Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

818 S.W. Hell and A. Schönle
hr
hr
fr
c
v
v
()
()
()=
++
−
(
)
∑
11
1
ς
ς
ς
ν
(32)
transformation
ok
hk
kgggggg
c
()
=
()
+
⊗
()
++ ⊗+ ⊗⊗+
()
ˆ
1
23
ζ
δξξ ξ
… (33)
At low intensities, ζ and therefore ξ is so small that only the linear term
is relevant and the convolution extends the support to 6k as discussed
above. The larger the maximum intensity, the more important higher
orders of the Taylor series will become. These involve multiple auto-
convolutions of the function g extending the support further and
further.
While a quantitative treatment in frequency space is more compli-
cated and less intuitive than the one introduced in the previous section,
the following analysis gives a feel for the effect of the saturation factor
and also illustrates the possible vast expansion of the OTF support. For
the sake of simplicity we assume a Gaussian form of the light distribu-
tion function
f(x) = 1 − exp(−x
2
/2a
2
) (34)
The properly normalized m-fold auto-convolution of g is then given by
⊗= −
m
gk a m ak m
ˆ
() / ( /( ))22
22
π exp
(35)
Now let us assume that the useful support ends at a frequency where
the OTF is attenuated to a small fraction ε of its value at small frequen-
cies. For large saturation factors the infl uence of the convolution with
the confocal OTF on the cut-off frequency can be neglected and we
have to calculated the sum in brackets in (33). Substituting (35) into
equation (33) and approximating the sum by an integral we get for the
term in brackets
ok i iak,/lnexplnςπξ ξ
(
)
≅− −
()
2
(36)
For large saturation factors we can write lnξ = lnζ − ln(ζ + 1) ≅ −1/ζ
and obtain
ok ak,exp(/)ςπζ ς
(
)
≅−2
(37)
This means that the attenuation of the modulus of the OTF at large
frequencies is anti-proportional to the square-root of the saturation
factor. This is equivalent to saying that the resolution increases with
the square root of the saturation factor just as we expected from our
previous analysis.
4.1 STED Microscopy
STED microscopy produces subdiffraction resolution and subdiffrac-
tion-sized fl uorescence volumes in exactly the manner described above
by the depletion of the fl uorescent state of the dye. Depletion inherently
implies saturation of the depleting transition. At present, it is realized
in a (partially confocalized) spot-scanning system due to a number of
technical advantages, but it has been conceptually clear from the outset
With g(r) = 1 − f(r) and = ζ/( ζ + 1 ) we obtain the OTF after Fourier
ξ
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 819
490nm
244nm
STED
source
EXCT.
source
DET
DC
DC
PP
97nm
104nm
x
z
c)
d) e)
fluorescent
AA
BB
non-fluorescent
450 650
λ[nm]
Excitation
STED
Detection
a)
b)
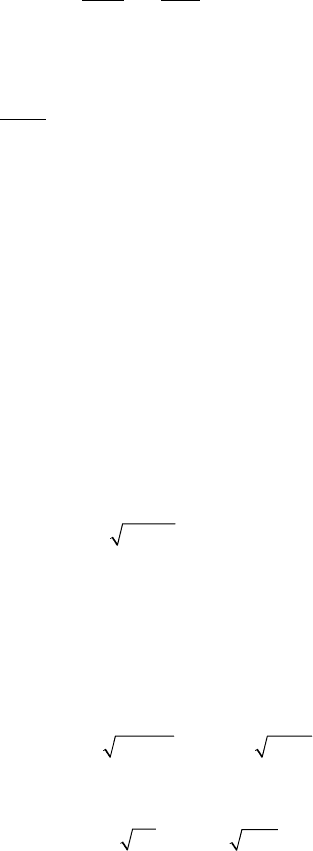
Figure 12–7. Stimulated emission depletion (STED) was the fi rst implementation of the RESOLFT
principle. (a) Dye molecules are excited into the S
1
(state A) by an excitation laser pulse. (b) Fluorescence
is detected over most of the emission spectrum. How ever, molecules can be quenched back into the
ground state S
0
(state B) using stimulated emission before they fl uoresce by irradiating them with a light
pulse at the edge of the emission spectrum shortly after the excitation pulse and before they are able to
emit a fl uorescence photon. Saturation is realized by increasing the intensity of the depletion pulse and
consequently inhibiting fl uorescence everywhere except at the “zero points” of the focal distribution of
the depletion light. (c) Schematic of a point-scanning STED microscope. Excitation and depletion beams
are combined using appropriate dichroic mirrors (DC). The excitation beam forms a diffraction-limited
excitation spot in the sample (inset in d) while the depletion beam is manipulated using a phase-plate
(PP) or any other device to tailor the wavefront in such a way that it forms an intensity distribution with
a nodal point in the excitation maximum (left inset in e). The third inlay shows the resulting quenching
probability when saturating the depletion process. (d) and (e) show an experimental comparison
between the confocal PSF and the effective PSF after switching on the depleting beam. Note the doubled
lateral and fi ve-fold improved axial resolution. The reduction in dimensions (x, y, z) yields ultrasmall
volumes of subdiffraction size, here 0.67 al (Klar et al., 2000), corresponding to an 18-fold reduction
compared to its confocal counterpart. The spot size is not limited on principle grounds but by practical
circumstances such as the quality of the zero and the saturation factor of depletion. (See color plate.)
