Heinrich J.G., Aldinger F. (Eds.) Ceramic Materials and Components for Engines
Подождите немного. Документ загружается.

particle size lpm, Denki Kagaku Kogyo Co.
Ltd., Japan), C (2600# grade, particle size 13
nm, Mitsubishi Chemical Co., Japan), A1,0,
(particle size O.lpm, purity 99.99%, Daimei
Chemical Co., Japan), Y203 (particle size
1.06pm, purity 99.9%, Shin-etsu Chemical Co.,
Japan), a-Sic (A2 grade, mean particle size
0.63pm, purity 99.1%, Showa Denko Co.,
Japan) and h-BN (SP-2 grade, particle size
4pm, Denki Kagaku
Kogyo
Co.Ltd., Japan).
For improving the sinterability in the reaction
process, 10
wt%
A1,03-Y203 additives (7:3
mixture of Al,03 and Y203) related to the Sic
contents in the composites were used.
Composites with no A1203-Y203 additives
were also produced for companson. About
sample designation, for example A-B25 means
that the BN content in the composite is 25
vol% and with A1203-Y,03 additives. Whereas,
N-B25 means that the BN content in the
composite is 25 vol% and without Al,03-Y,03
additives. However, the sample only from
reaction (l), i.e. without Sic addition, is
denoted as B55. The powders were ball-milled
for 24
h
in ethanol using Zr02(Y203) balls and
subsequently dried. Hot pressing of the mixed
powders was conducted in an argon
atmosphere under 30 MPa in a graphite die
with BN coating. For studying the phase
formation mechanism, hot press at different
temperatures for 60 min was conducted and
then the phase composition was determined by
X-ray diffraction (XRD) using CuKa radiation.
The final composites were hot pressed at
2000
"C for 60 min. The linear dimensional change
of the specimens during hot pressing was
measured by a displacement gauge. The
obtained data were used to calculate the
densification behavior, which is represented by
the relative density to the final phase
composition of the composite. Reaction
behavior was investigated by differential
thermal analysis (DTA) up to 1700 "C using a
heating rate
of
10 Wmin. Three-point bending
strength was tested on bars of 2.5 mm
x
3 mm
x
20
mm using a span of 16
mm
and a
crosshead speed of 0.5 mdmin. Fracture
toughness was measured
by
SENB method.
The strength and toughness data were average
of 5 measurements. Young's modulus, E,
parallel to the hot pressing direction, was
measured by the pulse echo method. The
microstructure of the composite was observed
by scanning electron microscopy (SEM).
RESULTS AND DISCUSSION
Effect
of
AI2O3-
Y203
Addition on Reaction
Process
The possible reactions in the Si3N4-B4C-C
system during the hot pressing process are:
Si3N4
+
B,C
+
2C
=
3SiC
+
4BN..
. . .
.(
1)
Si3N4
+
3B4C
=
3SiC
+
2N2
+
12B..
.
(3)
Si3N,
+
3B4C
=
3SiC
+
4BN
+
8B..
.
(4)
Si3N4
+
3C +4B
=
3SiC
+
4BN..
. . . .
(6)
Si3N4
+
3C
+
3SiC
+
2N2
............
(2)
4B
+
C
=
B4C
...
.
. .
.
.
.
. . . .
.
. . . .
.
. .
.
. .
.
.
.
(5)
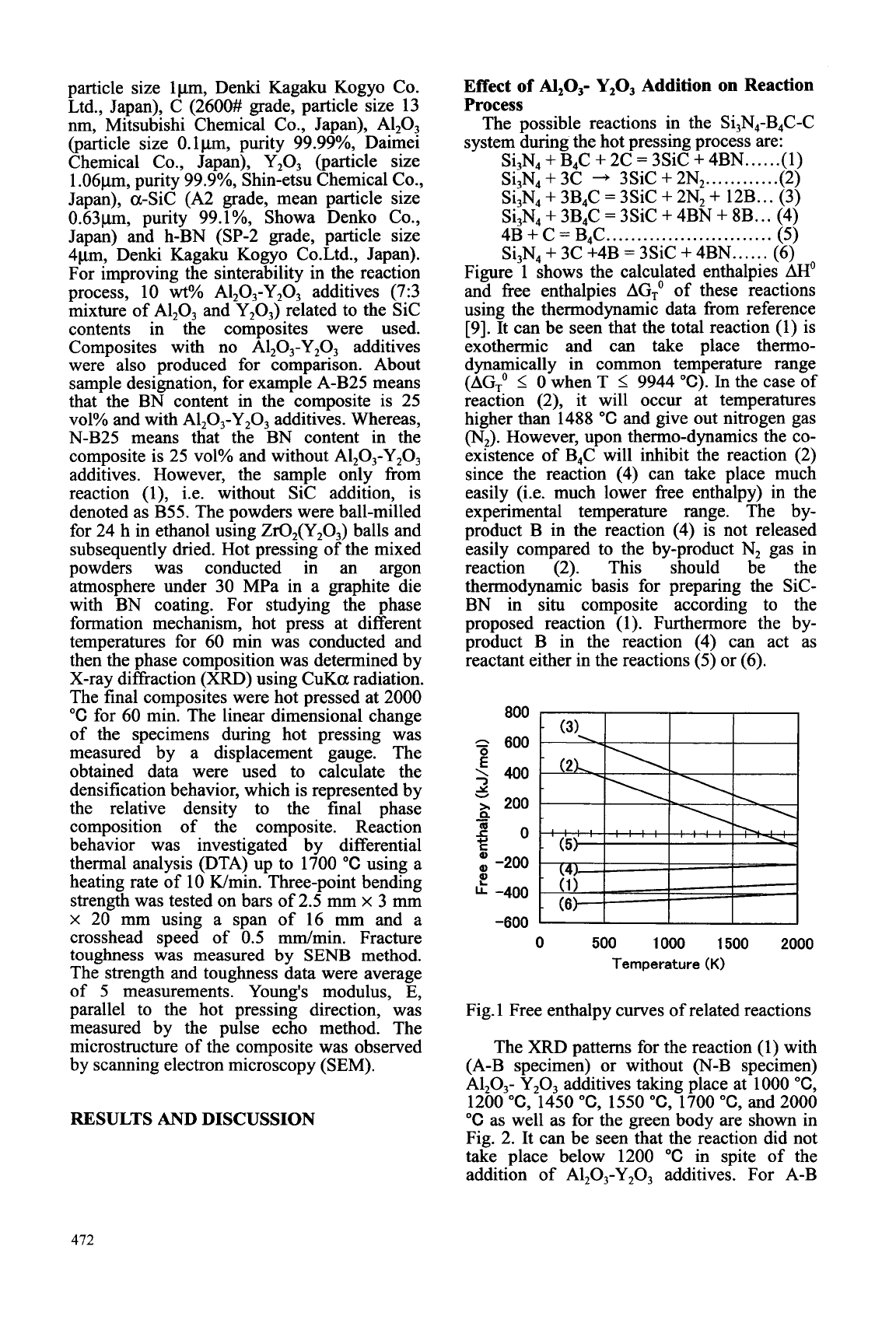
Figure 1 shows the calculated enthalpies
AHo
and free enthalpies
AG:
of these reactions
using the thermodynamic data from reference
[9]. It can be seen that the total reaction (1) is
exothermic and can take place thermo-
dynamically in common temperature range
(AG;
I
0
when T
I
9944 "C). In the case of
reaction (2), it will occur at temperatures
higher than 1488
"C
and give out nitrogen gas
(N,).
