Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

798 S.W. Hell and A. Schönle
and Stelzer, 1992b; Gustafsson et al., 1995) if light is detected coherently
through both lenses. That is, the intensity maxima for excitation and
detection are located at different points in space.
Three major types of 4 Pi microscopy have been reported (Hell and
Stelzer, 1992a). They differ on whether the spherical wavefronts are
coherently added for illumination, for detection, or for both simultane-
ously; they are referred to as type A, B, and C, respectively. Usually
the detection has been confocalized, but in conjunction with TPE suc-
cessful axial separation with nonconfocal detection has also been
reported. Here we will concentrate on the TPE 4 Pi (type A), the 4 Pi
type C, and the TPE 4 Pi type C confocal microscopes. Of these three,
the TPE 4 Pi confocal microscope has been applied to the largest number
of imaging problems. It uses the very effective lobe-reducing measure
of TPE combined with “point-like” detection. In reality the size of the
“point-like” detector amounts to about the size of the main maximum
of the diffraction-limited fl uorescence spot (Airydisk), when imaged
into the focal plane of the objective lens.
Clearly, nonconfocal wide-fi eld detection and regular illumination
would make 4 Pi micro scopy more versatile. Therefore, the related
approach of I
5
M (Gustafsson et al., 1995, 1996, 1999; Gustafsson, 1999)
confi nes itself to using the simultaneous interference of both the excita-
tion and the (Stokes-shifted) fl uorescence wavefront pairs; the latter
are spherical as in a 4 Pi microscope. The potential benefi ts of I
5
M are
readily stated: single-photon excitation with arguably less photobleach-
ing, an additional 20–50% gain in fl uorescence signal, and lower cost.
This method has so far yielded 3D images of actin fi laments with an
axial resolution slightly better than 100 nm in fi xed cells (Gustafsson
et al., 1999). To remove the side-lobe artifacts, I
5
M-recorded data are
deconvolved offl ine. While the consideration of the OTF support in
Figure 12–3 suggests that this single mechanism is indeed suffi cient, it
turns out that the relaxation of the side-lobe suppression comes at the
expense of an increased vulnerability to sample-induced aberrations,
especially with nonsparse objects (Nagorni and Hell, 2001a, 2001b).
Thus I
5
M imaging, which has so far relied on oil immersion lenses, has
required mounting the cell in a medium with n = 1.5 (Gustafsson et al.,
1999). Live cells inevitably necessitate aqueous media (n = 1.34). More-
over, water immersion lenses have an inferior focusing angle and
therefore larger lobes to begin with (Bahlmann et al., 2001). Potential
strategies for improving the tolerance of I
5
M are the implementation of
a nonlinear excitation mode and its combination with pseudoconfocal
or patterned illumination (Gustafsson, 2000). While these measures
again add physical complexity, they may have the potential to render
I
5
M more suitable for live cells.
However, at this stage, the implementation of at least two of the
mechanisms above proved more reliable: After initial demonstration
of TPE 4 Pi confocal microscopy (Schrader and Hell, 1996), superre-
solved axial separation was applied to fi xed cells (Hell et al., 1997). The
image quality could be improved further by applying nonlinear resto-
ration (Holmes, 1988; Carrington et al., 1995; Holmes et al., 1995). Under
biological imaging conditions, this typically improves the resolution
up to a factor of two in both the transverse and the axial direction.
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 799
Therefore, in combination with image restoration, TPE 4 Pi confocal
microscopy has resulted in a resolution of ∼100 nm in all directions, as
fi rst witnessed by the imaging of fi lamentous actin
19
and immunofl uo-
rescently labeled microtubules (Nagorni and Hell, 1998; Hell and
Nagorni, 1998) in mouse fi broblasts.
While a very useful and explanative comparison of the OTF supports
has been published (Gustafsson, 1999) it is obvious from the above that
consideration of the supports alone is not suffi cient to understand the
respective benefi ts and limitations of SWM, I
5
M, and 4 Pi microscopy.