that nonconfocalized detection is viable as well (Hell and Wichmann,
1994). The principal idea, a schematic setup and an exemplary mea-
surement of the resolution increase, is shown in Figure 12–7. The fl uo-
rophore in the fl uorescent state S
1
(state A) is stimulated to the ground
820 S.W. Hell and A. Schönle
state S
0
(state B) with a doughnut-shaped beam. The saturated deple-
tion of S
1
confi nes fl uorescence to the central intensity zero. With typical
saturation intensities ranging from 1 to 100 MW/cm
2
, saturation factors
of up to 120 have been reported (Klar et al., 2000, 2001). This should
yield a 10-fold resolution improvement over the diffraction barrier, but
imperfections in the doughnut have limited the improvement to 5 to
7-fold in experiments (Klar et al., 2001).
As already stated, light microscopy resolution can be described either
in real space or in terms of spatial frequencies. In real space, the resolu-
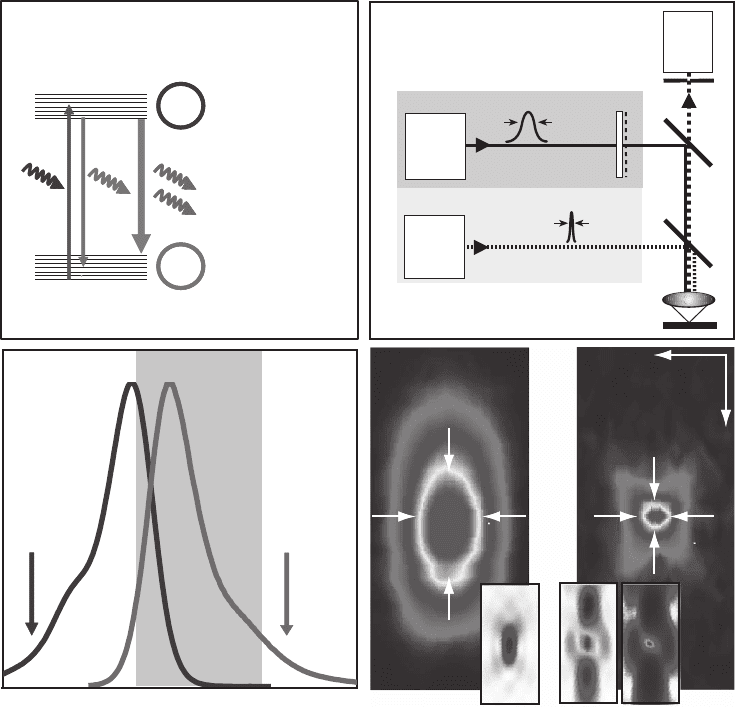
tion is assessed by the FWHM of the focal spot. The measurements
depicted in Figure 12–8 were carried out with an excitation wavelength
of λ = 635 nm, an oil immersion lens with a numerical aperture of 1.4,
and with the smallest possible probe: a single fl uorescent molecule
(Westphal and Hell, 2005; Westphal et al., 2003). Figure 12–8a shows
the measured profi le of the PSF in the focal plane (x) for a conventional
10
0.01
0.1
1
03040
Convent.