However, upon thenno-dynamics the co-
existence of B4C will inhibit the reaction (2)
since the reaction (4) can take place much
easily (i.e. much lower free enthalpy) in the
experimental temperature range. The by-
product B in the reaction (4) is not released
easily compared to the by-product N, gas in
reaction (2). This should be the
thermodynamic basis for preparing the SiC-
BN in situ composite according to the
proposed reaction (1). Furthermore the by-
product B in the reaction (4) can act as
reactant either in the reactions (5) or (6).
800
c
600
\
400
E
2
200
$0
3
v
-
C
Q)
Q)
-200
e!
LL
-400
Fig. 1 Free enthalpy curves of related reactions
The
XRD
patterns for the reaction
(1)
with
(A-B specimen) or without (N-B specimen)
A1203- Y203 additives taking place at 1000 "C,
1200"C, 1450"C, 1550"C, 1700"C, and2000
"C as well as for the green body are shown in
Fig. 2. It can be seen that the reaction did not
take place below 1200 "C in spite of the
addition of A1203-Y@3 additives. For A-B
472
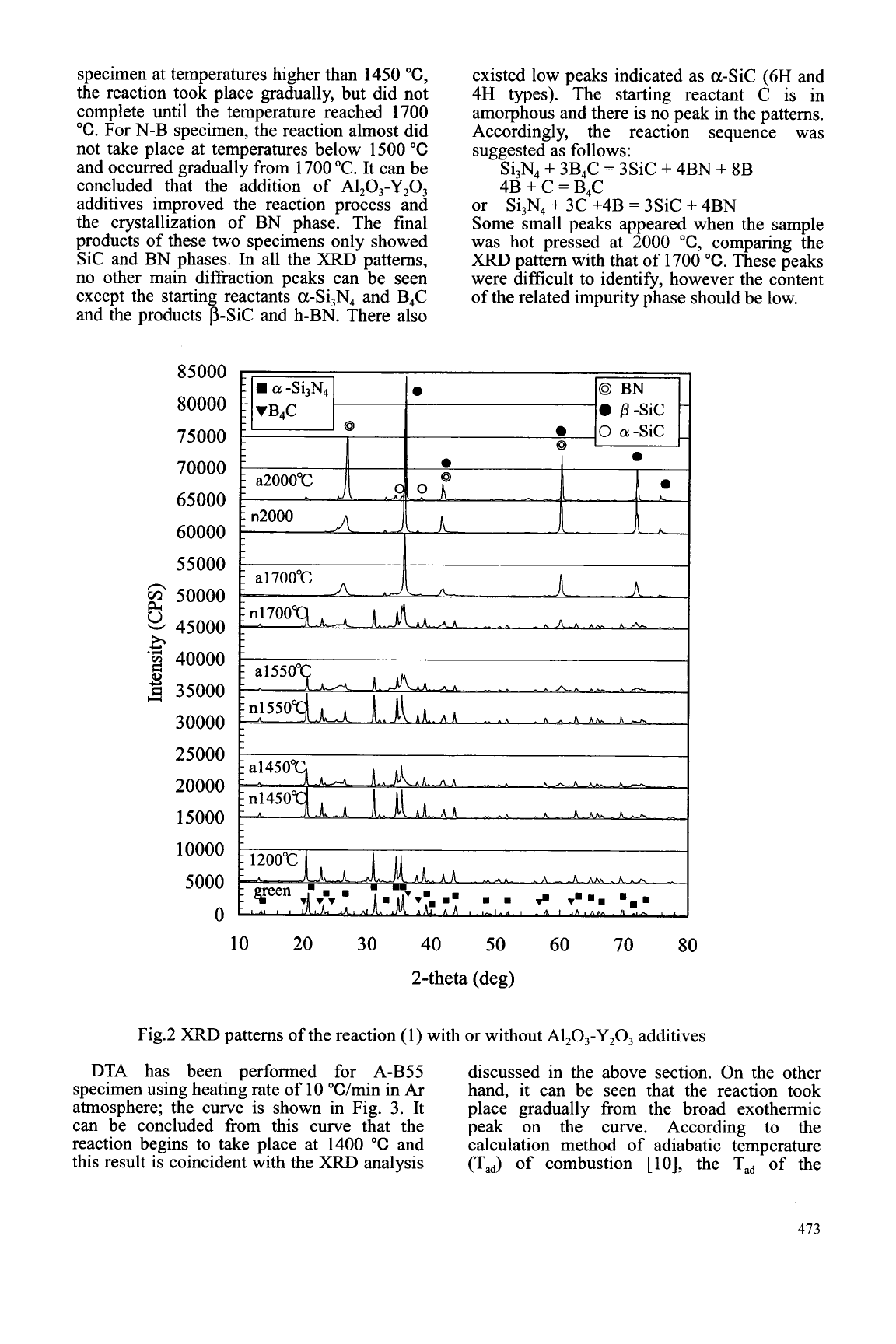
specimen at temperatures higher than 1450
"C,
the reaction took place gradually, but did not
complete until the temperature reached 1700
"C.
For N-B specimen, the reaction almost did
not take place at temperatures below 1500
"C
and occurred gradually from 1700 "C. It can be
concluded that the addition of Al,O,-Y,O,
additives improved the reaction process and
the crystallization of BN phase. The final
products of these two specimens only showed
Sic and BN phases. In all the XRD patterns,
no other main diffraction peaks can be seen
except the starting reactants a-Si,N4 and B4C
and
the products p-Sic and h-BN. There also
~~
I
H
a
-Si,N,
0
0
BN
T
VB,C
0
B-Sic
-
0
a-Sic
-
0
1
0
I
I
0
0
85000
80000
75000
70000
65000
60000
55000
g
50000
2
45000
0
'5;
40000
8
U
5
35000
30000
25000
20000
15000
10000
5000
0
existed low peaks indicated as a-Sic (6H and
4H types). The starting reactant C is in
amorphous and there is no peak in the patterns.
Accordingly, the reaction sequence was
suggested as follows:
Si,N,
+
3B4C
=
3SiC
+
4BN
+
8B
4B
+
C
=
B4C
Si,N,
+
3C +4B
=
3SiC
+
4BN or
Some small peaks appeared when the sample
was hot pressed at 2000
"C,
comparing the
XRD
pattern with that of 1700
"C.
These peaks
were difficult to identify, however the content
of the related impurity phase should be low.