Rather than comparing their technical implementation (Gustafsson et
al., 1999), a quantitative analysis of their PSFs and OTFs is needed to
clarify under which conditions these microscopes will be able to deliver
3D-resolved images with superior resolution. The success of increasing
the axial resolution with coherently used opposing lenses not only
depends on the achievable bandwidth, but also on the strength with
which the respective systems transfer the spatial frequencies within
this bandwidth. Gaps and weak parts that occur in some systems
(Gustafsson et al., 1995; Krishnamurthi et al., 1996) must be quantifi ed
for a particular optical setting because they may render the removal of
artifacts impossible, thus precluding an increase in axial resolution.
Below we demonstrate that these gaps are intimately connected with
the optical arrangement and therefore are inherent to some of the
methods described.
3.1 The Optical Transfer Function of 4 Pi-Microscopy and
Related Systems
Before expanding on the analysis of PSFs and OTFs, we quickly review
scope, as well as the detection PSF of the wide-fi eld, confocal, SWM, and
4 Pi-type microscopes, are regular intensity PSFs. Assuming that exci-
tation and emission involve a dipole transition of the dye, the excitation
and emission PSF of a single lens is well approximated by the absolute
square of the electric fi eld in the focal region:
h = |E
1
(z, r, φ)|
2
(3)
r, respectively) and the polar angle φ. The fi eld is usually calculated
using the vectorial theory of Richards and Wolf (1959). In our case, we
assumed circular polarization of the light. In frequency space the Fourier
transform of the electrical fi eld is then simply given by a spherical cap
of radius k
ex
(Figure 12–2). This is equivalent to approximating the
spherical wavefronts emerging at the exit pupil as plane waves close to
the focal spot. The absolute square in Eq. (3) corresponds to an autocor-
relation in frequency space. When calculating the OTF, this convolution
can be carried out directly (Schönle and Hell, 2002; Sheppard et al., 1993)
or the OTF can be determined by Fourier transformation of Eq. (3).
For the excitation PSF of the 4 Pi confocal microscopes (type A and
C) and for the detection PSF of the I
5
M and the 4 Pi confocal (type B
and C), a calculation of constructive interference between the two
spherical wavefronts is required. The PSF is therefore given by the
coherent addition of two beams
It depends on the axial and radial distance to the geometric focus (z and
their theoretical derivation. The excitation PSF of the confocal micro-
800 S.W. Hell and A. Schönle
h = |E
1
(z, r, φ) + E
2
(z, r, φ)|
2
(4)
The fi eld of the opposing lens is given by
E
2
(r) = ME
1
(M
−1
r) (5)
M is the coordinate transform from the system of lens number 2 to lens
number 1 and is a diagonal matrix inverting the z-components for a
triangular cavity and the y- and z-components for a rectangular cavity
(Bahlmann and Hell, 2000). In frequency space, the Fourier transform
of the electric fi eld is now given by two caps corresponding to the two
focused wavefronts. Consequently, the OTF consists of an autocorrela-