STED
STED deconv.
PSFa)
x [nm]
-200 -100 0 100 200
Convent.
222 nm
STED
40 nm
OTF
I [a.u.]
0.0
1.0
x
-1
[1/µm]
-100 0 100
62 nm
Mol.
no.1
Mol.
no.2
33 nm
b)
Figure 12–8. (a) Comparison of the effective PSF’s lateral intensity profi le for
confocal and STED microscopy indicating an ∼5.5-fold resolution increase in
the latter. (b) Lateral cuts through the effective OTFs giving the bandwidth of
the lateral spatial frequencies passed to the image. The data plotted in (a) and
(b) are gained by probing the fl uorescent spot of a scanning microscope with
a single molecule of the fl uorophore JA 26 using a numerical aperture of 1.4
(oil) objective lens and at wavelengths of 635 nm (excitation), 650–720 nm (fl uo-
rescence collection), and 790 nm (STED). The inset demonstrates subdiffrac-
tion resolution with STED microscopy. Two identical molecules located in the
focal plane that are only 62 nm apart can be entirely separated by their inten-
sity profi le in the image. A similar clear separation by conventional micro-
scopy would require the molecules to be at least 300 nm apart. (Date adapted
from Westphal et al.)
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 821
fl uorescence microscope along with its sharper subdiffraction STED
fl uorescence counterpart. STED leads to improvement in resolution by
a factor of approximately 5.5.
Figure 12–8b shows the OTF of a conventional microscope along with
the enlarged OTF of the STED fl uorescence microscope. As expected,
the effective OTF’s support in the confocal case ends at approximately
(2/635 nm + 2/720 nm) = 5.91/µm. For STED, we included the OTF after
successful linear deconvolution, which restores higher spatial frequen-
cies that are not swamped by noise. The region of usable OTF support
is approximately marked by the region where frequencies are enhanced
by the deconvolution process without producing artifacts and is ∼5.5
times larger than for the confocal case. This marks a fundamental
breaking of Abbe’s diffraction barrier in the focal plane. The inlay dem-
onstrates the resulting subdiffraction resolution exemplifi ed by the
linearly deconvolved STED image of two molecules at a distance of
62 nm. They are distinguished in full by two narrow peaks (Westphal
et al., 2003). As a result of deconvolution, the individual peaks are
sharper (33 nm FWHM) than the initial peak of 40 nm FWHM.
Very recently, utilizing STED wavelengths of λ = 750–800 nm, a lateral
FWHM of down to 16 nm has been achieved in experiments with single
JA 26 molecules spin-coated on a glass slide.
14
Measuring the resolution
as a function of the applied STED intensity confi rmed the predicted
increase of the resolving power with the square root of the saturation
factor (see Figure 12–9). Of course the cutoffs presented are based on a
somewhat arbitrary defi nition of what can be considered “usable fre-
quencies” at a certain signal-to-noise ratio. However low this threshold
is set, the confocal support cannot extend beyond ∼6/µm while the
STED-OTF’s support is theoretically unlimited.
The one-dimensional (1 D) phase-plate yielding 16 nm FWHM is opti-
mized for maximum resolution improvement in the lateral direction
perpendicular to the polarization of the depleting light and leads to an
intensity distribution with two strong peaks at either side of the excita-
tion maximum (Keller et al., in preparation). Because the depleting light
is polarized, the resolution gain depends on the orientation of the mole-
cules. However, a considerable increase in resolution is still possible for
the second phase-plate, which yields a doughnut-shaped intensity distri-
bution and thus an almost isotropic resolution increase in the lateral
directions when using circularly polarized light.
To “squeeze” the fl uorescence spot in both lateral directions two
STED beams aberrated with 1 D phase-plates oriented at 90° to each
other can be combined. Together with circularly polarized excitation,
almost uniform resolution in the focal plane is achieved as shown in
Figure 12–10. A series of xy images acquired with different STED beam
powers demonstrate the resolution increase and concomitant widening
of the OTF when the applied saturation factor increases (Schönle et al.,
in preparation). This combination of two incoherent beams causes the
resolution to depend on the orientation of the transition dipole and
results in spikes along the x and y direction of the OTF when imaging
randomly oriented fl uorophores (see Figure 12–10). New phase-plates
have been proposed to avoid such effects and to improve the effective
822 S.W. Hell and A. Schönle
saturation factors at a given total STED power. Incoherent combination
can then be used to improve the resolution in all three spatial dimen-
sions. The resulting PSFs exhibit very weak dependence on dipole ori-
entation (Keller et al., in preparation) and allow application of STED to
the imaging of biological specimen and reliable subsequent linear
deconvolution (Willing et al., in preparation).