n2000
Ak
I
10 20 30
40
50 60 70
80
2-theta (deg)
Fig.2
XRD
patterns of the reaction (1) with or without Al,O,-Y,O, additives
DTA has been performed for A-B55 discussed in the above section. On the other
specimen using heating rate of 10 "C/min in Ar hand, it can be seen that the reaction took
atmosphere; the curve is shown in Fig. 3. It place gradually from the broad exothermic
can be concluded fi-om this curve that the peak on the curve. According to the
reaction begins to take place at 1400
"C
and calculation method of adiabatic temperature
this result is coincident with the XRD analysis
(T,)
of
combustion
[
101, the
Tad
of the
473
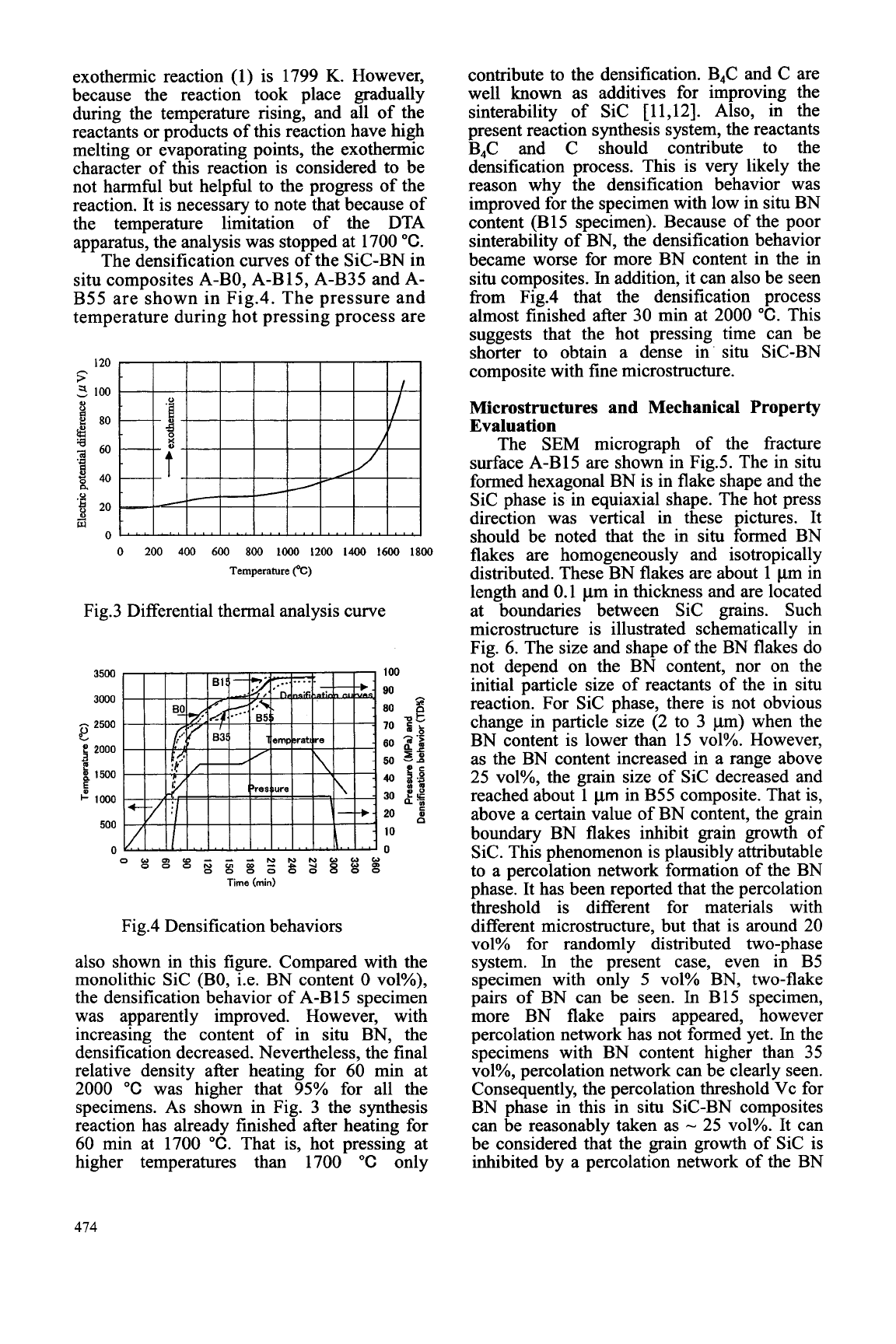
exothermic reaction (1) is 1799
K.
However,
because the reaction took place gradually
during the temperature rising, and all of the
reactants or products of this reaction have high
melting or evaporating points, the exothermic
character of this reaction is considered to be
not harmful but helpful to the progress of the
reaction. It is necessary to note that because of
the temperature limitation of the DTA
apparatus, the analysis was stopped at 1700
"C.
The densification curves of the SIC-BN in
situ composites A-BO, A-B15, A-B35 and A-
B55 are shown in Fig.4. The pressure and
temperature during hot pressing process are
120
r
I
I
I
I
I
I I
I
h
>
5
100
QJ
80
60
g
40
Q
B
8
20
i3
0
0
200
400
600 800 1000 1200
1400
1600 1800
Temperature
("c)
Fig.3 Differential thermal analysis curve
3500
3000
-
2500
e
5
2000
1500
I-
1000
c
500
0
Fig.4 Densification behaviors
also shown in this figure. Compared with the
monolithic Sic (BOY i.e. BN content
0
vol%),
the densification behavior of A-B 15 specimen
was apparently improved. However, with
increasing the content of in situ BN, the
densification decreased. Nevertheless, the final
relative density after heating for
60
min at
2000
"C
was higher that
95%
for all the
specimens. As shown in Fig. 3 the synthesis
reaction has already finished after heating for
60
min at 1700
"C.
That is, hot pressing at
higher temperatures than 1700
"C
only
contribute to the densification. B4C and C are
well
known
as additives for improving the
sinterability of Sic [11,12]. Also, in the
present reaction synthesis system, the reactants
B4C and C should contribute to the
densification process. This is very likely the
reason why the densification behavior was
improved for the specimen with low in situ BN
content (B15 specimen). Because of the poor
sinterability of BN, the densification behavior
became worse for more BN content in the in
situ composites.
In
addition, it can also be seen
from Fig.4 that the densification process
almost finished after 30 min at 2000
"C.
This
suggests that the hot pressing time can be
shorter to obtain a dense in situ Sic-BN
composite with fine microstructure.
Microstructures and Mechanical Property
Evaluation
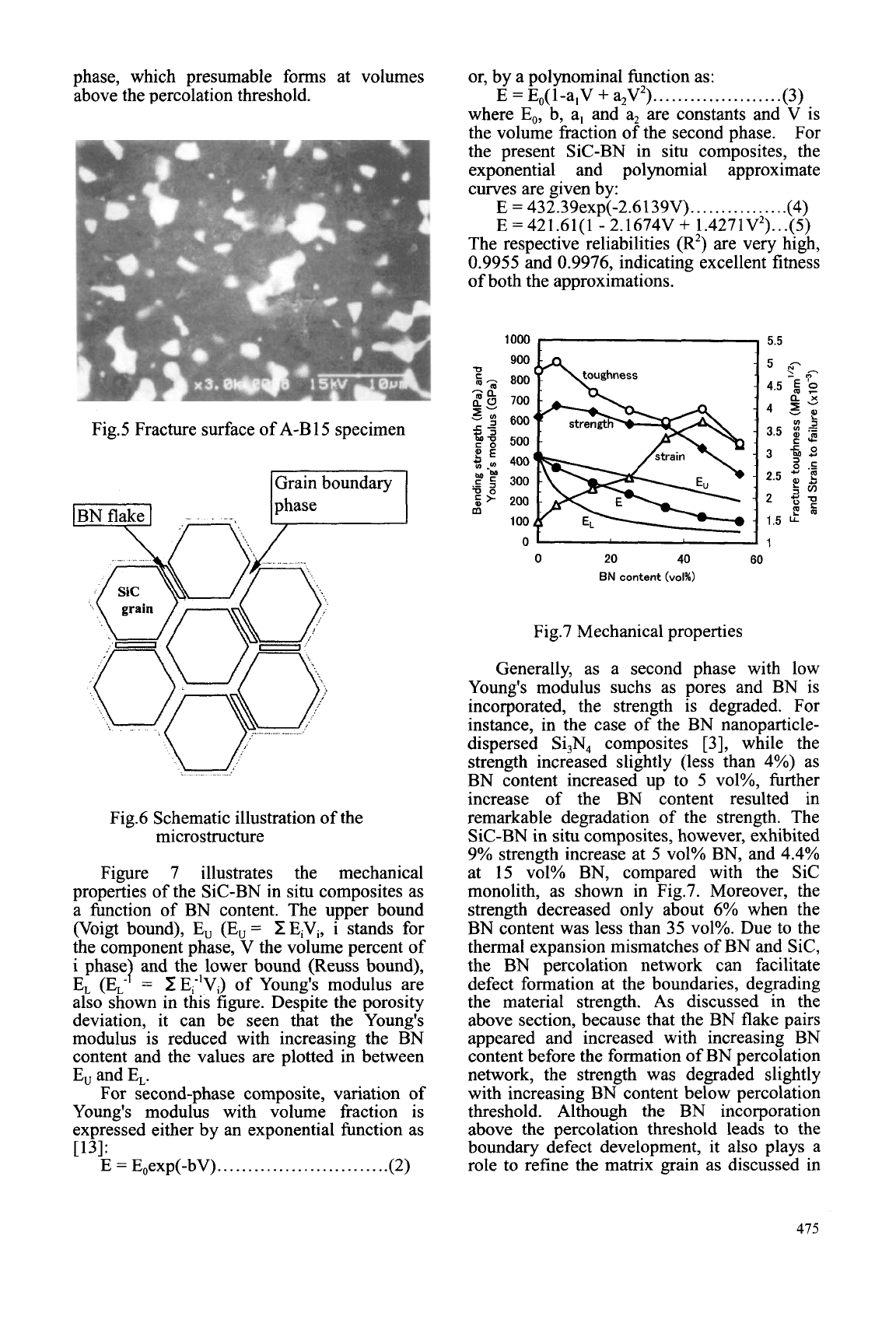
The
SEM
micrograph of the fracture
surface A-B15 are shown in Fig.5. The in situ
formed hexagonal BN is in flake shape and the
Sic phase is in equiaxial shape. The hot press
direction was vertical in these pictures. It
should be noted that the in situ formed BN
flakes are homogeneously and isotropically
distributed. These BN flakes are about 1 pm in
length and 0.1 pm in thickness and are located
at boundaries between Sic grains. Such
microstructure is illustrated schematically in
Fig.
