Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.


768 A. Diaspro et al.
(Born and Wolf, 1980). Using excitation light with wavelength λ, the
intensity distribution at the focal region of an objective with numerical
aperture NA = sin(α) is described [see also Eq. (2)] in the paraxial regime
(Born and Wolf, 1980; Sheppard and Gu, 1990) by
Iuv J v e d
iu
(,) ( )
(/ )
=
−
∫
2
0
2
0
1
2
2
ρρρ
ρ
(16)
where rho is a dimensionless radial, J
o
is the zeroth-order Bessel func-
tion, ρ is a radial coordinate in the pupil plane, and u = 8π sin
2
(α/2)z/λ
and v = 2π sin(α)r/λ are dimensionless axial and radial coordinates,
respectively, normalized to the wavelength (Wilson and Sheppard,
1984). Now, the intensity of fl uorescence distribution within the focal
region has an I(u, v) behavior for the one-photon case and I
2
(u/2, v/2)
for the TPE case as demonstrated above. The arguments of I
2
(u/2, v/2)
take into proper account the fact that in the latter case wavelengths are
utilized that are approximatively twice those used for one-photon exci-
tation. As compared with the one-photon case, the TPE intensity dis-
tribution is axially confi ned (Nakamura, 1993; Gu and Sheppard, 1995;
Jonkman and Stelzer, 2002). In fact, considering the integral over v,
keeping u constant, its behavior is constant along z for one-photon and
has a half-bell shape for TPE. This behavior, better discussed in Wilson
(2002), Torok and Sheppard (2002), and Jonkman and Stelzer (2002),
explains the three-dimensional discrimination property in TPE.
Now, the most interesting aspect is that the excitation power falls off
as the square of the distance from the lens focal point, within the
approximation of a conical illumination geometry. In practice this
means that the quadratic relationship between the excitation power
and the fl uorescence intensity results in the fact that TPE falls off as
the fourth power of distance from the focal point of the objective. This
fact implies that those regions away from the focal volume of the objec-
tive lens, directly related to the numerical aperture of the objective
itself, therefore do not suffer photobleaching or phototoxicity effects
and do not contribute to the signal detected when a TPE scheme is
used. Because they are simply not involved in the excitation process, a
confocal-like effect is obtained without the necessity of a confocal
pinhole. It is also immediately evident that in this case an optical sec-
tioning effect is obtained. In fact, the observed image o(x,y,z) at a plane
j, produced by the true fl uorescence distribution i(x,y,z) at plane j, dis-
torted by the microscope through s, plus noise n, again corresponds to
the confocal ideal situation where contributions from adjacent k planes
can be set to zero as in the confocal situation: o
j
= i
j
∗ s
j
+ n.
This means that TPE microscopy is intrinsically three dimensional.
It is worth noting that the optical sectioning effect is obtained in a very
different way with respect to the confocal solution. No fl uorescence has
to be removed from the detection pathway. In this case it should be
possible to collect as much fl uorescence is possible. In fact fl uorescence
can come only and exclusively from the small focal volume traced in
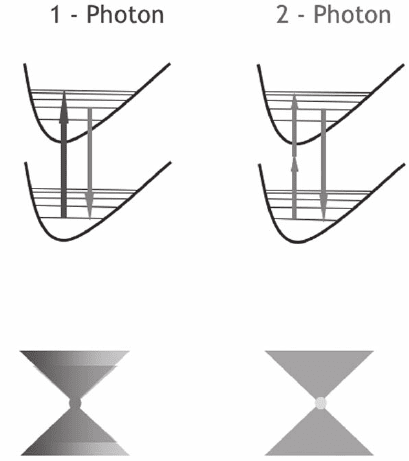
Figure 11–7, which also shows a comparison with the confocal mode,
that is of the order of a fraction of a femtoliter.