STED microscopy has also been successfully applied to the imaging
of biological samples. Subdiffraction images with three-fold enhanced
axial and doubled lateral resolution have been obtained with mem-
brane-labeled bacteria and live budding yeast cells (Klar et al., 2000).
While there is some evidence for increased nonlinear photobleaching
of some dyes when increasing the depletion intensity (Dyba and Hell,
2003), there is no reason to believe that the intensities currently applied
would be detrimental to live cells. This is not surprising since the
intensities are two to three orders of magnitude lower than those used
in multiphoton microscopy (Denk et al., 1990). Moreover, STED has
proven to be single molecule sensitive, despite the proximity of the
b)
Conf.
1
0
0.5
0.05
4.4 21 34
1/∆x [µm
-1
]
0 10 30
ς
1+NA
λ
c)
I
max
[MW/cm
2
]
0 200
200
0
235nm
131nm
72nm
40nm
31nm
26nm
800 1000
12
18
24
30
36
42
δ [nm]
n
0
30
δ [nm]
0.45
a)
Conf. STED
x [nm]
-100-200 100 200
1
0.5
16nm
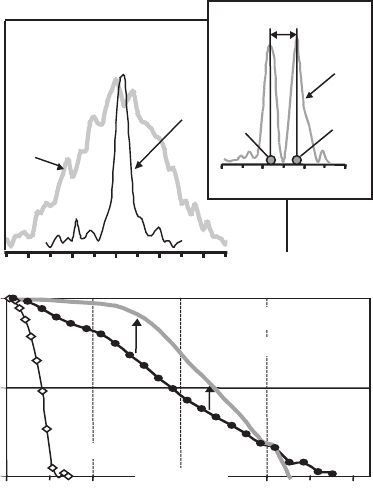
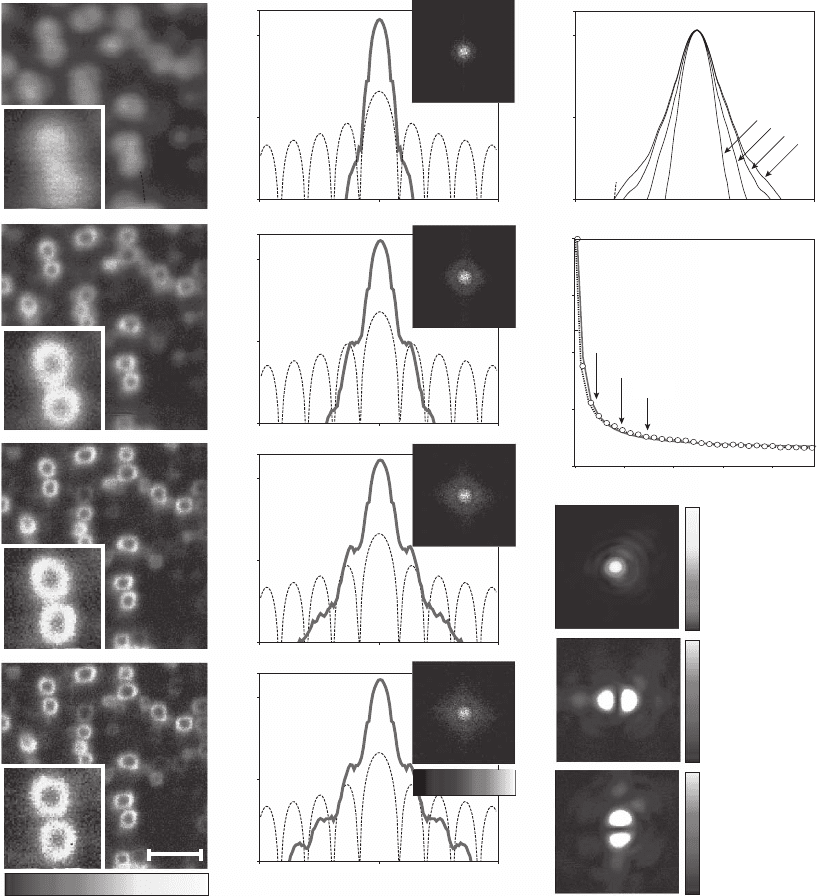
Figure 12–9. STED microscopy reduces the fl uo-
rescence focal spot size to a degree far below the
diffraction limit: (a) spot of a confocal micro-
scope (left) compared with that in an STED
microscope (right) utilizing a y-oriented inten-
sity valley for STED (upper right inset, not to
scale) squeezing the spot in the x direction to
16 n m widt h. (b) As also observed in Figure 12–8,
the bandwidth in STED is fundamentally
increased over confocal microscopy. The graph
shows the normalized magnitude of optical
transfer function (OTF) as a function of inverse
distance. For the “1D” depletion scheme, the
usable support of the OTF is increased by almost
a factor of 8. When using a doughnut-shaped
depletion beam with a “wider” intensity zero, the
OTF support is still extended almost fi ve-fold. (c)
The average focal spot size decreases with the
STED intensity following a square-root law, in
agreement with Eq. (27). Because the resolution
depends on molecule orientation, the spot sizes
were measured for several tens of single mole-
cules. The curves follow the mean values
