Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

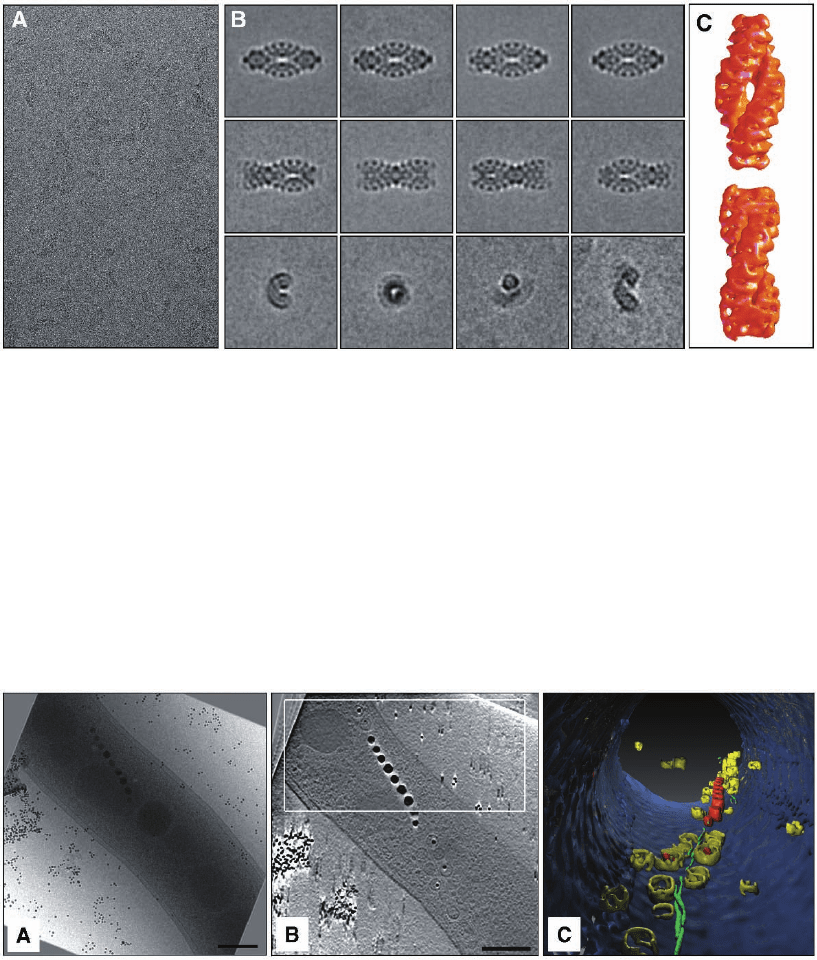
Figure 7–3. Single particle investigation of the giant protein complex TPP II from Drosophila mela-
nogaster embedded in vitrifi ed ice. In eukaryotes, tripeptidyl peptidase II (TPPII) is a crucial compo-
nent of the protein degradation pathway. The 150-kDa subunits of Drosophila TPPII assemble into a
giant proteolytic complex of 6 MDa with a remarkable architecture consisting of two segmented and
twisted strands that form a spindle-shaped structure (length 56 nm, width 24 nm). a) Cryo-electron
micrograph of isolated TPP II complexes illustrating the very weak image contrast and the high level
of noise. b) Averaging and classifi cation of a large number of equivalent projections of separate mole-
cules. Once a large set of views is available, a preliminary 3D reconstruction can be computed and
refi ned iteratively. c) The 3D model obtained by cryo-electron microscopy, reveals details of the
molecular architecture and, in conjunction with biochemical data, provides insight into the assembly
mechanism. The building blocks of this complex are apparently dimers, within which the 150 kDa
monomers are oriented head to head. Stacking of these dimers leads to the formation of twisted single
strands, two of which comprise the fully assembled spindle (Rockel et al., 2002 and 2005).