6.
The size and shape of the BN flakes do
not depend on the BN content, nor on the
initial particle size of reactants of the in situ
reaction. For Sic phase, there is not obvious
change in particle size (2 to 3 pm) when the
BN content is lower than 15 ~01%. However,
as the BN content increased in a range above
25 vol%, the grain size of Sic decreased and
reached about 1
pm
in B55 composite. That is,
above a certain value of BN content, the grain
boundary BN flakes inhibit grain growth of
Sic. This phenomenon is plausibly attributable
to a percolation network formation of the BN
phase. It has been reported that the percolation
threshold is different for materials with
different microstructure, but that is around 20
vol% for randomly distributed two-phase
system.
In
the present case, even in B5
specimen with only
5
vol% BN, two-flake
pairs of BN can be seen.
In
B15 specimen,
more BN flake pairs appeared, however
percolation network has not formed yet. In the
specimens with BN content higher than 35
vol%, percolation network can be clearly seen.
Consequently, the percolation threshold Vc for
BN phase in this in situ SIC-BN composites
can be reasonably taken as
-
25 ~01%. It can
be considered that the grain growth of Sic is
inhibited by a percolation network of the BN
474
phase, which presumable forms at volumes
above the percolation threshold.
Fig.5 Fracture surface of A-B 15 specimen
or, by a polynominal function as:
where
E,,
b, a, and a, are constants and V is
the volume fraction
of
the second phase. For
the present SIC-BN in situ composites, the
exponential and polynomial approximate
curves are given by:
The respective reliabilities
(R2)
are very high,
0.9955 and 0.9976, indicating excellent fitness
of both the approximations.
E
=
E,(1-a,V
+
a,V2)
.....................
(3)
...............
E
=
432.39exp(-2.6139V). (4)
E
=
421.61(1
-
2.1674V
+
1.4271V2)
...(
5)
1000
I
5.5
/Grain boundary
I
0
20
40
60
BN
content
(~01%)
Fig.7 Mechanical properties
-"
Fig.6 Schematic illustration of the
microstructure
Figure 7 illustrates the mechanical
properties of the Sic-BN in situ composites as
a function
of
BN content. The upper bound
(Voigt bound),
E,
(E,
=
1
EiVi, i stands for
the component phase, V the volume percent of
i phase and the lower bound (Reuss bound),
EL
(E;
=
ZEi-'Vi) of Young's modulus are
also shown in this figure. Despite the porosity
deviation, it can be seen that the Young's
modulus is reduced with increasing the BN
content and the values are plotted in between
E, and
EL.
For second-phase composite, variation
of
Young's modulus with volume fraction is
expressed either by an exponential function as
[13]:
..........................
E
=
E,exp(-bV).. (2)
Generally, as a second phase with low
Young's modulus suchs as pores and BN is
incorporated, the strength is degraded. For
instance, in the case of the BN nanoparticle-
dispersed Si,N, composites [3], while the
strength increased slightly (less than 4%) as
BN content increased up to 5 vol%, further
increase
of
the BN content resulted in
remarkable degradation
of
the strength. The
Sic-BN in situ composites, however, exhibited
9%
strength increase at
5
vol% BN, and 4.4%
at 15 vol% BN, compared with the Sic
monolith, as shown in Fig.7. Moreover, the
strength decreased only about 6% when the
BN content was less than 35 ~01%. Due to the
thermal expansion mismatches of BN and Sic,
the BN percolation network can facilitate
defect formation at the boundaries, degrading
the material strength.
As
discussed in the
above section, because that the BN flake pairs
appeared and increased with increasing BN
content before the formation of BN percolation
network, the strength was degraded slightly
with increasing BN content below percolation
threshold. Although the
BN
incorporation
above the percolation threshold leads to the
boundary defect development, it also plays a
role to refine the matrix grain as discussed in
475

the previous section and improves the material
strength. These two contradictory factors may
cause the gradual strength decrease in the BN
content range from 15 to
35
vol%
,
as shown
in Fig. 7. The fracture toughness increased a
little for B5 and then decreased gradually and
the details of the mechanism are under
investigation.
The strain to failure of the in situ Sic-BN
composites calculated from
&
=
0
E-' is also
shown in Fig.6. For improving the reliability
of structural ceramics, particularly when they
are used in conjunction with different
materials like metals, large strain-to-failure, or
strain tolerance, is an essentially important
property. In the present case, the strain-to-
failure increased remarkably with increasing
the BN content and reached the maximum,
which was about 2.5 times larger than that of
the Sic monolith, at 45 vol% BN content.
CONCLUSIONS
SIC-BN in
situ
composites were prepared
based on the in situ reaction of Si3N4, B4C and
C by hot pressing at
2000
"C
for 60 min
under 30 MPa. The effect of BN content on the
densification behavior, microstructure, elastic
modulus, bending strength and fracture
toughness of the SIC-BN in situ composites
was studied. The change of elastic modulus
with BN content obeyed both of the
exponential and polynomial rules. The
densification behavior of this in situ system
was excellent even at high BN content. The
grain growth of Sic was inhibited by the in
situ formed BN flakes at high BN contents
above
25
~01%. The bending strength
increased slightly with increasing BN content
up to 15 vol% and then decreased gradually.
Above 35 vol%, the strength decrease was
accelerated. These results were explained
according the BN percolation network
formation. The markedly low elastic modulus
as well as the relatively high strength were
indicative of excellent strain tolerance of this
material.
ACKNOWLEDGMENTS
This work has been supported by AIST,
MITI, Japan, as part of the Synergy Ceramics
Project.
of
B,03 with
AIN
and/or Si3N4
to
Form
BN-
Toughened Composites,
J.
Am. Ceram. SOC.,
(2)
E.
H. Lutz
and
M.
V.
Swain, Fracture
Toughness and Thermal Shock Behavior of
Silicon Nitride-Boron Nitride Ceramics,
J.