tion part equivalent to that for single lens excitation or detection and
a cross-correlation of two opposite spherical caps represented by the
outer “brackets” in Figures 12–2, 12–3, and 12–4. For TPE the excitation
PSF is simply given by squaring the one-photon PSF scaled to the
z
r
k
z
k
r
2%
4% 100%
PSF OTFPSF OTF
SWM I
5
M 4Pi C
PSF OTF
o
det
o
o
ex
=
h
det
h
h
ex
=
x
TPE 4Pi CTPE 4Pi A
Widefield Confocal
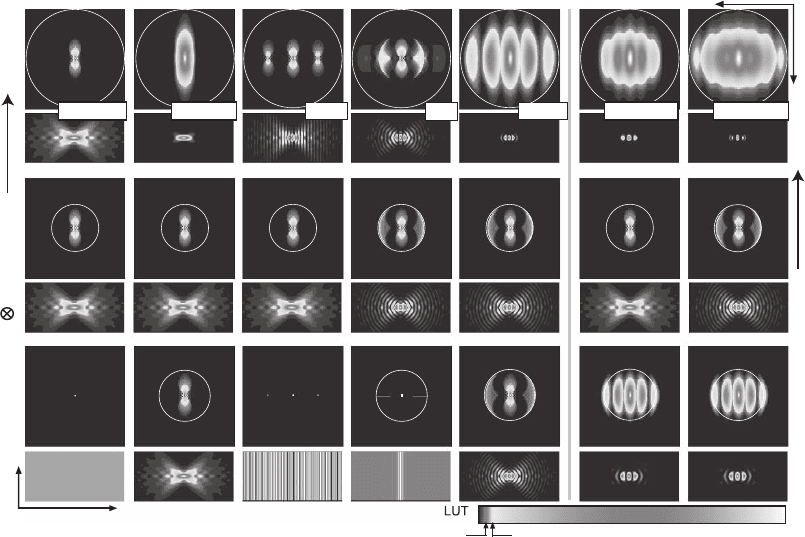
Figure 12–4. Overview of the excitation (bottom), detection (center), and effective (top) OTFs’ modulus
and the corresponding PSFs of the wide-fi eld, confocal, standing wave (SWM), I
5
M, 4Pi confocal type
C, TPE 4Pi confocal type A, and TPE 4Pi confocal type C microscopes. The color look-up table (LUT)
has been designed to emphasize the important weak OTF regions. The OTFs are shown in the squares
above the corresponding PSFs; the zero frequency point is in the center and the largest frequency
displayed is 2π/80 nm
−1
. The circles represent the maximum possible carrier as explained in Figure
12–3. For TPE, the excitation OTFs slightly extend over these circles because the excitation wavelength
of 800 nm is less than double the one-photon excitation wavelength of 488 nm. While all these methods
extend the OTF along the axial direction, they fundamentally differ in contiguity and absolute strength
within the support region. For example, there are pronounced frequency gaps for the SWM and
depressions for the I
5
M. The rectangular images of the PSFs represent a region of 5 × 2.5 µm with the
geometric focus in the center. (See color plate.)
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 801
appropriate TPE wavelength and similarly the OTF is the autoconvolu-
tion of the scaled one-photon OTF (Denk et al., 1990).
The excitation intensity of the SWM is given by a plane standing
wave along the optic axis,
h = I
0
cos
2
(k ) (6)
0
constructive interference to occur at the common geometric focus. The
excitation OTF is its Fourier transform and given by
o = I
0
[δ(k) + δ(k − 2k
ex
)/2+ δ(k + 2k
ex
)/2]/2 (7)
Again, this is the result of auto-correlating the electric fi eld’s Fourier
transform that consists of delta peaks at ±2k
ex
.
While the 4 Pi microscope uses a spatially coherent point-like laser
illumination, in the I
5
M microscope wide-fi eld illumination is used,
normally in the Köhler mode, either with a lamp or a laser. The physical
consequences of this difference are best explained as follows. If the two
spherical wavefronts of the 4 Pi illumination are decomposed into
plane waves incident from different angles and corresponding to dif-
ferent points of the illumination apertures, all plane waves of the aper-
ture interfere with each other in the focal region. In the I
5
M the
illumination light is not coherent throughout the aperture. Therefore
only pairs of plane waves originating from corresponding points (mirror
images about the focal plane) of the illumination apertures are mutu-
ally coherent, forming a standing wave in the focal region. The period
of these standing waves scales with the cosine of the azimuth angle θ.