Figure 7–4. Cellular cryo-electron tomography of the magnetotactic microorganism Magnetospirillum
griphiswaldense. The entire bacterium is oriented like a compass needle inside the magnetic fi eld in its
search for optimal living conditions. The miniature cellular compass is made by a chain of single
nano-magnets, called magnetosomes (the scale bar represents 200 nm). a) The two-dimensional image
represents one projection (at 0°) from an angular tilt-series. b) x–y slices along the z axis through a
typical three-dimensional reconstruction (tomogram). c) Surface-rendered representation of the inside
of the cell showing the membrane (blue), vesicles (yellow), magnetite crystals (red) and a fi lamentous
structure (green). Until now, it was not clear how the cells organise magnetosomes into a stable chain,
against their physical tendency to collapse by magnetic attraction. However, the biochemical analysis
revealed a protein responsible for the chain formation and the 3D investigation a cytoskeletal struc-
ture, which aligns the magnetosomes like pearls on a string (Scheffel et al., 2005).
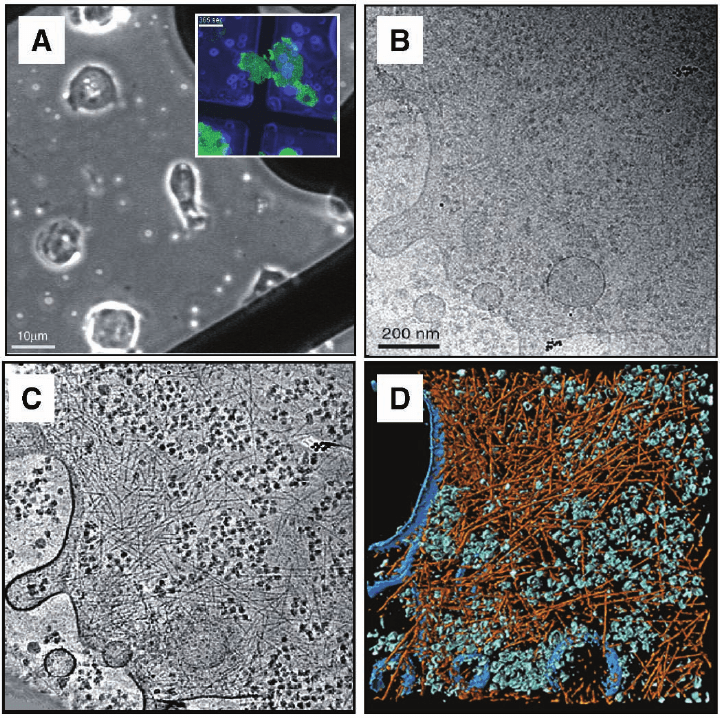
Figure 7–5. First electron tomographic investigation of a eukaryotic cell; the slime mould Dictyostel-
ium discoideum embedded in vitrifi ed ice. a) Phase contrast image and corresponding fl uorescence
image (inset) of cells on TEM grids. b) Cryo-electron micrograph at 0° tilt (conventional 2D
projection) of a ∼200 nm thin peripheral region of the cell. c) Tomographic reconstruction from a
complete tilt-series (120 images) and d) visualization by segmentation. Large macromolecular com-
plexes, e.g. Ribosomes are shown in a green color, the actin fi lament network in orange-red and the
cells’ membrane in blue. Cryo-tomograms of Dictyostelium discoideum cells grown directly on carbon
support fi lms have provided unprecedented insights into the organization of actin fi laments in an
unperturbed cellular environment. The tomograms show, on the level of individual fi laments, their
modes of interaction (isotropic networks, bundles, etc.), they allow us to determine the branching
angles precisely (in 3-D), and they reveal the structure of membrane attachment sites (Medalia
et al., 2002).