(squares) and the inset discloses the histogram
of the measured spot sizes at 1100 (MW/cm
2
)
with the minimum FWHM at 16 nm and a 26 nm
average.
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 823
1µm
0.001
0.2
0.1
0.01
32
-32
1/∆x [µm
-1
]
0.001
0.2
0.1
0.01
0.001
0.2
0.1
0.01
0.001
0.2
0.1
0.01
0 a.u. 1
a)
b)
c)
d)
0.001
0.2
0.1
0.01
32
-32
1/∆x [µm
-1
]
a)
b)
c)
d)
e)
I [MW/cm
2
]
0
1
0.2
0.75
0.25
0
F/F
0
f)
g)
h)
i)
0
1
0
0.3
0
0.3
800
200
b) [84 MW/cm
2
]
c) [187 MW/cm
2
]
d) [290 MW/cm
2
]
0.001 0.2
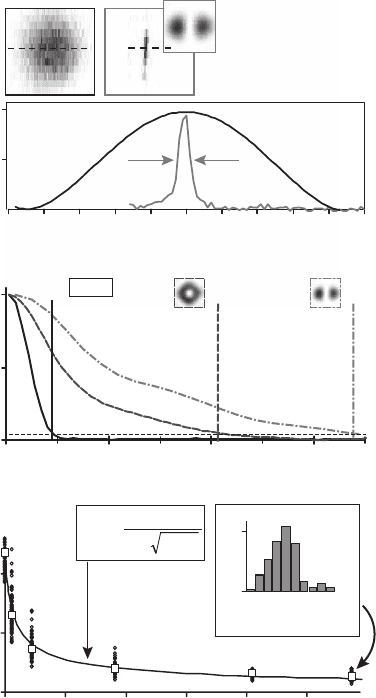
Figure 12–10. Images of a wetted Al
2
O
3
matrix featuring z-oriented holes (Whatman plc, Brentford,
UK) with a spin cast of a dyed (JA 26) polymethyl methacrylate solution. The rings formed in this way
are ∼250 nm in diameter and are barely resolved in confocal mode. (a–d) The confocal image (a) and
STED images with two depleting beams perpendicularly polarized and aberrated by “1D” phase-plates
(b–d). The excitation PSF (g) and the STED PSF for y polarization (h) and x polarization (i) are shown
on the right. The STED intensity was chosen at the spots marked in the saturation curve (f). The smaller
effective spot size also results in an extended OTF as seen in the second column. Here, the insets show
the 2D Fourier transformation of the images in the left and the graphs show a profi le along the x direc-
tion. Note the logarithmic scales. The Fourier transform of the image is given by the product of OTF
and the Fourier transform of the object [Eq. (2)]. For such regular structures, an estimate for the
modulus of the OTF can therefore be gained by estimating the latter and solving for the OTF. The
dashed line shows the Fourier transform of a ring with a diameter of 275 nm and a width of 50 nm and
the estimated OTF is presented in (e). (f) The suppression of fl uorescence resulting from stimulated
emission. The phase-plates were removed and the ratio of fl uorescence without STED light (F
0
) and
with the STED beams switched on (F) was recorded. The intensities are pulse intensities per beam at
the global maximum. (See color plate.)