The excitation PSF of the I
5
M can then be calculated by adding the
intensity of these plane standing waves. Assuming uniform intensity
throughout the exit pupil of the lens, the PSF is given by
hz I d d k z
Id kz
()=
()
=
()
∫∫
0
0
2
0
2
2
φθθ θ
πθθ θ
α
sin cos cos
sin cos cos
ex
ex
00
α
∫
(8)
The support of the excitation OTF is readily inferred: For each θ the
integrand in Eq. (8) is the excitation PSF of the SWM and thus the total
OTF consists of a superposition of expressions of Eq. (6) for wave
vectors ranging from k
ex
cos(α) to k
ex
. Loosely speaking, this incoherent
superposition smears out the delta peaks at the sides, forming the lines
in Figures 12–3 and 12–4. This excitation mode contributes to avoiding
the gaps that remain in the SWM’s support after convolution with the
detection OTF. If, on the other hand, the incoherent light source is
imaged into the focal plane of the lens, i.e., critical illumination, mutu-
ally coherent points form wavefronts focused onto and interfering at
the conjugate point in the image of the light source. The individual 4 Pi
PSFs produced by each point of the light source as a result are incoher-
ently summed up, giving an integral of the 4 Pi excitation PSF over the
fi eld of view in the focal plane. The OTF becomes nonzero exclusively
on the inverse optical axis where it is given by the values of the 4 Pi
OTF, altogether leading to a result not very much different from that
predicted by Eq. (8). However, critical illumination is problematic due
ex
z
where I denotes a constant,
k
ex
is the wavenumber; and we assumed
802 S.W. Hell and A. Schönle
to potential nonuniformities in the light source and will be omitted in
our analysis.
In any case, once the fl uorescence light is generated in the sample,
the I
5
M collects the spherical fl uorescent wavefronts just as in a 4 Pi
microscope of type B or C. The two counterpropagating spherical
wavefronts of fl uore scence collected by each lens interfere construc-
tively in a common point on the camera. Disturbance of the interfer-
ence pattern of neighboring points of fl uorescence emission is damped
by the spatial incoherence in the focal plane: the radius of spatial coher-
ence is largely given by the Airy disk associated with the fl uorescence
light at the aperture in use. Thus the I
5
M implements the highest pos-
sible degree of parallelization of 4 Pi detection.
Figure 12–4 shows the numerically calculated excitation, detection,
and effective PSFs/OTFs of the 4 Pi, I
5
M, and SWM setups, along with
those of the conventional epifl uorescence and confocal microscope. The
epifl uorescence micro scope features uniform illumination intensity
throughout the sample volume; its OTF is a single deltapeak at the
origin. To obtain a practically relevant comparison, we assumed a
numerical aperture of NA = 1.35 and oil immersion with a refractive
index n = 1.51. In the case of single-photon excitation of the dye, an
excitation and detection (i.e., central fl uorescence) wavelength of 488 nm
and 530 nm, respectively, is assumed. For TPE, an excitation wavelength
of 800 nm was chosen. Finite-sized pinholes can be taken into account
by convolving the detection PSF with the image of the pinholes in the
focal plane: a disk of radius r
PH
. In frequency space this corresponds to
a multiplication with the disk’s Fourier transform given by
h
ˆ
PH
= J
1
(kr
PH
)/k (9)
The fi rst root of the Bessel function is at ∼3.83 and thus the detection
OTF becomes zero at k = 3.83/r
PH
. However, the largest frequency
present in the detection OTF is given by half the wavelength and
therefore its support is unaltered if the pinhole radius is smaller than
3.83 λ/(4πNA) ≅ 0.3 λ/NA, which corresponds to half the Airy disk
radius. Pinholes smaller than this can virtually be neglected in the
computation, while for sizes around this value and larger, the PSF will
widen laterally, suppressing the OTF at higher lateral frequencies. The
effect of the pinhole size on axial resolution remains small as long as
it does not exceed that of the Airy disk. We will therefore neglect the
pinhole in our further analysis. All PSFs were numerically computed
in a volume of 128 × 128 × 512 pixels in x-, y-, and z-directions, respec-
tively, for cubic pixels with 20 nm length. The OTFs were calculated
by Fourier transformation; of the 512 pixels in the z-direction only data
based on the central 256 pixels are shown in Figure 12–4. The color
look-up-table (LUT) has been chosen so that the regions of weak signal
are emphasized for both PSF and OTF. This reveals important differ-
ences between the systems. Areas of low but nonnegligible intensity
are important since they cover a large volume and substantially contri-
bute to the image formation.