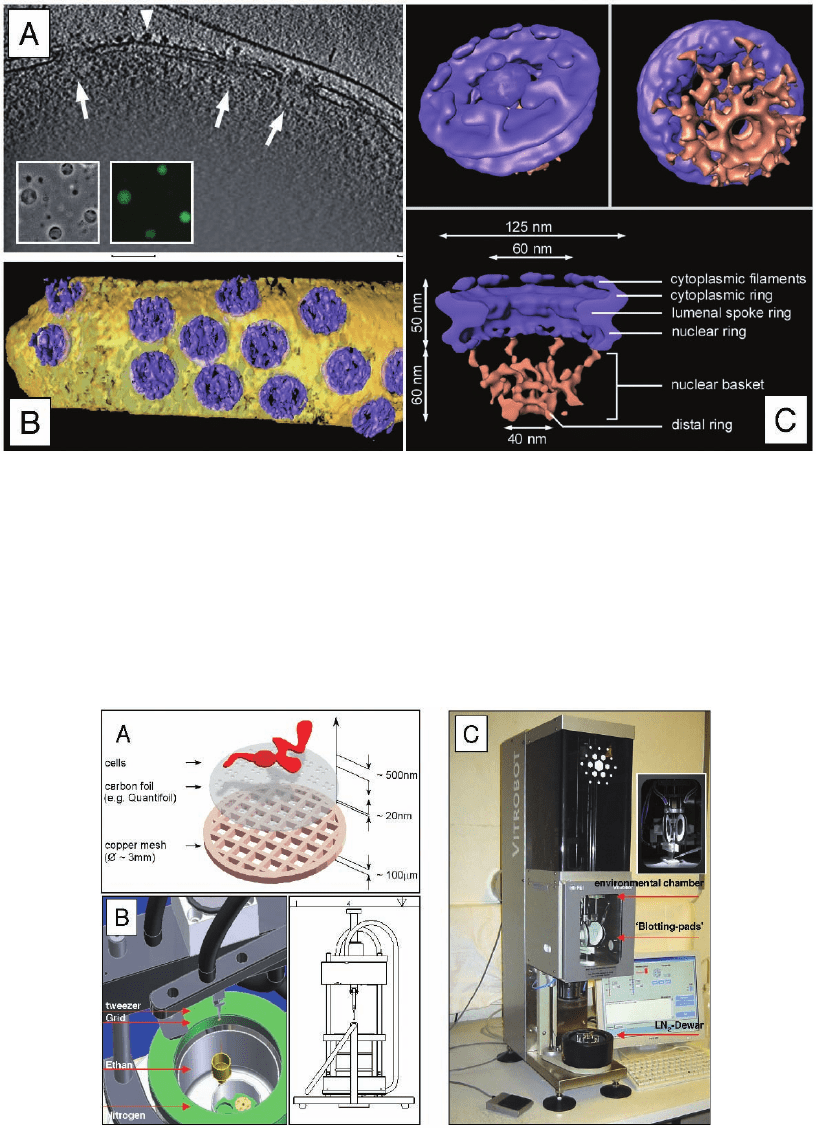
Figure 7–6. CET in combination with the single particle approach of transport-competent Dictyostel-
ium discoideum nuclei. a) Three-dimensional reconstruction of the peripheral rim of an intact nucleus.
X-y slice of 10 nm thickness along the z axis through a typical tomogram. Side views of nuclear pore
complexes (NPCs) are indicated by arrows. Ribosomes connected to the outer nuclear membrane are
visible (arrowheads). Inset displays a phase-contrast image and the corresponding fl uorescence image.
b) Surface rendered representation of a segment of nuclear envelope (NPCs in blue, membranes in
yellow). c) Structure of the Dictyostelium NPC after classifi cation and averaging of subtomograms.
Cytoplasmic face of the NPC (upper left); the cytoplasmic fi laments are arranged around the central
channel. Nuclear face of the NPC (upper right); the distal ring of the basket is connected to the nuclear
ring by the nuclear fi laments. Cross sectional view of the NPC (bottom). The dimensions of the main
features are indicated. All views are surface-rendered (nuclear basket in brown; Beck et al. 2004).
