mater. Res.,
11, (1996) 120-126
(5)
J.
T.
Smith and C.
L.
Quakenbush, Phase Effects
in
Si3N4 Containing
Y2O3
and Ce02:I Strenght. Am.
Ceram. SOC. Bull.,
59 (1980), 529-532
(6)
C. M. Wang,
X.
Pan and
M.
J.
Hoffmann. Grain
Boundary Films in Rare-Earth Glass-Based Silicon
Nitride.
J.
Am.
Ceram. SOC.
79, (1996)
(7)
W. A. Sanders and D. M. Mieskowski, Strength an
Microstructures of Sintered Si3N4 with Rare-Earth
Oxide Additions.
J.
Am. Ceram. SOC.
64 (1985) 304-
309
(8)
B. B.Mandelbrot, D. E. Passoja and A.
J.
Paully,
Fractal Character of Fracture Surfaces of Metals.
Nature
308 (1984) 721-722
(9)
David Avnir and Dina Farin, Molecular Fractal
Surfaces. Nature
308 (1984) 261-263
(10)
L.
J.
Huang,
J.
R. Ding and H. D. Li, Growth of the
Fractal Patterns in Ni-Zr Thin Films During Ion-Solid
Interaction.
J.
Appl. Phys.
83 (1988) 2879-2881
Table
1
:
Characteristics of Si3N4 Powder*
Phase Content of elements
(wt.%)
Particle
(wt.%)
Size
a
N
0
Si Fe
(cc)
88 31,3 2,O 58,O
<
0.4
1.2
*
Purchased from Zhuzhou Cemented Carbide Works
Zhuzhou,
4 12000
China
Table
2:
The Selected Compositions
(wt.%)
Samples MgO+ CeOz++ Si3N4
1 10
0
90
2 8 2 90
3 6 4 90
4 4 6 90
5
2 8 90
6
0
10 90
7
5
5
90
+
Purchased from Tianjing Chemicals, Tianjing
3000000
China
++
Purchased from Hunan Rate Earth Institute
Changsha
4 10000
China
Fig.
1:
Relative density
vs.
MgO/Ce02
(10%
MgO-
CeO2,
sintered at
1800
"C for
60
min)
444
Ce02
(wt.%)
Fig. 5: Micro cracks by a abnormal large grain
(sintered at 1850 "C for
60
min)
Fig.
2:
Bending strength
vs.
MgO/Ce02(10% MgO-
Ce02, sintered at 1800 "C for
60
min)
F&
-
3:
Typical microstructure of the Si3N4+5% MgO+
5% Ce02 ceramics
A
I
*a
Fin.
6:
Dislocations in a abnormal large grain
(sintered at 1850 "C for
60
min)
Fig.
4:
2
04
Deg)
XRD
pattern for the Si3N4+5% MgO+
min,
0,
a-Si3N4;
A,
8-
Si3N4;
0
MgO;
A,
CeOz;
0,WC
servation
5% Ce02 ceramics sintered at 1800 "C for
60
a)
b)
Fin.
7:
Fractal growth on Si3N4 grains during TEM ob-
a) Observing
2
minutes b) Observing
6
minutes
445

This Page Intentionally Left Blank

CHARACTERISATION
OF
MULTI-CATION STABILISED ALPHA
-
SIALON
MATERIALS
M.RTerner*, S.P.Swenser, Y.-B.Cheng,
Department
of
Materials Engineering, Monash University, Australia
ABSTRACT
Si3N4 based ceramics have many desirable
engineering properties including high hardness,
corrosion resistance, and strength retention at elevated
temperatures. Reaction sintering to produce these
materials requires high purity, synthesised starting
powders that result in a costly but homogenous product
best suited for specialised high performance
applications. Presently, a carbothermal reduction-
nitridation process is being investigated as a low-cost
alternative for a-sialon production. One of the main
advantages of this is the ability to utilise lower grade,
impure starting minerals, whereby impurities present
may be incorporated into the a’ structure. The effect of
various potential stabilising cations present in this
system is investigated via electron microscopy and
EDXS techniques.