Am.
Ceram. SOC.,
75, (1992) 67-70.
(3) T. Kusunose,
Y.
H.
Choa, T. Sekino and
K.
Niihara, Mechanical Properties
of
Si,N,/BN
Composites by Chemical Processing, Key
Engineering Materials, 16
1
-
1
63,
(
1999) 475-
80.
(4)
P.
G.
Valentine, A. N. Palazotto, R.
Ruh
and D.
C. Larsen, Thermal Shock Resistance
of
SiC-
BN Composites,
Adv.
Ceram. Mater.,
1,
(1986)
(5)
R.
Ruh,
A.
Zangvil and
R.
R.
Wills, Phase and
Property Studies
of
Sic-BN Composites, Adv.
Ceram. Mater., 3,
(1988)
411-15.
(6) R.
Ruh,
L.
D.
Bentsen
and
D. P.
H.
Hasselman,
Thermal
Difisivity
Anisotropy
of
SiC/Bhr
Composites,
J.
Am.
Ceram.
SOC.,
67, (1984)
C-
(7)
R.
Riedel, A. Kienzle, W. Dressler,
L.
Ruwisch,
J.
Bill
and
F.
Aldinger,
A
Silicoboron
Carbonitride
Ceramic
Stable
to
2000
"C,
Nature (London), 382, (1996) 796-798.
(8)
B. Baufeld, H. Gu,
J.
Bill,
F.
Wakai and F.
Aldinger, High Temperature Deformation
of Precursor-derived Amorphous Si-B-C-N
Ceramics,
J.
Eur. Ceram. SOC., 19, (1999)
(9)
I.
Barin and
0.
Knacke, Thermochemical
Properties of Inorganic Substances,
Spinger-Verlag, BerlidHeidelberg and
Verlag Stahleisen m.b.H., Dusseldorf,
Germany, 1973.
(10)
Z.
A. Munir, Synthesis of High
Temperature Materials by Self-propagating
Combustion Methods, Amer. Ceram. SOC.
Y.
Zhou, H. Tanaka,
S.
Otani and
Y.
Bando, Low-Temeprature Pressureless
Sintering of a-Sic with A14C3-B,C-C
Additions,
J.
Am.
Ceram. SOC.,
82, (1999)
(12)
H.
Gu,
Y.
Shinoda and
F.
Wakai,
Detection of Boron Segregation to Grain
Boundaries in Silicon Carbide by Spatially
Resolved Electron Energy-Loss
Spectroscopy,
J.
Am. Ceram. SOC., 82,
W. D. Kingery, H.
K.
Bowen and D.
R. Uhlmann, Introduction to Ceramics,
Second Edition, John Wiley
&
Sons, Inc.,
New
York,
USA, (1976) 768-777.
7
1,
(1 988)
1080-85.
8
1-87.
83-C-84.
2797-2814.
Bull., 67, (1988) 342-49.
(1 1)
1959-64.
(1 999) 469-72.
(13)
REFERENCES
(1)
W.
S.
Coblenz
and
D.
Lewis
,
In
Situ
Reaction
476
COATING EXPERIMENTS ON CARBON FIBERS USING
A
CONTINUOUS
LIQUID COATING PROCESS
N.
Doslik*,
R
Gadow
Institute for Manufacturing Technologies of Ceramic Components and Composites,
University of Stuttgart, Allmandring
5b,
D-70569 Stuttgart, Germany
ABSTRACT
Carbon fibers are found today in a wide range of applica-
tions as ceramic and metal matrix composite component
with characteristic features as high tenacity. Commer-
cially available fibers aren't appropriate for application at
high
temperatures because of the decay of tenacity
caused
by oxidative degradation.
The best prospects for new high temperature stable coat-
ing materials show the
use
of SiBCN- and SiCN-systems.
The realization is
carried
out
by
a liquid polymer impreg-
nation with
polyorgano-(boro)silazanes
in a continuous
INTRODUCTION
The
high
potential
of
carbon fibers as refractoq reinforc-
ing component in ceramic and metal
matrix
composites"]
strongly depends on the chemical and physical compati-
bility to the surrounding
matrix.
Therefore, new precur-
sor systems based on
polyorgano-(boro)silazanes
are
applied for coatings. One of the best prospects show the
use of SiBCN-systems, especially to achieve high tem-
perature stable materials. For carbon fiber coating, the
knowledge of the rheological and wetting behavior of the
precursor solvents is one
of
the most important
require-
ments to get coatings without defects.[*] The
precursors
are optimized in viscosity by addition
of
solvents and
Wer compounds and after detailed rheological investi-
gations they are adapted in the coating process. By opti-
mization of the rheological properties homogeneous and
crack-free coatings on carbon monofilaments
are
ob-
taine~i[~]
The
realization of the continuous fiber coating is
camed out
by
a liquid polymer impregnation. The liquid
coating is followed by a
two
step thermal treatment
process which is carried out in inert atmosphere. The
first
step allows drymg, curing and polymerization of the
pre-
cursors and the second
performs
controlled pyrolysis to
ceramize the intermediate inorganic polymer coating
leading
to
systems which are resistive against oxidation
and chemical attack e.g. in metal melts at high tempera-
tures.
r
I
L
Fig.1:
Liquid phase coating and impregnation
@PI)
fiber coating process. These precursors are optimized in
viscosity
by
the addtion of solvents and further com-
pounds after detailed rheological investigations and
adapted in the coating process. In a continuous them
treatment and
fiber
trzlnsport
the precursor coatings are
dried,
polymerized and ceramized leading to systems
which
are
resistive against oxidation and chemical attack
e.g. in metal melts at high temperatures. The oxidation
resistance of the coated composites was evaluated using
thermogravimetric analysis in atmosphere.