Let us fi rst consider the PSFs (Figure 12–4, narrow columns). Imme-
diately, some differences between the various approaches become
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 803
apparent. While the excitation modes of the SWM and I
5
M are similar,
the local minima are not zero for the latter due to the incoherent addi-
tion of the standing wave spectrum. The most important difference is
observed when comparing the 4 Pi microscopes. As a result of focus-
ing, their PSFs are confi ned in the lateral direction so that contributions
from the outer lateral parts of the focal region are reduced. The confi ne-
ment has important consequences. Whereas the 4 Pi confocal micro-
scope, especially its two-photon version, exhibits only two pronounced
but rather low lobes, the I
5
M and even more so the SWM feature a
multitude of lobes and fringes on either side of the focal plane, despite
the fact that all of them rely on the same aperture. The second conse-
quence is that due to its quadratic or cubic dependence on the excitation
distributions, the 4 Pi confocal PSFs can be separated into an axial
and a radial function in good approximation (Hell et al., 1995; Schrader
et al., 1998):
h(r, z) ≅ c(r)h
l
(z) (10)
Separability is a particular feature of the 4 Pi confocal and multiphoton
arrangements and we shall see later that it is the prerequisite for simple
online removal of sidelobe effects in the image. TPE leads to a further
suppression of the outer parts of the excitation focus and thus of the
side lobes of the 4 Pi illumination mode. Figures 12–3 and 12–4 also
reveal that in conjunction with coherent detection (type C), TPE 4 Pi
confocal microscopy features an almost lobe-free PSF. Its OTF almost
fi lls the maxi mum support region. In the SWM and the I
5
M, the number
and relative heights of the lobes increase dramatically when moving
away from the focal point because an effective suppression mechanism
is missing. In the SWM the lobes become even higher than the central
peak itself. In the I
5
M the secondary maxima are as high as the fi rst
maxima of the single-photon 4 Pi confocal microscope of type C.
3.2 Removing Periodic Artifacts through Deconvolution
Even if they are small, side lobes and resulting ghost images in the raw
data remain a common feature of all methods employing two lenses
coherently. Next, we turn to the OTF to understand the circumstances
under which image processing can effectively be used to remove the
artifacts induced by the lobes. It is obvious from Eq. (2) that if the OTF
were nonzero everywhere, we could divide the Fourier transform of
the image by it. Subsequent Fourier back-transformation of the data
would render the object. In practice, the OTF is limited in bandwidth
and has weak regions. As division outside the OTF support is impos-
sible, these frequencies are lost. But even in regions where the OTF is
small, division strongly amplifi es noise-producing artifacts.
Image restoration techniques, whether linear or nonlinear, aim at
restoring as many frequencies as possible while trying to avoid this
effect. Linear deconvolution is based on the division approach but
introduces a special treatment for frequencies not transmitted by the
OTF. It is capable of restoring frequencies only where the OTF is above
the noise level. If frequencies are missing, a correct representation of
804 S.W. Hell and A. Schönle
the object in the image can be given only if these frequencies are
extracted from a priori knowledge of the object. This extraction is
mathematically more complex and often not viable. Linear deconvolu-
tion, on the other hand, is computationally facile and fast. Speed is of
particular importance because the interference artifacts are ideally
removed online making the fi nal image readily accessible. Therefore,
one of the most prominent advantages of a uniformly strong OTF is
the possibility of applying a linear deconvolution.