INTRODUCTION
Alpha sialons (a’) possess many desirable
engineering properties such as high hardness, corrosion
resistance, wear resistance, and high strength retention
at elevated temperatures. Despite these attractive
properties, the high cost of production coupled with low
fracture toughness has limited the use of a’ materials to
specialised, high performance areas such as metal-
cutting tools. One of the main production costs is that of
the raw materials
-
the reaction sintering process
utilises costly, high-purity, synthesised nitride and oxide
powders, and high firing temperatures
(1
600-
1
SOOOC).
Sialons are formed from the substitution of
Al
and
0
into Si3N4, and in the a’ case there is extra substitution
of
A13+
beyond that of the
0’-
content, which results in
an overall excess of negative charge. Charge
compensation occurs by adding various stabilising
cations such as Li+, Mg2+, Ca”, Nd3+, Sm3+, Y” to the
system, usually via oxide powders. These cations can
inhabit
two
large interstices, or ‘cages’ located within
the a’ unit cell.
An
alternative processing route is that of
simultaneous carbothermal reduction and nitridation
(CRN) of oxide starting powders in the presence of a
carbonaceous reductant and nitrogen atmosphere. This
process has been successhlly used to manufacture
Si3N4 from silica [l], and
f3-
and 0-sialons from clays
[Z-31,
but has received less attention for the production
of a-sialons
[4-61.
A
major attraction of this process is
the possibility of utilising lower-grade and thus
relatively inexpensive starting powders. Naturally
occumng aluminosilicate minerals with significant Mg
or Ca contents may be potential candidates for
a’
formation. Furthermore, other impurity elements in the
raw minerals may potentially be incorporated into the
a‘
structure, thus producing multi-cation stabilised
a-
sialons.
The impurities may however result in a high
quantity of residual glass and thus have a deleterious
effect on mechanical properties, especially at high
temperatures. Hence
this
material may be best suited to
low-medium temperature applications that can benefit
from the intrinsically high hardness and good corrosion
resistance of a-sialon.
It is apparent that impurities in the starting minerals
play a critical role in the development of the final
material microstructure and mechanical properties. This
paper presents the results of a characterisation study of
an
a’
material produced by CRN of low-grade minerals.
METHOD
The material to be investigated, designated ‘Cl’, was
produced via simultaneous carbothennal-reduction of a
mixture of
two
locally available aluminosilicate based
minerals. The composition contained CaO in significant
quantity, and minor amounts of MgO, Ti02 and F@O3
were present in addition to a few other trace elements.
This composition was chosen because it contained
several possible stabilising elements
-
Ca, Mg, Ti, and
Fe, though only Ca and Mg are currently
known
to act as
a’ stabilisers. Furthermore, it was known from previous
work that the CRN of this composition would produce a
predominantly a-sialon powder.
To facilitate hrther analysis, the
a’
powder produced
was hot pressed to form a dense pellet. X-ray diffraction
was performed using a Rigaku-Geigerflex Bragg-
Brentano difiactomer with Ni-filtered Cu ka radiation.
The microstructure was viewed after etching the sample
in molten NaOH using a Jeol JSM 6300F FEG SEM,
and a Philips CM20 TEM equipped with an Oxford
Pentafet
EDXS
detector was used to analyse an ion-
beam thinned foil.