In TPE over 80% of the total intensity of fl uorescence comes from a
700- to 1000-nm-thick region about the focal point for objectives with
numerical apertures in the range of 1.2–1.4 (Brakenhoff et al., 1979;
Chapter 11 Two-Photon Excitation Fluorescence Microscopy 769
Wilson and Sheppard, 1984; Wilson, 2002; Jonkman and Stelzer, 2002;
Torok and Sheppard, 2002). This fact implies a reduction in back-
ground that allows compensation of the poorer spatial resolution com-
pared to the single-photon confocal mode due to the longer wavelength
utilized. However, the utilization of an infrared wavelength instead of
UV-visible ones also allows deeper penetration than in the conven-
tional case (So et al., 2001; Periasamy et al., 2002; König and Tirlapur,
2002). The long wavelengths used in TPE, or in general in multiphoton
excitation, will be scattered less than the ultraviolet–visible wave-
lengths used for conventional excitation (de Grauw and Gerritsen,
2001). Hence deeper targets within a thick sample can be reached. Of
course, for fl uorescence light, scattering on the way back can be over-
come by acquiring the emitted fl uorescence using a large area detector
and collecting not only ballistic photons (Soeller and Cannel, 1999;
Bauhler et al., 1999; Girkin and Wokosin, 2002).
7 The Optical Setup
A TPE architecture including confocal modality includes the following:
a high peak-power laser delivering moderate average power (femto second
or picosecond pulsed at a relatively high repetition rate) emitting infrared
or near-infrared wavelengths (650–1100 nm), CW laser sources for confo-
cal modes, a laser beam scanning system or a confocal laser scanning
head, high numerical aperture objectives (>1), a high-throughput
Figure 11–7. Illustration of the two different modalities for selecting 3D infor-
mation under a confocal (left) and TPE regime (right). In the confocal case the
selection is realized during the emission process. The different case of two-
photon excitation shows how the 3D selection can be realized during the fl uo-
rescence excitation process.
770 A. Diaspro et al.
microscope pathway, and a high-sensitivity detection system (Denk et al.,
1995; So et al., 1996; Soeller and Cannell, 1996; Wokosin and White, 1997;
Centonze and White, 1998; Potter et al., 1996; Wolleschensky et al., 1998;
Diaspro et al., 1999a,b; Wier et al., 2000; Soeller and Cannell, 1999; Tan et
al., 1999; Mainen et al., 1999; Majewska et al., 2000; Diaspro, 2002; Girkin
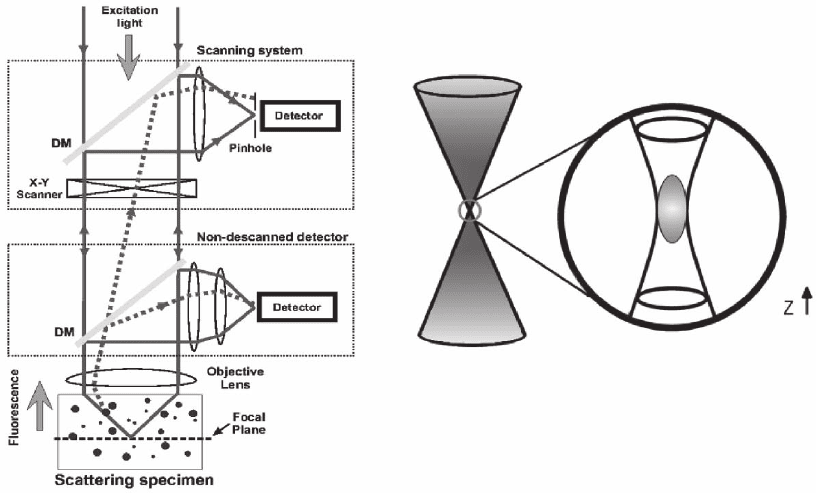
and Wokosin, 2002; Iyer et al., 2002). Figure 11–8 shows a general scheme
for a TPE microscope incorporating a confocal mode.
In typical TPE or confocal microscopes, images are built by raster
scanning the x–y mirrors of a galvanometrically driven mechanical
scanner (Webb, 1996). This fact implies that image formation speed is
mainly determined by the mechanical properties of the scanner, i.e., for
single line scanning it is of the order of milli seconds. Faster beam-
scanning schemes can be realized, even if the “eternal triangle of com-
promise” should be considered for sensitivity, spatial resolution, and
temporal resolution. While the x–y scanners provide lateral focal-point
scanning, axial scanning can be achieved by means of different posi-
tioning devices, the most popular being a belt-driven system using a DC
motor and a single objective piezo nano positioner, such as the PIFOC
(Physik Instrumente, Germany). Usually, it is possible to switc h bet ween
confocal and TPE modes retaining x–y–z positioning on the sample
being imaged (Diaspro, 2001; Diaspro and Chirico, 2003). Acquisition
and visualization are generally completely computer controlled by ded-
icated software. Figure 11–9 shows a TPE microscope.