The comparison of the effective OTFs in Figure 12–4 highlights the
severe gaps in the SWM, making deconvolution impossible. Linear
deconvolution is reportedly possible in the I
5
M (Gustafsson et al., 1999).
However, as the gaps in the I
5
M are fi lled with rather low amplitudes,
this method will create image artifacts for objects that are not sparse,
not very bright, or objects containing spatial frequencies coinciding
with the gap. Owing to the contiguity of its support and the strong
amplitudes of the OTF, 4Pi confocal microscopy fulfi lls the precondi-
tions for linear deconvolution. In fact, linear deconvolution along the
axial direction based on the separability of the PSF has been applied
for the removal of interference artifacts arising in the recording of
complex objects. Thus superresolved axial imaging has been shown in
the dense fi lamentous actin
55
and in the microtubular network (Nagorni
and Hell, 1998) of a mouse fi broblast cell. We will have a closer look at
this important procedure in the subsequent section.
Approximating the 4Pi confocal PSF as in Eq. (10) allows us to
perform a computationally inexpensive one-dimensinal linear deconvo-
lution that simply eliminates the effect of the lobes in the image. The
axial factor of the PSF can basically be decomposed into the convolu-
tion of a function h
p
(z) describing the shape of a single peak, and a lobe
function l(z) containing the position and relative heights of the lobes:
h
l
(z) ≅ h
p
(z) ⊗ l(z) (11)
h
p
quantifi es the blur and l describes the replication that is responsible
for the “ghost images” in unprocessed 4Pi images. The effect of the
lobe function can be eliminated using algebraic inversion. We use a
discrete notation, where each lobe is represented by a component l
i
of
the vector l with l
0
denoting the strength of the central lobe and the
index running from −n to n. Negative indices denote lobes to the left
of the central peak. If the lobe distance in pixels is denoted by d, the
values of the object along the line are given by O
j
and those of the
image by I
j
. Thus, the convolution is given by
IlO
jkjdk
k
=
−
∑
(12)
Looking for a fi lter l
−1
inverting this convolution we need
OlI llO
jsjdsskjdsdk
ss
==
−
−
−
−−
∑∑
11
(13)
for all possible objects. The inverse fi lter needs to fulfi ll the condition
ll
sjs j
s
−
−
=
∑
1
0
δ
(14)
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 805
At this point we can arbitrarily choose the length of the inverse fi lter,
assuming an index running from −m to m. The index j in Eq. (13) can
take values from −m − n to m + n. Therefore we have a system of 2(m
+ n) + 1 equations with 2m + 1 unknowns, which is usually not solvable.
An approximation is found by considering the equations for j =
−m . . . m only. Now, the problem is equivalent to solving a linear
Toeplitz problem with the Toeplitz matrix given by the vector l (Nagorni
and Hell, 2001; Press et al., 1993). The approximation is good if the
edges of the inverse fi lters are small, since the remaining equations are
nearly satisfi ed. This holds if the fi rst-order lobes are <45%; in this case,
the error is practically not observable. A typical length of the inverse
fi lter is 11. In conjunction with a lobe height of 45%, the edges of the
inverse fi lter feature a modulus below 1% of the fi lter maximum. For
lobes of 35% relative height, this value drops to a value of only about
0.08%. Thus, using this technique, the separability of the 4Pi confocal
PSF provides a quick way to obtain a fi nal image that is equivalent to
imaging with an “ideal” optical microscope that has a single narrow
main maximum at the focal point. The inverse fi lter is discrete and
nonzero only at a few points. This very effective side lobe removal
method is therefore referred to as point deconvolution. Exploiting the
characteristics of the PSF of the 4Pi microscope, it is applicable only in
conjunction with this method.