Figure 7–7. Plunge freezing instrumentation. a) Typical arrangement of cells cultured on TEM grid just
prior to plunging. The schematic indicates the dimensions. b) CAD (computer aide d design) image of
a home-build plunge freezing apparatus (Images courtesy of R. Gatz, MPI of Biochemistry, Martinsried
(near Munich), Germany). The small reservoir (yellow) in the middle of the dewar (green) contains the
liquid ethane, which is chilled by a surrounding bath of liquid nitrogen. The forceps, which hold the
grid are attached to a weighted arm. When the arm is released by means of a foot-trigger, the grid is
gravity-plunged into the ethane. The cross-sectional scetch (left part of image B) illustrates the ‘guillo-
tine-like’ arrangement. c) The Vitrobot (FEI company, Eindhoven, The Netherlands), a ‘robot’ for vitri-
fi cation, allows the control of environmental and all necessary processing parameters.
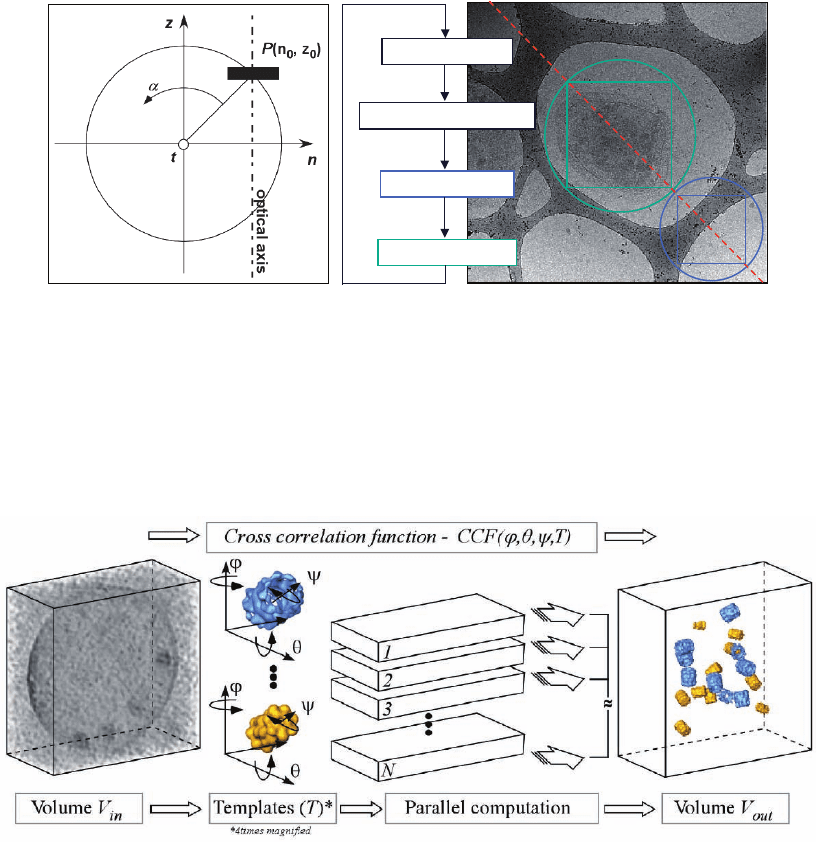
Figure 7–22. Detection and identifi cation of individual macromolecules in cellular tomograms is
based on their structural signature. Because of the crowded nature of the cytoplasm and ‘contamina-
tion’ with noise, an interactive segmentation and feature extraction is not feasible. It requires sophis-
ticated pattern recognition techniques to exploit the information contained in the tomograms. A
volume rendered presentation of a 3D image is presented on the left. Even though some high-density
features may be visible, an unambiguous identifi cation of individual structures would be diffi cult if
not impossible given the residual noise. An approach, which has proven to work, is based on template
matching. Templates of the macromolecules under scrutiny are obtained by a high- or medium resolu-
tion technique (X-ray crystallography, NMR, electron crystallography or single particle analysis).
These templates (4 times magnifi ed in this fi gure; 20S proteasome and thermosome) are then used
to search the entire volume of the tomogram systematically for matching structures by three-
dimensional cross-correlation and the result is refi ned by multivariate statistical analysis. In principle
the 3D image has to be scanned for all possible Eulerian angles ϕ,ψ and θ around three different axes,
with templates of all the different protein structures one is interested in e.g. the thermosome (blue)
and proteasome (yellow). The search procedure is computationally very demanding but can be paral-
lelized with respect to the different angular combinations in a highly effi cient manner. Finally, the
position and orientation of the different complexes can be mapped directly in the 3D image.