Let us now consider two popular approaches that can be used to
perform TPE microscopy, namely, the descanned and nondescanned
Figure 11–8. Optical confi guration for a TPE microscope operating in a descanned (upper inset box)
and nondescanned (lower inset box) mode; see text. (Courtesy of M. Cannel and C. Soeller.)
Chapter 11 Two-Photon Excitation Fluorescence Microscopy 771
modes. They are skectched in Figure 11–8. The former uses the very
same optical pathway and mechanism employed in confocal laser
scanning microscopy. The latter mainly optimizes the optical pathway
by minimizing the number of optical elements encountered on the
way from the sample to detectors, and increases the detector area. The
TPE nondescanned mode provides very good performances giving a
superior signal-to-noise ratio inside strongly scattering samples
(Masters et al., 1997; Daria et al., 1998; Centonze and White, 1998;
So et al., 2000).
In the descanned approach pinholes are removed or set to their
maximum aperture and the emission signal is captured using an exci-
tation scanning device on the back pathway. For this reason it is called
the descanned mode. In the latter, the confocal architecture has to be
modifi ed in order to increase the collection effi ciency: pinholes are
removed and the emitted radiation is collected using dichroic mirrors
on the emission path or external detectors without passing through the
galvanometric scanning mirrors. A high-sensitivity detection system
is another critical issue (Wokosin et al., 1998; So et al., 2000; Girkin and
Wokosin, 2002).
The fl uorescence emitted is collected by the objective and transferred
to the detection system through a dichroic mirror along the emission
path (Figure 11–8). Due to the high excitation intensity, an additional
barrier fi lter is needed to avoid mixing the excitation and emission light
at the detection system that is differently placed depending on the
acquisition scheme being used. Photodetectors that can be used include
photomultiplier tubes, avalanche photodiodes, and CCD cameras
(Denk et al., 1995; Murphy, 2001). Photomultiplier tubes are the most
commonly used. This is due to their low cost, good sensitivity in the
blue-green spectral region, high dynamic range, large size of the sensi-
tive area, and single-photon counting mode availability (Hamamatsu
Photonics, 1999). They have a quantum effi ciency around 20–40% in
the blue-green spectral region that drops down to <1% moving to the
red region. This is a good condition, especially in MPE mode, because
it is desirable to reject as much as possible wavelengths above 680 nm
Figure 11–9. The TPE setup at LAMBS, Micro ScoBio Research Center of the
University of Genoa (from left to right: Ilaria Testa, Paolo Bianchini, and
Davide Mazza).
772 A. Diaspro et al.
that are mainly used for excitation. Another advantage is that the large
size of the sensitive area of photomultiplier tubes allows effi cient col-
lection of signal in the nondescanned mode within a dynamic range
of the order of 10
8
. Avalanche photodiodes are excellent in terms of
sensitivity exhibiting quantum effi ciency close to 70–80% in the visible
spectral range. Unfortunately they are high in cost and the small active
photosensitive area, <1 mm size, could introduce drawbacks in the
detection scheme and requires special descanning optics (Farrer et al.,
1999). CCD cameras are used in video rate multifocal imaging (Fuijta
and Takamatsu, 2002; Girkin and Wokosin, 2002). However, once the
best quality image possible has been obtained then image restoration
algorithms can be applied to enhance the features of interest to the
biological researcher and to improve the quality of data to be used
for three-dimensional modeling, such as those used for single-
photon optical sectioning microscopy, available at http://www.
powermicroscope.com (van der Voort et al., 1995; Shotton, 1995; Diaspro
et al., 1990, 2000; Boccacci and Bertero, 2002; Carrington, 2002; Difato
et al., 2004; Bonetto et al., 2004).