RESULTS
/
DISCUSSION
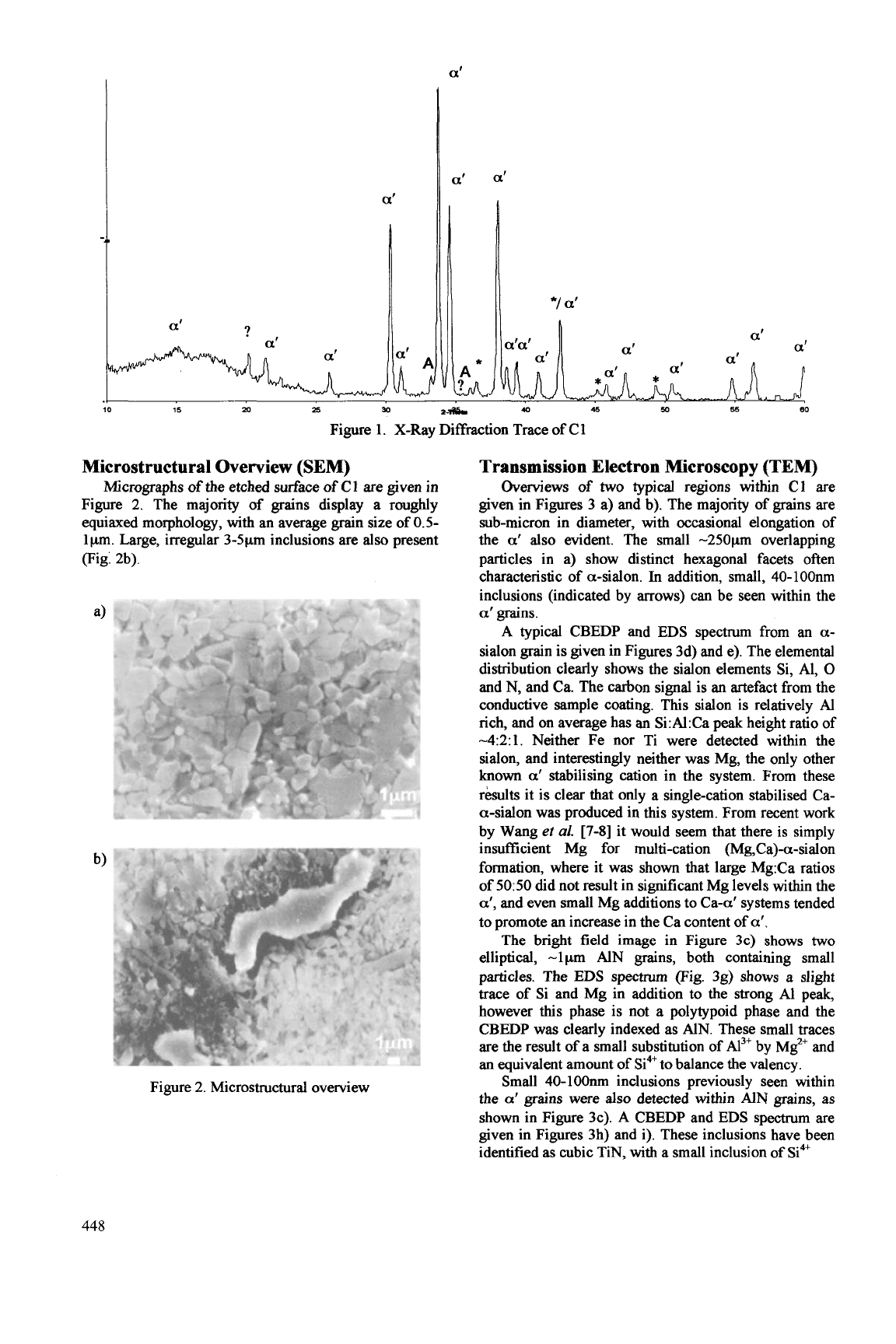
X-ray Diffraction
The
XRD
trace in Figure 1. shows that a-sialon is
the dominant phase formed, along with a minor amount
of
AlN
and glass. Several unidentified peaks remain in
the spectrum.
447
a’
1
I
a’
a’
a’
a’
a’
a’
10
1s
20
25
30
2-*
4a
45
so
55
(10
?
a’
a’
a’
Figure 1. X-Ray Diffraction Trace of C 1
a’
a’
a’
*fa‘
Microstructural
Overview
(SEM)
Micrographs
of
the
etched surface
of
C
1
are given in
Figure 2. The majority
of
grains display a roughly
equiaxed morphology, with an average grain size of
0.5-
1 pm. Large, irregular 3-5pm inclusions are
also
present
(Fig. 2b).