824 S.W. Hell and A. Schönle
STED wavelength to the emission peak. In fact, individual molecules
have been switched on and off by STED upon command (Kastrup and
Hell, 2004; Westphal et al., 2003).
The power of STED and 4Pi microscopy has been synergistically com-
bined to demonstrate for the fi rst time an axial resolution of 30–40 nm in
focusing light microscopy (Dyba and Hell, 2002). The intensity distribu-
tion of the depleting light is formed by a 4Pi setting with destructive
interference at the geometric focus leading to a zero intensity there and
two neighboring maxima at a distance of approximately λ/4. This
results in superior xz images, and the technique has initially been suc-
cessfully applied to membrane-labeled bacteria (Dyba and Hell, 2002).
More recently, STED-4Pi microscopy has been extended to i mmunofl uo-
rescence imaging (Figure 12–11). A spatial resolution of ∼50 nm has been
demonstrated in the imaging of the microtubular meshwork of a mam-
malian cell.
76
These results indicate that the basic physical obstacles to
attaining a 3D resolution of the order of a few tens of nanometers have
been overcome. Since the samples were mounted in an aqueous buffer
(Dyba and Hell, 2002; Dyba et al., 2003), the results indicate that the
optical conditions for obtaining subdiffraction resolution are met under
the physical conditions encountered in live cell imaging.
It is to be expected that ultrasmall detection volumes created by
STED will also be useful in a number of sensitive bioanalytical tech-
niques. Fluorescence correlation spectroscopy (FCS) (Magde et al.,
1972) relies on small focal volumes to detect rare mole cular species or
interactions in concentrated solutions (Eigen and Rigler, 2001; Elson
and Rigler, 2001). While volume reduction can be obtained by nano-
fabricated structures (Levene et al., 2003), STED may prove instrumen-
tal in attaining ultrasmall spherical volumes at the nanoscale inside
samples that do not allow for mechanical confi nement. The latter fact
is particularly important to avoid an alteration of the measured fl uctua-
tions by the nanofrabricated sur face walls.
In fact, the viability of STED FCS has recently been shown in an
experiment (Kastrup et al., 2005). In a particular implementation STED
FCS has witnessed a reduction of the focal volume by a factor of fi ve
along the optic axis and a concomitant reduction of the axial diffusion
time. The initial experiments showed that for particular dyewavelength
combinations the evaluation of the STED FCS data might be complicated
by a seemingly uncorrelated background at the outer wings of the fl uo-
rescence spot where STED may not completely suppress the signal.
Further investigations will show whether this challenge is easily over-
come in the near future. In any case, published results suggest a further
decrease of the volume by another order of magnitude (Westphal et al.,
2003; Irie et al., 2002). An inherent disadvantage of STED is the necessity
of an additional pulsed light train that is tuned to the red edge of the
emission spectrum of the dye. Nevertheless STED is to date the only
known method for “squeezing” a fl uorescence volume to the zeptoliter
scale without making mechanical contact. Thus, the creation of ultrasmall
volumes, tens of nanometers in diameter, by STED may be a pathway to
improving the sensitivity of fl uorescence-based bioanalytical tech-
niques (Weiss, 2000; Laurence and Weiss, 2003).