Laser sources, as often happened in optical microscopy, represent an
important resource, especially in fl uorescence microscopy (Gratton
and van de Ven, 1995; Svelto, 1998). For nonresonant TPE, owing to the
comparatively low TPE cross-sections of fl uorophores, high photon fl ux
densities are required, >10
24
photons cm
−2
s
−1
(König, 2000). Using radia-
tion in the spectral range of 600–1100 nm for TPE, excitation intensities
in the MW–GW cm
−2
range are required. This high energy can be
obtained by the combined use of focusing lens objectives and CW
(Hanninen and Hell, 1994; König et al., 1995) or pulsed (Denk et al.,
1990) laser radiation of 50 mW mean power or less (Girkin and Wokosin,
2002; Diaspro and Sheppard, 2002). TPE microscopes have been real-
ized using CW, femtosecond, and picosecond laser sources (Periasamy,
2001; Diaspro, 2001, 2002; Masters, 2002). Since the original successful
experiments in TPE microscopy, advances have been made in the tech-
nological fi eld of ultrashort pulsed lasers. Today laser sources suitable
for TPE can be described as “turnkey” compact systems (Fisher et al.,
1997; Wokosin et al., 1996; Diaspro, 2001).
Figure 11–10 shows a new generation ultrafast Ti:sapphire laser
source. The emission range between 700 and 1050 nm of the Ti:sapphire
laser allows a large number of commonly used fl uorescent molecules
to be excited. Other laser sources used for TPE are Cr-LiSAF, pulse-
compressed Nd-YLF in the femtosecond regime, and mode-locked
Nd-YAG and picosecond Ti-sapphire lasers in the picosecond regime
(Gratton and Van de Ven, 1995; Wokosin et al., 1996). Most of the laser
sources used for TPE operate in a mode-locking mode. This endows
the laser with the ability to generate a train of very short pulses by
modulating the gain or excitation of a laser at a frequency with a period
equal to the roundtrip time of a photon within the laser cavity (Fisher
et al., 1997; Svelto, 1998) (Figure 11–11). The resulting pulsewidth is in
the 50–150 fs regime. The parameters that are more relevant in the
selection of the laser source are average power, pulsewidth and re-
petition rate, and wavelength also according to Eq. (15). The most

Chapter 11 Two-Photon Excitation Fluorescence Microscopy 773
popular features for an infrared pulsed laser are 700 mW–1 W average
power, 80–100 MHz repetition rate, and 100–150 fs pulse width.
At present, the use of short pulses and small duty cycles are manda-
tory to allow image acquisition in a reasonable time while using power
levels that are biologically tolerable (Denk et al., 1994; Denk, 1996;
Koester et al., 1999; König et al., 1996, 1998; König, 2000; König and
Tirlapur, 2002). To minimize pulse width dis persion problems König
(2000) suggested work ing with pulses around 150–200 nm, and this
constitutes a very good compromise both for pulse stretching and
sample viability. It should always be remembered that a shorter pulse
broadens more than a longer one. Pulse width measurement is a very
delicate issue. In fact, because it is not very easy to measure it at the
focal volume within the sample, little can be defi nitely said about it
(Hanninen and Hell, 1994; Guild et al., 1997; Wolleschensky et al., 2002).
Although users do not perform measurement of the pulse width at the
Figure 11–10. Typical laser sources in use for TPE microscopy.
Figure 11–11. Laser emission time scale for TPE excitation: a short pulse at
high photon density is released for approximately 100 fs; this laser shot is able
to prime fl uorescence without damaging the sample so fl uorescence occurs in
the next few nanoseconds. The laser is silent for 10 ns and then delivers a new
high-density photon pulse. This modality allows TPE to be experienced at
tolerable time-averaged power (see text).
774 A. Diaspro et al.
sample when they use two-photon microscopy, which would require
a specifi c procedure that, even if not too complex for a researcher in
the fi eld, could be irksome for the majority of users, it is a reasonable
approximation to assume that at the focal volume, 1.5–2. times tempo-
ral pulse broadening occurs using high-quality optics (Wolleschensky,
2002; Girkin and Wokosin, 2002). As an example, for a measured laser
pulse width of about 100 fs, an estimate at the sample is about 150–180 fs
under favorable experimental conditions, sample characteristics
included. Sample properties are mentioned because for thick samples
the role played by thickness, also in terms of pulse width broadening,
is not so obvious (de Grauw and Gerritsen, 2002; So et al., 2001; Gu
et al., 2000; Saloma et al., 1998).