Figure 2. Microstructural overview
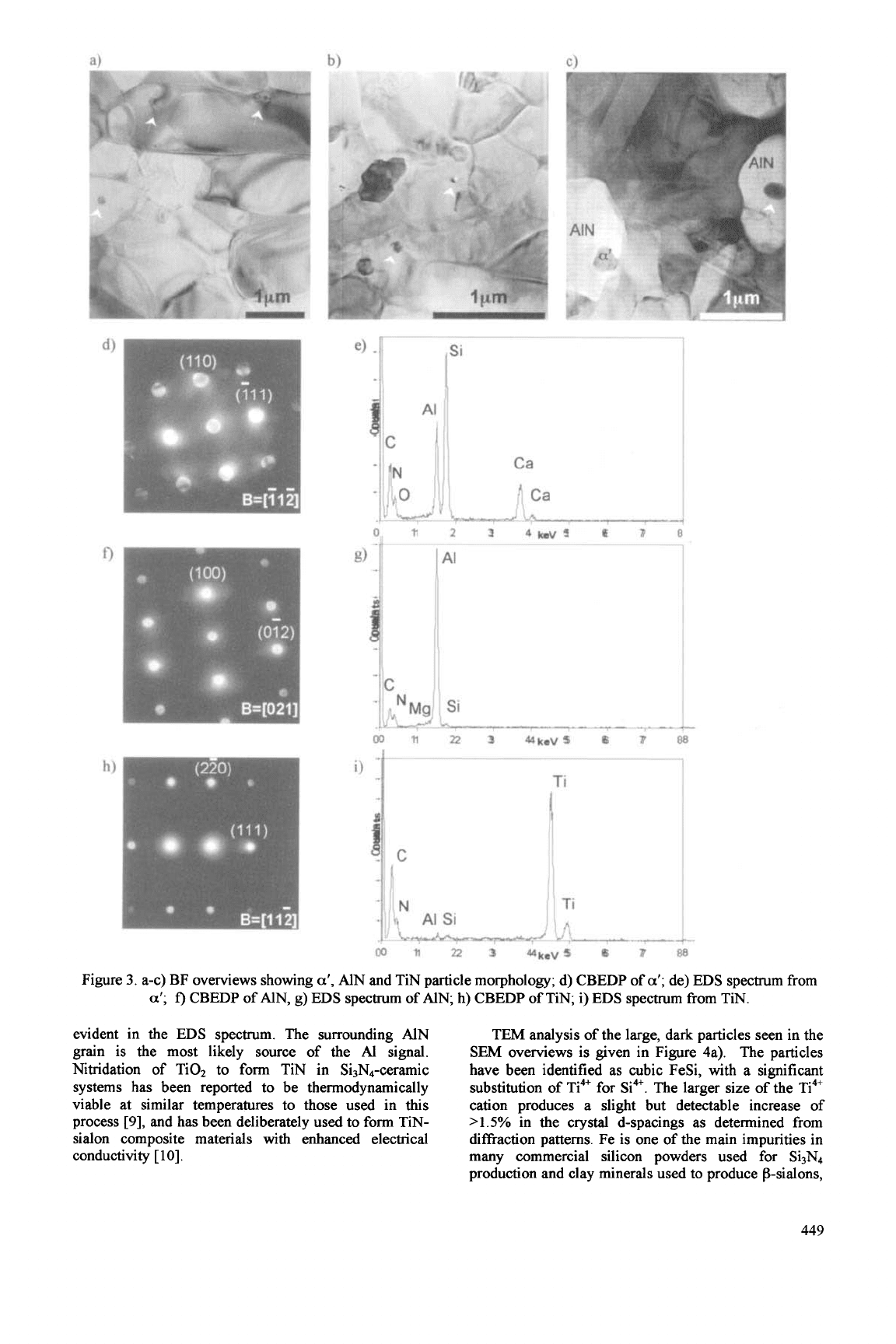
Transmission Electron Microscopy (TEM)
Overviews
of
two
typical regions within C1 are
given in Figures 3 a) and b). The majority
of
grains are
sub-micron in diameter, with occasional elongation of
the a’ also evident. The small -250pm overlapping
particles in a) show distinct hexagonal facets often
characteristic of a-sialon.