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 825
0
400
800
1200
1600
01234
Z / µm
Counts
Z / µm
FWHM
53nm
01234
1
2
3
z
x
Confocal
c)
e)
STED-4Pi
d)
f)
b)
25nm
60nm
Immunolabeled
microtubule
Overview
a)
Y
X
Monolayer
Microtubules
Monolayer
Microtubules
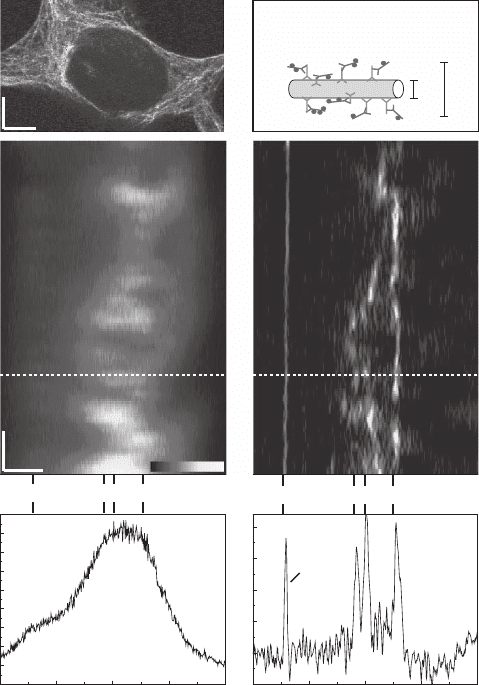
Figure 12–11. Subdiffraction immunofl uorescence imaging with STED-4Pi microscopy. (a) Overview
image (xy) of the microtubular network of an HEK cell. (b) Sketch of typical dimensions of a labeled
microtubule fl uorescently decorated via a secondary antibody. (c) and (d) Standard confocal and STED-
4Pi xz image recorded at the same site of the cell; the straight line close to the cell stems from a mono-
molecular fl uorescent layer attached to the adjacent coverslip. In both images, the pixel size was 95 ×
9.8 n m in the x and z direction, respectively; the dwell time per pixel was 2 ms. Note the fundamentally
improved clarity in (d). The STED-4Pi microscope’s PSF features two low side lobes caused by the sec-
ondary minima STED intensity distribution. These lobes are <25% and were removed in the STED-4Pi
image using linear fi ltering as outlined in the text [see Eq. (16ff)]. (e) and (f) Corresponding profi les of
the image data along the dashed lines in (b) and (c) quantify the improved axial resolution of the STED-
4Pi microscopy mode (f) over the confocal benchmark. Peaks 1, 2, and 3 due to microtubules are broader
than the response to the monolayer. Note the ability of the STED-4Pi microscope to distinguish adjacent
features. (See color plate.)
An important step toward far-reaching applicability of STED micro-
scopy was the demonstration of the suitability of laser diodes both for
excitation and for depletion (Westphal et al., 2003). However, several
issues remain to be addressed. Due to the considerably smaller detec-
tion volumes, the signal per pixel is reduced and the amount of pixels
to be recorded increases. Therefore, it will be important to incorporate
STED into fast, ideally parallelized scanning systems.
826 S.W. Hell and A. Schönle
While the transition to shorter wavelengths will further increase
resolution by a factor of ∼1.5, it is most likely that the ultimate resolu-
tion limit in STED will be set by the stability of the marker used. The
photostability of current markers was considerably improved by
stretching the depleting pulse to >300 ps (Dyba and Hell, 2003), but it
might not be easily possible to attain saturation factors ζ > 200 in the
near future. Nevertheless, according to Eq. (27) ζ = 200 should already
yield an improvement by one order of magnitude, provided that the
actual intensity value at the “intensity zero” is indeed negligible at this
saturation level.
As explained above (Eq. 30), the actual “depth” of the zero codeter-
mines the attainable resolution, because for relatively high saturation
factors the saturable transition also becomes effective at the zero point
or points. So far, typical depths were in the range of γ = 1–2.5% of the
global maximum of the depleting intensity I(r). The zero could be a
single point, as in a single beam scanning system, but in the case of a
parallelized system, it may also be a line or an array of points or lines.
The actual depth of the zeros will certainly depend on the particular
setup and the quality of optical components and proper alignment.
Independently of implementation details, active optical elements such
as wavefront phase modulators will be a valuable tool to further “deepen”
the zeros, which in turn will allow the full potential of the attained satu-
ration level to be exploited for improvement in resolution.
4.2 Variations of RESOLFT Microscopy and Producing Large
Saturation Factors at Low Power
At this point, we reiterate that RESOLFT is not restricted to the process
of stimulated emission, but can exploit any reversible (linear) transition
driven by light; the attainable resolution is determined by the ratio of
driving intensity and the competing transition rate k
BA
. If the applicable
intensity is limited by the onset of photodamage to the marker or even
to the sample, marker constructs must be found where high saturation
levels are attained at lower intensities. This is certainly the case if the
rate competing with the transition to be saturated is lower.