8 Conclusion
Confocal microscopy, in the authors’ opinion, constitutes one of the
most signifi cant advances in optical microscopy within the past
decades, and has become a powerful investigative tool for the molecu-
lar, cellular, and developmental biologist, the materials scientist, the
biophysicist, and the electronic engineer. It is entirely compatible with
the range of “classical” light microscopic techniques, and, at least in
scanned beam instruments, can be applied to the same specimens on
the same optical microscope stage. Its peculiar advantages result in its
ability to generate multidimensional (x–y–z–t) images by noninvasive
optical sectioning with a virtual absence of out-of-focus blur, its capac-
ity for multiparametric imaging of multiply labeled samples, and its
property of investigating at microscopic resolution large objects as a
result of the rejection of scattered light. So far, the advent of confocal
microscopy in the mid-1980s favored the rapid spreading of two- and
multiphoton excitation microscopy, since Denk’s report at the begin-
ning of the 1990s, bringing dramatic changes in designing experiments
that utilize fl uorescent molecules and, more specifi cally, in fl uorescence
3D optical microscopy.
While confocal microscopy is moving to spectral and fast-scanning
architectures in terms of acquisition, it is mainly two-photon micro-
scopy that occupies the scene of advances in fl uorescence optical
microscopy. TPE micro scopy, with its intrinsic three-dimensional reso-
lution, the absence of background fl uorescence, and the attractive pos-
sibility of exciting UV excitable fl uorescent molecules, thus increasing
sample penetration, constitutes signifi cant progress in science. In fact,
in a TPE scheme two 720-nm photons combine to produce the very
same fl uorescence conventionally primed at ∼360 nm, and to be utilized
in a classical confocal microscope using conventional excitation of fl uo-
rescent molecules. The excitation of the fl uorescent molecules bound
to the specifi c components of the biological systems being studied
mainly takes place (80%) in an excitation volume of the order of mag-
nitude of 0.1 fl . This results in an intrinsic 3D optical sectioning effect.
What is invaluable for cell imaging and, in particular, for live-cell
imaging is the fact that weak endogenous one-photon absorption and
Chapter 11 Two-Photon Excitation Fluorescence Microscopy 775
highly localized spatial confi nement of the TPE process dramatically
reduce phototoxicity stress. To summarize the unique characteristics
and advantages of TPE we recall the following properties:
1. Spatially confi ned fl uorescence excitation in the focal plane of the
specimen can be con sidered the key feature of TPE microscopy. It is one
of the advantages over confocal micro scopy, where fl uorescence emis-
sion occurs across the entire thickness of the sample being excited by the
scanning laser beam. A strong implication is that there is no photon