In
addition, small, 40-100nm
inclusions (indicated by arrows) can be seen within the
a’grains.
A typical CBEDP and
EDS
spectrum from an
a-
sialon grain is given in Figures 3d) and
e).
The elemental
distribution clearly shows the sialon elements Si, Al,
0
and N, and Ca. The carbon signal
is
an artefact from the
conductive sample coating. This sialon is relatively
Al
rich, and on average has
an
Si:Al:Ca peak height ratio
of
-4:2:1. Neither Fe nor Ti were detected within the
sialon, and interestingly neither was Mg, the only other
known
a‘
stabilising cation in the system. From these
results it is clear that only a single-cation stabilised Ca-
a-sialon was produced in this system. From recent work
by Wang
et
al.
[7-81
it would seem that there is simply
insufficient Mg for multi-cation (Mg,Ca)-a-sialon
formation, where it was shown that large Mg:Ca ratios
of
5050
did not result in significant Mg levels within the
a’, and even small Mg additions to Ca-a’ systems tended
to promote
an
increase in the Ca content of a’.
The bright
field
image in Figure 3c) shows
two
elliptical, -lpm
AlN
grains, both containing small
particles. The
EDS
spectrum (Fig. 3g) shows a slight
trace of Si and Mg in addition to the strong A1 peak,
however
this
phase is not a polytypoid phase and the
CBEDP was clearly indexed as AN. These small traces
are the result of a small substitution of A13+
by
Mgz+ and
an equivalent amount of Si4+ to balance the valency.
Small 40-1OOnm inclusions previously seen within
the a’ grains were also detected within
AlN
grains, as
shown in Figure 3c). A CBEDP and
EDS
spectrum are
given in Figures 3h) and i). These inclusions have been
identified as cubic TiN, with a small inclusion of Si4+
448
00
n
22
3
@kaV5
6
??
88
Figure
3.
a-c) BF overviews showing
a',
AlN and TiN particle morphology; d) CBEDP of
a';
de) EDS spectrum from
a';
f)
CBEDP of AlN, g)
EDS
spectrum of AIN; h) CBEDP of TiN;
i)
EDS spectrum from TIN.
evident in the
EDS
spectrum. The surrounding AlN
grain is the most likely source of the
Al
signal.
Nitridation of TiOz to form TiN in Si3N4-ceramic
systems has been reported to be thermodynamically
viable at similar temperatures to those used in this
process
[9],
and
has
been deliberately
used
to form TiN-
sialon composite materials
with
enhanced electrical
conductivity
[lo].