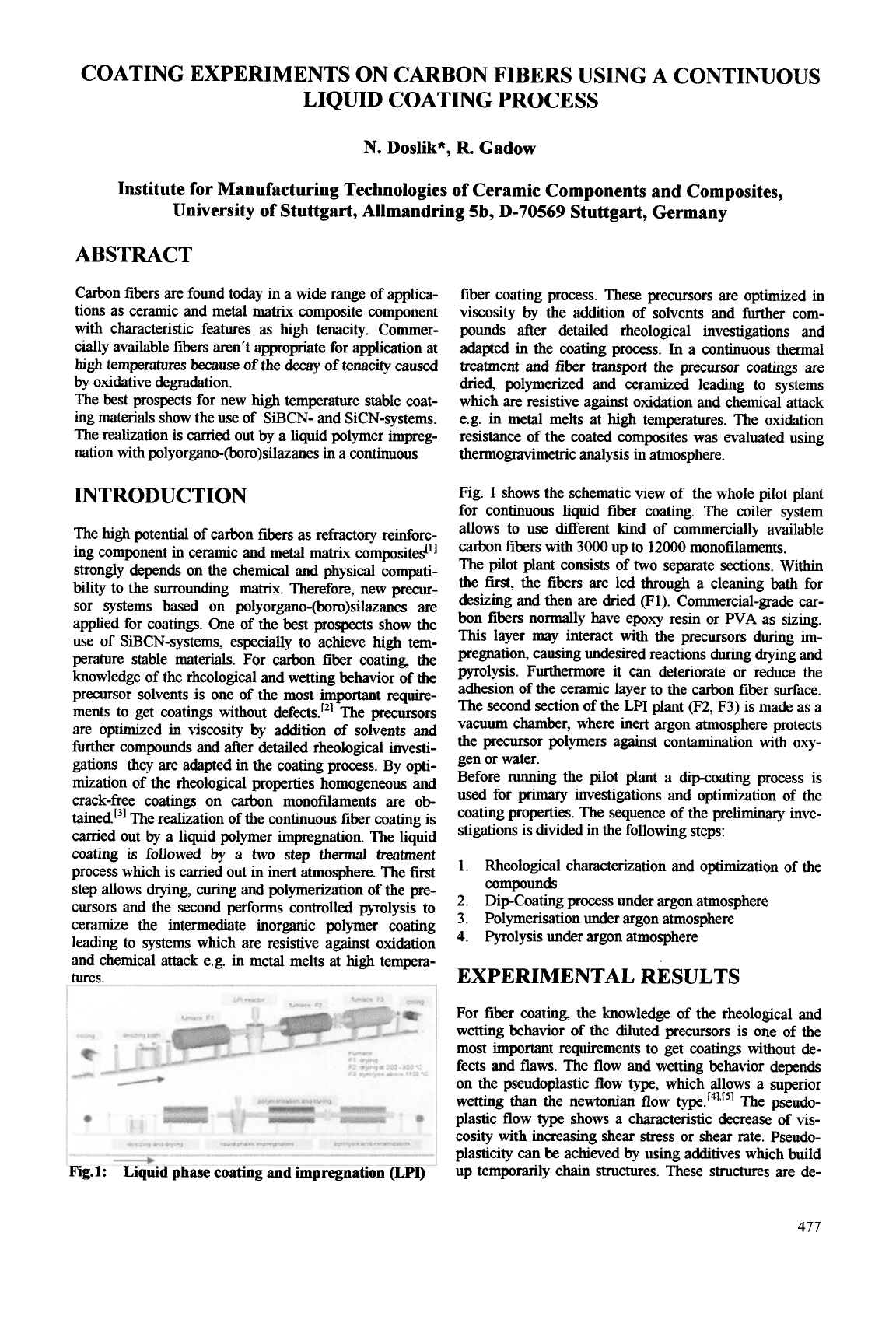
Fig.
1
shows the schematic view
of
the whole pilot plant
for continuous liquid fiber coating. The coiler system
allows to
use
different kind of commercially available
carbon fibers with
3000
up to 12000 monofilaments.
The pilot plant consists of
two
separate sections. Within
the first, the fibers
are
led through a cleaning bath for
desizing and then
are
dried (Fl). Commercial-grade car-
bon fibers normally have epoxy resin
or
PVA as sizing.
This
layer may interact with the precursors during im-
pregnation, causing undesired reactions dunng drymg and
pyrolysis. Furthermore it
can
deteriorate or reduce the
adhesion
of
the ceramic layer to the carbon fiber surface.
The second section of the
LPI
plant (F2, F3) is made as a
vacuum chamber, where inert argon atmosphere protects
the
precursor
polymers against contamination with oxy-
gen or water.
Before running the pilot plant a dipcoating process is
used
for
primary
investigations and optimization of the
coating properties. The sequence of the preliminary inve-
stigations is divided in the following steps:
1. Rheological characterization and optimization of the
compounds
2. Dip-Coating process under argon atmosphere
3.
Polymerisation under argon atmosphere
4.
Pyrolysis under argon atmosphere
EXPERIMENTAL RESULTS
For fiber coating, the knowledge
of
the rheological and
wetting behavior of the diluted precursors is one of the
most important requirements to get coatings without de-
fects and flaws. The flow and wetting behavior depends
on the pseudoplastic flow
type,
which allows a superior
wetting
than
the newtonian flow
type.1413[51
The pseudo-
plastic
flow
type
shows a characteristic decrease
of
vis-
cosity with increasing shear
stress
or
shear
rate. Pseudo-
plasticity
can
be achieved
by
using additives which build
up temporarily chain structures. These structures are de-
477

stroyed in a reversible process with increasing shear
stress.
A
new measurement method to determine the rheological
behavior of the precursors is the rotative oscillation
method, which analyzes the viscuelastic properties.
Vis-
coelasticity
stands
for the ratio of the elastic and plastic
(viscous)
part
of flow properties. For measurin& a sample
is
poured
in a gap between a cone and a plate. The cone
makes a rotative oscillation. Torque and phase displace-
ment are measured, rheological
data
are
calculated. The
method of determination
used
for the compounds is the
amplitude sweep measuring method
(AMS,
frequency
=
constant, amplitude
=
variable). The amplitude
sweep
is
able to determine the linear viscoelastic behavior, the
flow point, the point
of
change of predominance of vis-
coelastic moduli and the stability of the additive. The
storage modulus
G
stands
for the elastic
part
of the vis-
coelasticity, the loss modulus
G'
for the plastic
part.
With increasing storage modulus the sample
shows
a
solid state like behavior, with increasing loss modulus the
sample shows a fluid like."
In Fig2 the SiBCN-precursor
Rt7],
diluted in the aprotic
solvent tetrahydrofiuane, shows newtonian flow behavior
with a constant low viscosity
lq*l=
0,016
Pas.
The loss
modulus
is
six
orders of
magnitude
higher
than
the stor-
age modulus, thus the sample is very
thin
fluid and has
non appropriate adhesion on the
substrate.
A
liquid with a
high
loss modulus and a low viscosity at low shear rates is
not able to stick to the surface of the substrate.
1E-4
1EJ
a
1E4
s,
0.01
c
-*--.-*
-*
--H
--*
*
a-
-
*-+W
*-a*
0.1
shear
stress
7
[pal
T
r
B
-
0.02
2
5
.E
H
--L
0.01
Fig.2
Newtonian
flow
behavior
of
pFecursor
P2
without additive
10,015
Fig.
3:
Structural
viscous flow behavior
of
precursor
P2
with additive PVB
Fig.3 shows the precursor
P2
with the additive polyvinyl-
butyral
(PVB).
This
sample
shows
pseudoplastic behavior
due to the reversible interaction with the additive. From
textile ingineering and sizing it is
known
that
pseudoplas-
ticity enhances the wetting and penetration of fiber
strands
by
liquid
coatings.
The higher viscosity at low
shear
stresses
Iq*l
=
0,037
Pas
enables the precursor to
wet the
substrate
and to stick on the surface (compound
mixture:
15,25
x
lo-'
mom
P2,
3,O
x
lo4
moVl
PVB,
17,74
x
lo4
mom
THF).
41
i
b
3
1N-
1E-4-
1M.
3
1Ed
-
1E-7.
h
t
It
1
Ed
. .
14014
0.M
a1
shear
stresa
%
pa]
Fig.4:
Newtonian flow behavior
of
SiCN-precursor
PCS
without additive
The SiCN-precursor
PCS['],
also
diluted in tetmhydroh-
rane, shows a comparable rheological behavior (Fig.4) to
sample
P2
without
PVB
additive (Fig.2) with a non ap-
propriate wetting characteristic.
The additive effect of
PVB
on the flow behavior of
PCS
is very significant (compound
mixture:
7,7
x
10"
moVl
PCS,
7,O
x
10"
mom
PVB,
17,74
x
lo4
moVl
TJXIF).
It
induces in addition to the pseudoplasticty (Fig.5).
1
030
-
025
0.01
lE-3
-
0.20
g
I
r
-
0.15
F
-
1E-4-
mpwraool*.-lkm*~
1Eb- 0*1°%
0.05
0.00
0.01 0.1
1
shear
stress
7
pa]
Fig.
5:
Hardening behavior
of
SiCN-precursor PCS
with additive PVB
The increasing storage modulus depends on the
observed
hardening
process
of
PVB.
Dumg
the
period
of shear
stress
variation (totally
ca.