signal from sources out of the geometric position of the optical focus
within the sample. Therefore, the signal-to-noise ratio increases, photo-
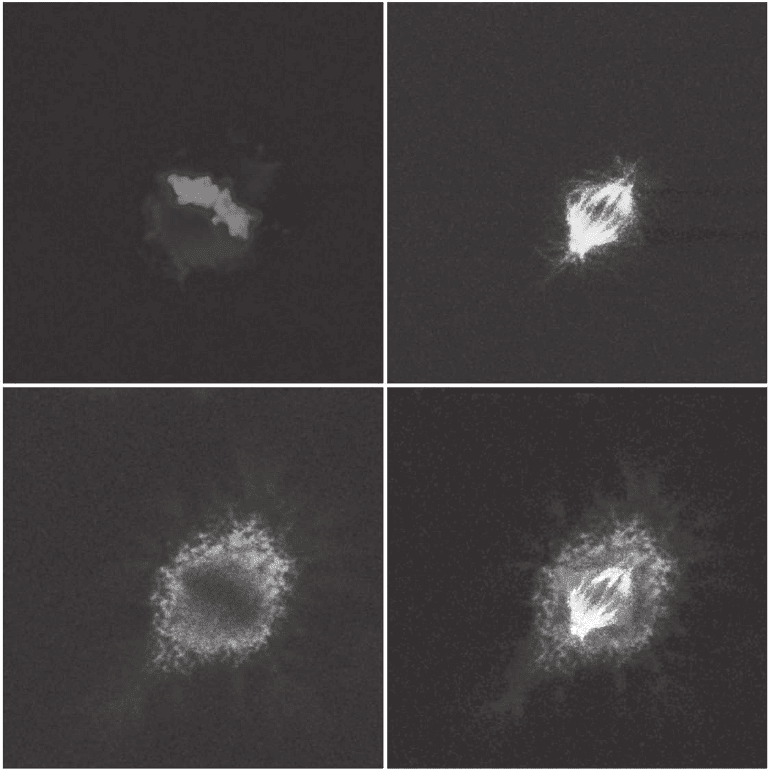
Figure 11–12. Multiple excitation of three fl uorescent dyes using 740 nm u nder a TPE regime. The
conventional excitation would have required the utilization of 360 or 405 nm, 488 nm, and 543 nm laser
lines. The fi nal image (lower right quadrant) is realized by merging the three subsets. (This image has
been acquired by students of the Biotechnology School during the course of Advanced Microscopy
Techniques activated at the University of Genoa, academic year 2005. Advisors: Grazia Tagliafi erro
and Alberto Diaspro.) (See color plate.)
776 A. Diaspro et al.
degradation effects decrease, and optical sectioning is immediately
available without the need for pinhole or deconvolution algorithms. In
addition, very effi cient acquisition schemes can be implemented such as
the nondescanned one operating at an excellent signal-to-noise ratio.
2. The use of near-infrared (IR)/IR wavelengths permits examina-
tion of thick specimens in depth. This is due to the fact that, apart from
some cases such as pigmented samples and portions of the absorption
spectral window of water, cells and tissues absorb poorly in the near-
IR/IR region. Cellular damage is globally minimized, thus allowing
cell viability to be prolonged with long-term 3D sessions. Moreover,
scattering is reduced and deeper targets can be reached with fewer
problems than in one-photon excitation. The depth of penetration can
be up to 0.5 mm. In addition, whereas in one-photon excitation, the
emission wavelength is comparatively close to the excitation one (about
50–200 nm longer), in TPE the fl uorescence emission occurs at a wave-
length substantially shorter and at a larger spectral distance than in
one-photon excitation. Thus separation of the excitation light and the
emitted light can be easily performed.
Continuing research in this fi eld is focused on very intriguing prob-
lems (www.focusonmicroscopy.org offers a complete scenario of the
evolution of three-dimensional microscopy in the past 5 years) such as
local heating from absorption of IR light by water at high laser power
(Schonle and Hell, 1998) and photothermal effects on fl uorescent mol-
ecules (Chirico et al., 2003a), phototoxicity from long wavelength IR
excitation and short wavelength fl uorescence emission (König et al.,
1996c; Tyrrel and Keyse, 1990; König, 2000; Hopt and Neher, 2001;
König and Tirlapur, 2002), photoactivation and photocycling of visible
fl uorescent proteins (Post et al., 2004; Chirico et al., 2004; Schnedier
et al., 2005), development of new fl uorochromes better suited for TPE
and multiphoton excitation (Albota et al., 1998a; Abbotto et al., 2005),
and the investigation of the cross-sections of uncharacterized mole-
cules (Gostkowski et al., 2004; Wokosin et al., 2004).