Axial lobes in the PSF entail suppressed OTF regions along the optic
axis. Thus, point deconvolution restores suppressed frequencies by
linear deconvolution even though the actual calculation takes place in
real space. For PSFs that cannot be written as in Eq. (10) point decon-
volution is insuffi cient, because the transverse directions (x, y) have to
be involved as well. In this case, it is mathematically clearly preferable
to pass the data through the frequency domain. Established linear
deconvolution algorithms rely on inverting Eq. (2) but also accommo-
date for the vanishing regions of the OTF. An estimate of the frequency
spectrum of the object can be obtained by using
E
ˆ
(k) = I
ˆ
(k)o*(k)/(|o(k)|
2
+ µ) (15)
The regularization parameter µ (Bertero et al., 1990) sets a lower thresh-
old on the denominator to avoid amplifi cation of frequencies where the
modulus of the OTF is so small that the frequency spectrum of the
image is dominated by noise. If the OTF is a convex function that is in
the absence of “gaps,” the effect of regularization is similar to smooth-
ing. In most cases considered in this chapter, however, the OTF is not
convex and the situation is more complicated. Small OTF values are
found not only at the OTF boundaries, but also within its region of
support, e.g., in the vicinity of the minima (Figure 12–4) or at the fre-
quency gaps (Figure 12–3). If the lobes are too high (typically >50%),
the level of the minima of the OTF is comparable or smaller than the
noise level. This is defi nitely the case in the SWM, but also in the pres-
ence of slight aberrations in the I
5
M, as well as in an aberrated 4Pi
microscope. Therefore, the necessity of implementing a certain value
of µ is closely connected with the lobe height and with the potential
artifacts induced by the lobes.
806 S.W. Hell and A. Schönle
If the PSF is separable in a peak function and an axial lobe function,
the lobe removal can be elegantly targeted:
h(r) = h
p
(r) ⊗ l(z) (16)
Contrary to the decomposition in Eq. (11), the peak function h
p
(r) also
contains the dependence of the peak on the lateral coordinates. We no
longer require the PSF to separate in a radial and an axial part, yet the
lobe function still gives the relative height of the lobes as well as their
location:
lz l z ds
s
s
()=−
(
)
∑
δ
(17)
with d now being the lobe distance in units of length. The frequency
spectrum of the image is then given by
IGoGhlk
pz
()
ˆ
() ()
ˆ
()
ˆ
()
ˆ
()kkkkk=⋅=⋅ ⋅ (18)
If the lobe function is symmetric with respect to the focal plane (i.e.,
for constructive interference), we can write
ˆ
() ( )lk l sdk
zsz
s
=+
>
∑
12
0
cos
(19)
This decomposition immediately discloses why the critical lobe height
is 50%: If the PSF consists of a main maximum and two primary lobes
of 50%, the right-hand side of (19) vanishes for the axial frequencies
associated with the distance d. So, if the lobes are >50% the frequency
represented by the lobes is not contained in the OTF, and hence not
transferred to the image. In reality, the critical lobe height is slightly
above 50%. The reason is the infl uence of the secondary lobes that have
been neglected in our reasoning. Nonetheless, the 50% threshold is an
excellent rule for the critical lobe height, which applies equally to
SWM, I
5
M, and 4Pi micro scopy, for fundamental reasons.
Equation (18) implies that the effect of the lobes can be removed by
direct Fourier inversion. This is the case if the Fourier transform of the
lobe function remains above the noise level throughout the relevant
frequency spectrum. The spectrum of the lobe-free image is then
obtained by dividing the image spectrum by the Fourier transform of
the lobe function. Figure 12–5 shows a typical data set acquired with
a TPE type A 4Pi confocal microscope and illustrates lobe removal both
in the spatial and the frequency domain. The avalanche photodiode
used as a detector had a typical dark count rate <1 count/pixel, which
is negligible (Hell et al., 1997). Hence, the only signifi cant source of
noise was the Poisson noise of the photon-counting process, manifest-
ing itself as white noise that is independent of the spatial frequency.
Since the primary lobes are well below 50%, the fi rst minima of the
OTF are at 19%, which is well above the noise level of typically 0.5–1%.