12
mia,
measurement each 30
sec)
an irreversible crosslinking of the precursor
/
additive
mixture
occurs
simultaneously. The expected pseudoplas-
ticity, expressed by decreasing viscosity under rising
shear
stress,
is overcompensated
by
the viscosity increase
due to the
crosslinking.
The storage modulus increases
478
from 1E-7
Pa
to 0,9 Pa. The loss modulus increases
smoothly from 0,l to
0,8
Pa.
The sample makes a transi-
tion to a slightly solid state characteristics (transition
point: 0,l Pa shear stress). The viscosity moves from
0,05
to
0,25
Pas after hardening. The sample
has
a good wet-
ting behavior due to the pseudoplasticity and a superior
adhesion due to the hardening process.
The SiCN-precursor
HPSr8]
is unlike the other examined
precursors a highly viscous liquid. Nevertheless the
rheological data show an newtonian flow behavior
(Fig.6). PVB addition was not expected to be
sumssful
because of the critically high viscosity caused by
crosslinking.
To
point out
if
the wetting properties of this
precursor are appropriate for a
satisfying
coating, the
multimode frequency sweep
(MFS)
method has to be
used because it provides evaluable data even for newto-
nian fluid coatings.
But
the mathematical model is only
valid under the condition of linear viscoelastic behavior
(G' parallel to G"
).
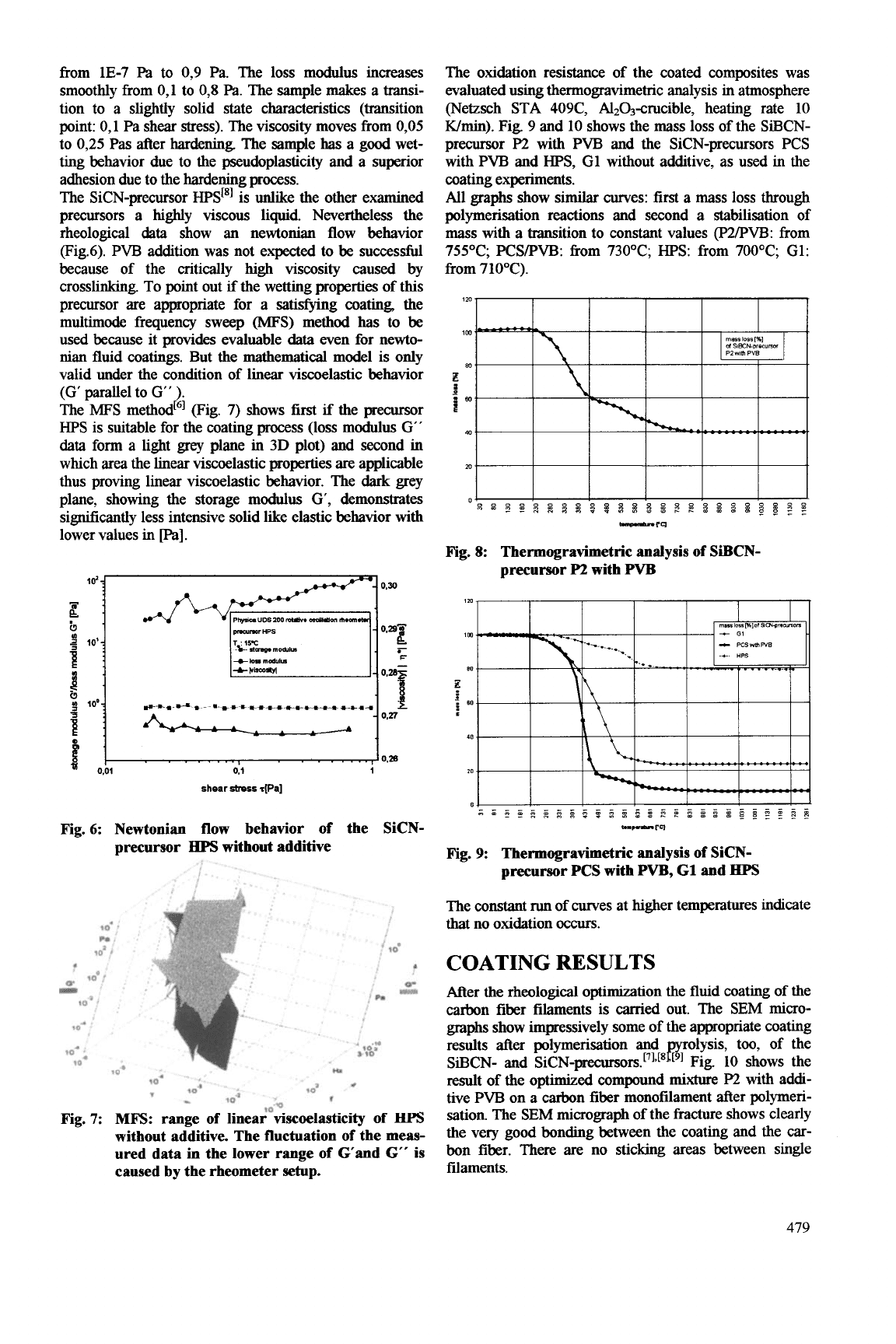
The
MFS
method6] pig. 7) shows first
if
the precursor
HPS
is suitable for the coating process
(loss
modulus
G"
data form a light grey plane in
3D
plot) and second in
which area the linear viscoelastic properties
are
applicable
thus proving linear viscoelastic behavior. The
dark
grey
plane, showing the storage modulus
G',
demonstrates
sigmiicantly less intensive solid like elastic behavior with
lower values in
[pa].
0.1
1
shear
mess
r[Pa]
Fig.6:
Newtonian flow behavior
of
the SiCN-
precursor
HPS
without additive
Fig.
7:
MFS:
range
of
linear viscoelasticity
of
EIPS
without additive. The fluctuation
of
the meas-
ured data in the lower range
of
Gand G" is
caused by the rheometer setup.
The oxidation resistance
of
the coated composites was
evaluated using thermogravimetric analysis in atmosphere
(Netzsch STA 409C, Al2G-crucib1e, heating rate
10
Wmin).
Fig.
9 and
10
shows the
mass
loss
of
the SiBCN-
precursor
P2
with
PVB
and
the SiCN-precursors PCS
with PVB and
HPS,
G1 without additive, as used in the
coating experiments.
All graphs show similar curves:
first
a
mass
loss through
polymerisation reactions and second a stabilisation of
mass
with a transition to constant values
(P2PVB:
from
755OC; PCSPVB: fiom 730OC;
HPS:
from 700°C; G1:
from 710°C).
rw
m
z
f
1"
40
Fig.
8:
Thermogravimetric analysis
of
SiBCN-
precursor
P2
with
PVB
Fig.
9:
Thermogravimetric analysis
of
SiCN-
precursor
PCS
with
PVB,
G1 and
HPS
The constant
run
of curves at higher temperatures indicate
that no oxidation
occurs.
COATING
RESULTS
After the rheological optimization the fluid coating
of
the
carbon fiber filaments is carried out. The
SEM
micro-
graphs show impressively some
of
the appropriate coating
results after polymerisation and rlysis, too, of the
SBCN- and SiCN-precursors.[71,[8
[
Fig. 10 shows the
result of the optimized compound
mixture
P2
with add-
tive PVB on a carbon fiber monofilament after polymeri-
sation The
SEM
micrograph of the fracture shows clearly
the very good bonding between the coating and the car-
bon fiber.
There
are
no sticking areas between single
filaments.
479

Fig. 11: SEM micrograph
of
SiCN-precursor PCS
with additive PVB
afetr
polymerization at
215OC under argon atmosphere
The polymerisation, to fix and
cure
the coating on the
carbon fiber monofilaments, is followed
by
the pyrolysis
at higher temperatures to build
up
the ceramic structure
of
the coating. Fig.