One of the major benefi ts in setting up an MPE microscope is the
fl exibility in choosing the measurement modality favored by the
simplifi cation of the optical design. In fact, a TPE microscope offers a
greater variety of measurement options without changing any optics
or hardware. This means that during the very same experiments real
multimodal information can be obtained from the specimen being
studied (Zoumi et al., 2002; Wang et al., 2004). Moreover, the usefulness
of the TPE scheme for spectroscopic and lifetime studies (So et al.,
1996; Sytsma et al., 1998; Schwille et al., 2000; Diaspro et al., 2001;
Wiseman et al., 2002), for optical data storage and microfabrication
(Cumpston et al., 1999; Kawata et al., 2001), and for single molecule
detection (Mertz et al., 1995; Farrer et al., 1999; So et al., 2000; Chirico
et al., 2001; Cannone et al., 2003b) has been well documented. Other
very interesting applications involve the study of impurities affecting
the growth of protein crystals (Caylor et al., 1999), TPE imaging in the
fi eld of plant biology (Tirlapur and König, 2002), and measurements in
living systems (Squirrel et al., 1999; Yoder and Kleinfeld, 2002; Diaspro
et al., 2002b,d; Post et al., 2004). Here the combination of MPE and
Chapter 11 Two-Photon Excitation Fluorescence Microscopy 777
second-harmonic generation offers the opportunity to investigate the
morphometric properties on the basis of the microstructure of blood
cells (Zoumi et al., 2004). Another promising fi eld is the investigation
of complex formation where the TPE properties will improve the infor-
mation accessible (Heinze et al., 2004). Another, more indirect usage
that provides a look at the sample with nanometer resolution is the
excitation of an evanescent wave at a metal surface (Novotny et al.,
1998). For microscopic purposes the evanescent wave needs to be local-
ized at a nanoparticle or a fi ne metal tip (Sánchez et al., 1999; Gerton
et al., 2004). The MPE microscope can also be used as an active device,
with increasing applications related to nanosurgery (König, 2000),
selective uncaging of caged compounds (Diaspro et al., 2003), and
photodynamic therapy (Bhalwalkar et al., 1997; So et al., 2000). Recently
TPE microscopy, even if in an evanescent-fi eld-induced confi guration,
has been extended to large area structures of the order of square cen-
timeters (Duveneck et al., 2001). This has application in the realization
of biosensing platforms such as genomic and proteomic microarrays
based upon large planar waveguides. It is easy to perceive that the
range of applicability of MPE microscopes is rapidly increasing in the
biomedical, biotechnological, and biophysical sciences and is expand-
ing to clinical applications (Diaspro, 2002; Masters, 2002; Periasamy
and Diaspro, 2003).
Acknowledgments. The fi rst Italian TPE architecture realized at LAMBS
has been supported by INFM grants. LAMBS-MicroScoBio is currently
funded by IFOM (Istituto FIRC di Oncologia Molecolare, FIRC Institute
of Molecular Oncology, Milano). This chapter is dedicated to the
memory of Osamu Nakamura, who passed away January 23, 2005 at
Handai Hospital.
References
Abbe, E. (1910). In: Die Lehre von der Bildentstehung in Mikroskop (O.
Lummer, Ed.). F. Reiche, Braunschweig.
Abbotto, A., Baldini, G., Beverina, L., Chirico, G., Collini, M., D’alfonso, L.,
Diaspro, A., Magrassi, R., Nardo, L. and Pagani, G.A. (2005). Dimethyl-
Pepep: A Dna probe in two-photon excitation cellular imaging. Biophys.
Chemi. 114(1), 35–41.
Agard, D.A. (1984). Optical sectioning microscopy: Cellular architecture in
three dimensions. Annu. Rev. Biophys. 13, 191–219.
Agard, D.A., Hiraoka, Y., Shaw, P.J. and Sedat, J.W. (1989). Fluorescence
microscopy in three-dimensions. Methods Cell. Biol. 30, 353–378.
Albota, M., Beljonne, D., Bredas, J.L., Ehrlich, J.E., Fu, J.Y., Heikal, A.A., Hess,
S.E., Kogej, T., Levin, M.D., Marder, S.R. and others. (1998a). Design of
organic molecules with large two-photon absorption cross sections. Science
281(5383), 1653–1656.
Albota, M.A., Xu, C. and Webb, W.W. (1998b). Two-photon fl uorescence excita-
tion cross sections of biomolecular probes from 690 to 960 nm. Appl. Opt. 37,
7352–7356.
Amos, B. (2000). Lessons from the history of light microscopy. Nature Cell Biol.
2, E151–E152.
Andrews, D.L. (1985). A simple statistical treatment of multiphoton absorption.
Am. J. Phys. 53, 1001–1002.