One such example is the GSD mentioned earlier. In this version of the
RESOLFT concept the ground state (now state A) is depleted by target-
ing an excited state (B) with a comparatively long lifetime (Hell and
Kroug, 1995; Hell, 1997), such as the meta-stable triplet state T
1
. In many
fl uorophores T
1
can be reached through the S
1
with a quantum effi ciency
of 1–10% (Lakowicz, 1983). Being a forbidden transition, the relaxation
of the T
1
is 10
3
–10
5
times slower than that of the S
1
, thus yielding I
s
= 0.1–
100 kW/cm
2
. The signal to be measured (from the intensity zero) is the
fl uorescence of the molecules that remained in the singlet system; this
measurement can be accomplished through a synchronized further
excitation (Hell and Kroug, 1995). For many fl uorophores, this approach
is not straightforward, because T
1
is involved in the process of photo-
bleaching, but there are potential alternatives such as the meta-stable
states of rare earth metal ions that are fed through chelates.
Also proposed has been depleting the ground state S
0
by populating
the S
1
(now B) (Heintzmann et al., 2002). This is the technically simplest
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 827
realization of saturated depletion, since it requires only excitation
wavelength matching. However, as the fl uorescence emission maps the
spatially extended “majority population” in state B, the super resolved
images (represented by state A) are negative images hidden under a
bright signal from B. Hence photon noise from the large signal might
swamp the fl uorescence minima that occur when intensity zeros, where
no fl uorescence is excited, are colocalized with fl uorophores. The sub-
sequent computational extraction of the positive image is therefore
very dependent on an excellent signal-to-noise ratio. The saturation
intensity is of the same order as in STED microscopy, because the satu-
ration of fl uorescence also competes with the spontaneous decay of S
1
.
This probably results in photostability issues similar to the case of
STED. In fact, the photobleaching should be exacerbated, since the satu-
rated transition is effected with higher energy photons that are gener-
ally more prone to facilitating photochemical reactions. Pumping the
dye to a higher state rather than into the ground state also favors pho-
tolability. Moreover, the fact that a large number of dye molecules
constantly undergo excitation–emission cycles to image a compara-
tively small spot adds to the problem. Finally, saturation of the S
1
will
be possible only if the long-lived triplet state is not allowed to build up
during repeated excitation. As most dyes feature a triple relaxation rate
of >1 µs (that strongly depends on the environment), effective triplet
relaxation requires a pulse repetition rate <500 kHz. Nevertheless, due
to the simplicity of raw data acquisition it may remain an attractive
method for the imaging of very bright and photostable samples.
One possible solution to the quest for large saturation factors at low
intensities should be compounds with two (semi)stable states (Hell
et al., 2003; Dyba and Hell, 2002). If the rate k
BA
(and the spontaneous
rate k
AB
) almost vanish, large saturation factors are attained at very low
intensities. The lowest useful intensity is then determined by the
slowest acceptable imaging speed, which is ultimately determined by
the switching rate. A favorable aspect is that in most bistable com-
pounds the speed of the actual switching mechanism, i.e., of the con-
formational change, is less than a few nanoseconds, which is much
faster than the typical pixel dwell time in scanning. In the ideal case,
the marker indeed is a bistable fl uorescent compound that can be pho-
toswitched at separate wavelengths, from a fl uorescent state A to a dark
state B, and vice versa, where spontaneous rates will not infl uence this
compromise.
Recently, a photoswitchable coupled molecular system, based on a
photochromic diary lethene derivative and a fl uorophore, has been
reported (Irie et al., 2002). Using the kinetic parameters reported, Eq.
(27) predicts that focusing of less than 100 µW of deep-blue “switchoff
light” to an area of 10
−8
cm
2
for 50 µs should yield better than 5 nm spatial
resolution. Future targeted optimization of photochromic or other com-
pounds to fatigue-free switching and visible light operation could there-
fore open up radically new avenues in microscopy and data storage (Hell
et al., 2003).
For live cell imaging, fl uorescent proteins have many advantages
over synthetic dyes. Many of them feature dark states with light-driven
transitions (Hell, 1997; Hell et al., 2003). If the spontaneous lifetimes of