12
shows the SiBCN-precursor
p2
after
pyrolysis at
1
100°C
under argon atmosphere. The result is
a homogeneous and crack-fkee ceramic coating with non-
sticking monofilaments.
Fig. 10: SEM micrograph
of
SiBCN-precursor
P2
with additive
PVB
after polymerization at
305OC under
argon
atmosphere
Similar results are reached for the polymerisation of the
optimized compound
mixture
of
the SiCN-precursor
PCS
(Fig.
11).
Fig. 12: SEM micrograph
of
SiBCN-precursor
P2
with
additive PVB after pyrolysis
at
llOO°C under
argon atmosphere (monofilament)
480
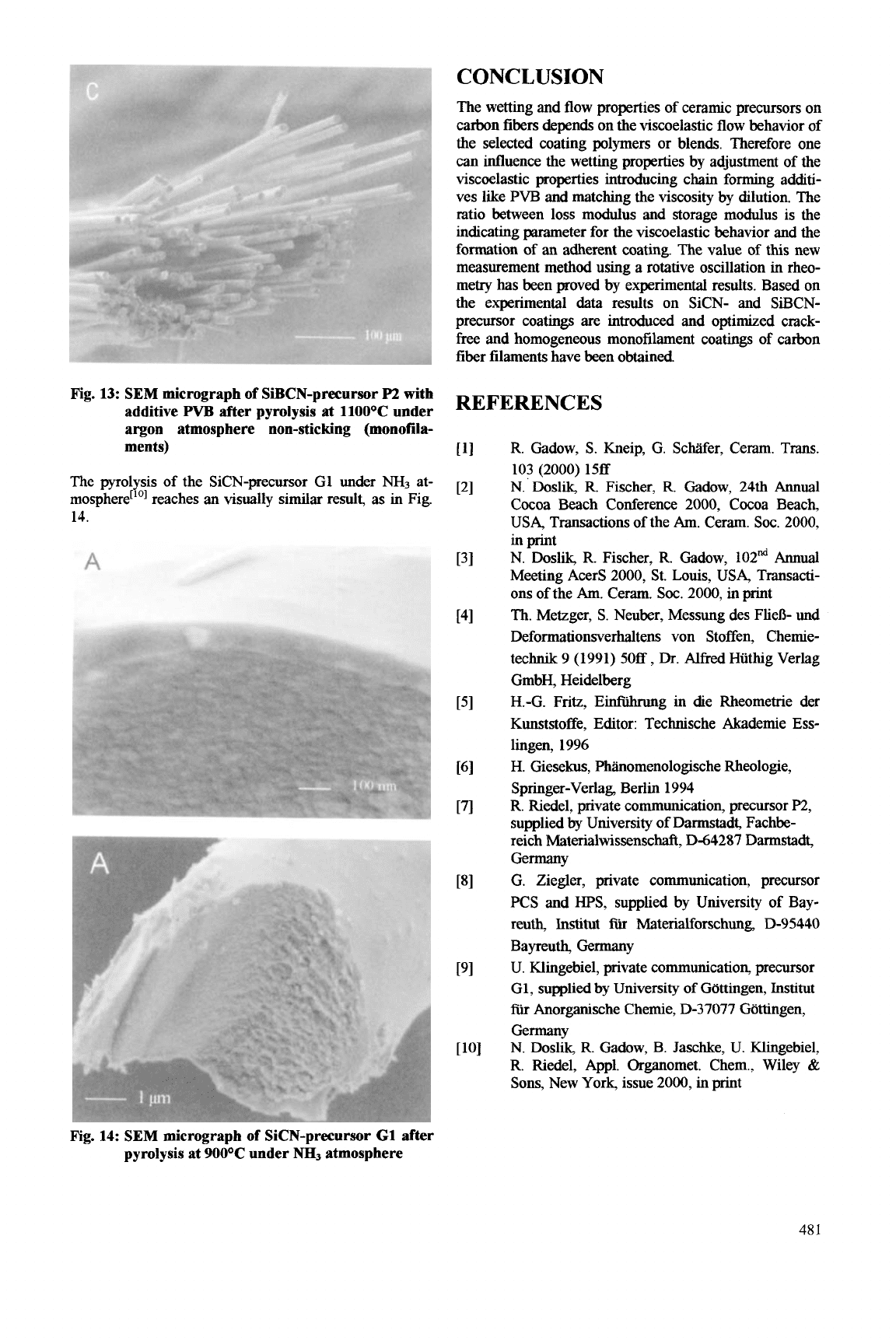
CONCLUSION
Fig. 13:
SEM
micrograph
of
SiBCN-precursor
P2
with
additive
PVB
after pyrolysis
at
llOO°C under
argon atmosphere non-sticking (monofila-
ments)
The pyrolysis of the SiCN-precursor G1 under
NH3
at-
mospherer'O1 reaches an visually similar
result,
as in Fig.
14.
The wetting and flow properties of ceramic precursors on
carbon fibers depends on the viscoelastic flow behavior of
the selected coating polymers or blends. Therefore one
can influence the wetting properties by adjustment of the
viscoelastic properties introducing chain forming additi-
ves like
PVB
and matching the viscosity by
dilution.
The
ratio between
loss
modulus and storage modulus is the
indicating parameter for the viscoelastic behavior and the
formation of
an
adherent coating. The value of
this
new
measurement method
using
a rotative oscillation in rheo-
metry
has
been proved by experimental results. Based
on
the experimental data results on SiCN- and SiBCN-
precursor coatings are introduced and optimized crack-
free and homogeneous monofilament coatings of carbon
fiber filaments have been obtained
REFERENCES
R.
Gadow,
S.
Kneip, G. ScMer, Ceram. Trans.
103
(2000)
15ff
N. Doslik,
R.
Fischer,
R.
Gadow, 24th Annual
Cocoa Beach Conference 2000, Cocoa Beach,
USA,
Transactions of the
Am.
Ceram.
Soc.
2000,
N. Doslik,
R.
Fischer,
R.
Gadow, 102"'
Annual
Meeting AcerS
2000,
St.
Louis,
USA,
Transacti-
ons of the
Am.
Ceram.
Soc.
2000,
in print
Th. Metzger,
S.
Neuber, Messung des Fliefi-
und
Deformationsverhaltens von Stoffen, Chemie-
technik 9 (1991) 50ff,
Dr.
Alfred Huthig Verlag
GmbH, Heidelberg
H.-G. Fritz, Einfikung in die Rheometrie der
Kunststoffe, Editor: Technische Akademie Ess-
lingen, 1996
H.
Giesekus, FWnomenologische Rheologie,
Springer-Verlag Berlin 1994
R.
Riedel, private communication, precursor
P2,
supplied
by
University of Darmstadt, Fachbe-
reich Materialwissenschaft, D-64287 Darmstadt,
G.
Ziegler, private communication, precursor
PCS
and
HPS,
supplied by University of Bay-
reuth,
Institut
fiir
Materialforschung D-95440
Bayreuth,
Germany
U.
Klingebiel, private communication, precursor
G1,
supplied
by
University
of
GiSttingen, Institut
fiir
Anorganische Chemie, D-37077 Gottingen,
N.
Doslik,
R.
Gadow, B. Jaschke,
U.
Klingebiel,
R.
Riedel, Appl. Organomet. Chem., Wiley
&
Sons,
New York, issue 2000, in print
in print
Ge-Y
Ge-Y
Fig. 14:
SEM
micrograph
of
SiCN-precursor G1
after
pyrolysis at 9OOOC under
NE13
atmosphere
48
1