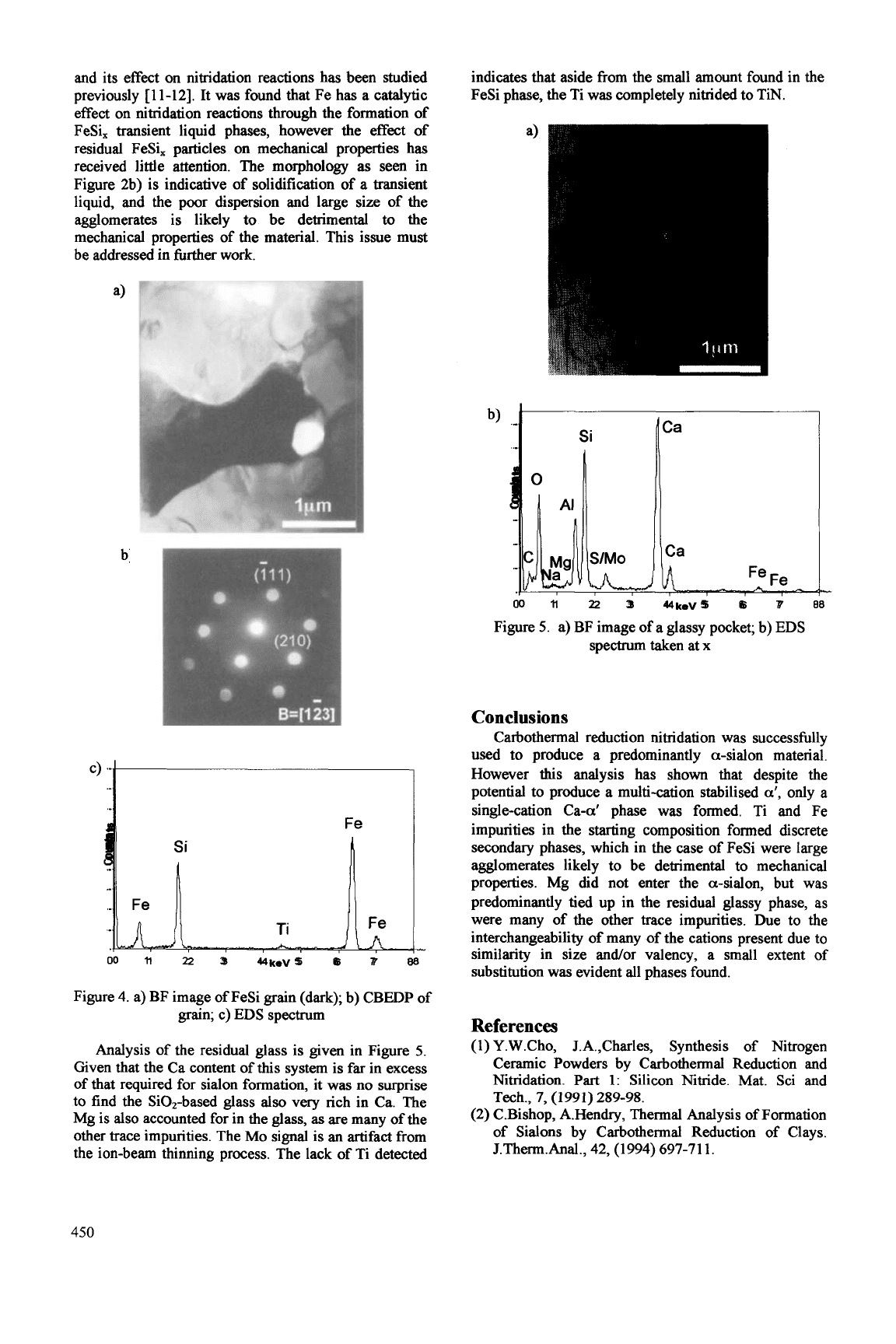
TEM analysis of the large, dark particles seen in the
SEM
overviews is given in Figure 4a). The particles
have been identified as cubic FeSi, with a significant
substitution of Ti4+ for Si4+. The larger size of the Ti4+
cation produces a slight but detectable increase of
>1.5%
in the crystal d-spacings as determined from
diffraction patterns. Fe is one of the main impurities in
many commercial silicon powders used for Si3N4
production and clay minerals used to produce p-sialons,
449
and its effect on nitridation reactions has been studied
previously [ll-121. It was found that Fe has a catalytic
effect on nitridation reactions through the formation of
indicates that aside from the small amount found in the
FeSi phase, the Ti was completely nitrided to TiN.