Direct lobe removal is straightforward in this example, underscoring
the importance of nonvanishing amplitudes in the OTF. If the OTF
exhibited regions close to zero, as is predicted for the I
5
M, the multi-
plication with a high number in this region would result in a strong
amplifi cation of noise, and compromise the obtained image. In the
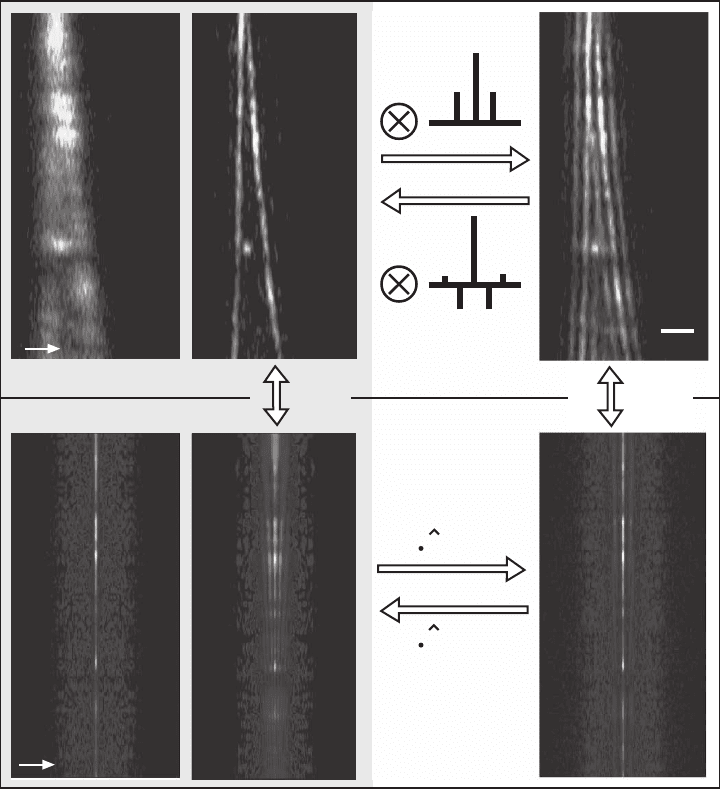
Chapter 12 Nanoscale Resolution in Far-Field Fluorescence Microscopy 807
1 µm
a) b) c)
z
k
z
d)
1)
l(k
z
)
l(k
z
)
-1
3)
FFT
z
4)
confocal
4pi (raw)
4pi
FFT
z
2)
e) f)
Figure 12–5. Lobe removal and deconvolution in 4Pi microscopy. The fi gure shows images of the same
pair of actin fi bers in a fi xed mouse fi broblast cell recorded in the TPE confocal (a) and TPE 4Pi type
A (b and c) mode. The corresponding Fourier transform along the optic axis is also shown (d, e, and
f). The fi ve-fold axial resolution increase (a vs. b) and the correspondingly extended OTF (d vs. e) are
immediately visible. The side lobes are well below 50% and the factorization of the PSF’s axial and
lateral dependence is possible in 4Pi microscopy. Therefore, an inverse discrete fi lter can be found and
its application to the raw data (c) yields a valid and almost artifact-free image (b). Alternatively, lobe
removal can be performed in the frequency domain. Equation (16) indicates that the Fourier transforms
of the raw data (f) is given by the product of the Fourier transform of the lobe-free image (e) and the
lobe function
l
(k
z
). Thus, (2) Fourier transforming the raw data, (3) multiplying with the inverse of
the lobe function’s Fourier transform,
l
−1
(k
z
), and (4) Fourier backtransforming lead to almost the
same lobe-free image. This method can be applied even if the separation of axial and lateral depen-
dence is impossible for the PSF. The Fourier transforms along the axial direction (3 and 4) merely have
to be replaced by their 3D counterparts. (See color plate.)