FeSi, transient liquid phases, however the effect
of
residual FeSi, particles on mechanical properties has
received little attention. The morphology as seen in
Figure 2b) is indicative of solidification
of
a transient
liquid, and the poor dispersion and large size of the
agglomerates is likely
to
be detrimental
to
the
mechanical properties of the material. This issue must
be addressed in hrther work.
Figure 4. a) BF image of FeSi grain (dark);
b)
CBEDP
of
grain; c)
EDS
spectrum
Analysis
of
the residual glass is given in Figure
5.
Given that the Ca content of
this
system is far in excess
of that required for sialon formation, it was
no
surprise
to find the Si02-based glass also very rich in Ca. The
Mg is also accounted for in the glass,
as
are many of the
other trace impurities. The Mo signal is an artifact from
the ion-beam thinning process. The lack of Ti detected
00
I'I
22
3
MkeVS
0
7
88
Figure
5.
a) BF image of a glassy pocket; b)
EDS
spectrum taken at x
Conclusions
Carbothermal reduction nitridation was successfidly
used to produce a predominantly a-sialon material.
However
this
analysis has shown that despite the
potential to produce a multi-cation stabilised a', only a
single-cation Ca-a' phase was formed. Ti and Fe
impurities in the starting composition formed discrete
secondary phases, which in the case of FeSi were large
agglomerates likely to be detrimental to mechanical
properties. Mg did not enter the a-sialon, but was
predominantly
tied
up in the residual glassy phase,
as
were many of the other trace impurities. Due to the
interchangeability of many
of
the cations present due to
similarity in size andor valency, a small extent
of
substitution was evident all phases found.
References
(1)
Y.W.Cho, J.A.,Charles, Synthesis of Nitrogen
Ceramic Powders by Carbothermal Reduction and
Nitridation.
Part
1:
Silicon Nitride. Mat. Sci and
(2) C.Bishop, A.Hendry, Thermal Analysis of Formation
of Sialons by Carbothermal Reduction of Clays.
Tech.,
7,
(1991) 289-98.
J.Tht~m.Anal., 42, (1994) 697-711.
450

(3) C.G.Barris, et
al.,
Reaction Bonded 0-Sialon and
0-
Sialon-Silicon Carbide. J. Aust. Cer.
Soc.,
33, (1997)
(4) M.Mitomo, M.Takeuchi, M.Ohmasa, Preparation
of
a-Sialon Powders by Carbothermal Reduction and
Nitridation. Cer. Int.,
14,
(1988) 43-8.
(5)
J.W.T. van Rutten, et
al.,
Carbothemal Preparation
and Characterisation
of
Ca-a-sialon. J.Eur. Cer. SOC.,
(6)R,Metselaar, et
al.,
The Synthesis
of
a-
and
p-
Sialons from Fly Ash. Key Eng. Mat., Proc. PacRim
2, Cairns, Australia, (1996).
(7)P.L.Wang, C.Zhang, W.Y.Sun, D.S.Yan, Formation
Behaviour
of
Multi-Cation a-sialons Containing
Calcium and Magnesium. Mat. Let., 38, (1999) 178-
185.
15-20.
15, (1995) 1-6.
(8) P.L.Wang, Private communication.
(9) M.B.Trigg, E.R.McCartney, Comparison
of
the
Reaction Systems Zr02-Si3N4 and Ti02-Si3N4.
Comm.Am.Cer.Soc., Nov, (198 1) C-15 1-1 52.
(1 O)F.Hong, R. J.Lumby, M.H.Lewis, TiN/Sialon
Composites via In-Situ Reaction Sintering.
J.
Eur.
Cer. SOC., 11, (1993) 237-239.
(1 l)S.M.Boyer, A. J.Moulson, A Mechanism
for
the
Nitidation of Fe-Contaminated Silicon. J.Mat. Sci.,
(12)A.D.Mazzoni, E.F.Aglietti, E.Pereira, p'-Sialon
Preparation
from
Kaolinitic Clays. Appl. Clay
Sci.,
7, (1 993) 407-420.
14, (1978) 1637-1646
45
1