Figure 7–18. a) Geometric model of specimen rotation around a tilt axis. b) Automated data collection
scheme for the ‘full-tracking/ full-focusing’ case. For tracking, focusing and the fi nal image acquisi-
tion the same magnifi cation is used and the beam is adjusted in a way that the areas where tracking,
focusing a and exposure is done do not overlapp (blue and green circle). Using very short exposure
times in combination with high binning values (4 × 8 or even 8 × 8) the exposure to the sample in
auto-tracking and auto-focusing can be minimized.
change tilt angle
‚image shift‘ correction
Focus correction
image acquisition
T
i
l
t
ax
i
s
Tracking
Focus
Exposure
A
B
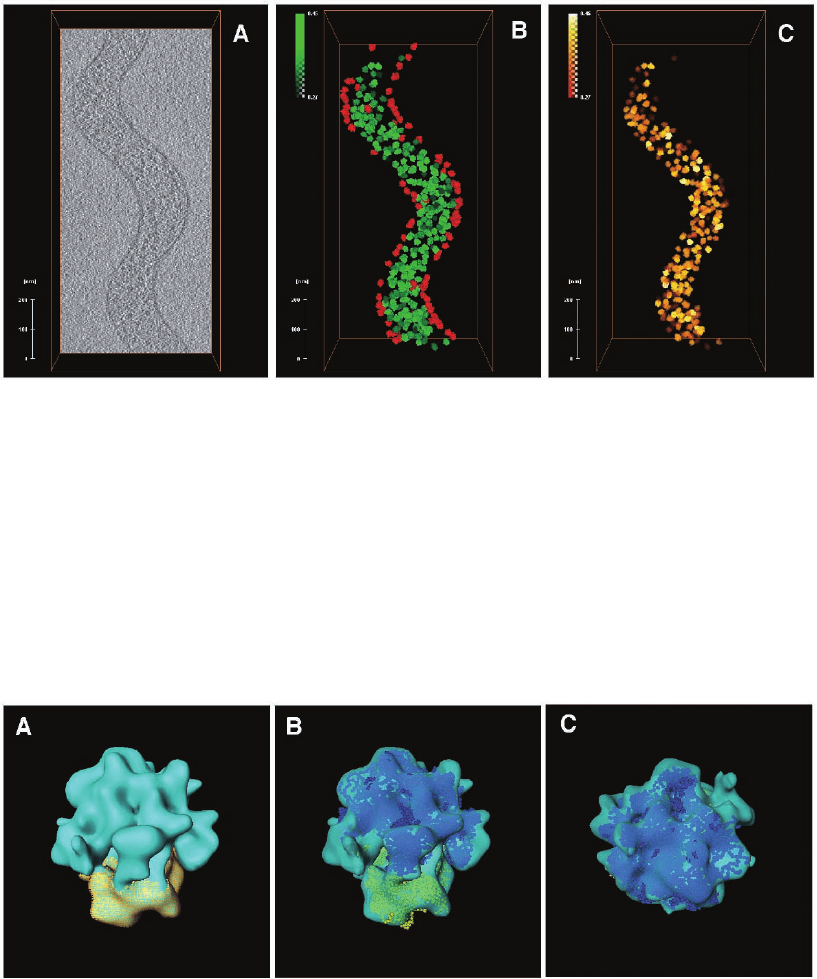
Figure 7–23. Mapping ribosomes in an intact S. melliferum cell. a) x–y slice from the corresponding
tomogram. Image analysis of this portion of the cell is displayed in subsequent panels. b) Locations
and orientation of all ribosomes detected by template matching. Each 70S ribosome is represented by
the averaged density (see Fig. 26) derived from the tomogram. The colour coding indicates the detec-
tion fi delity; green is high, yellow is intermediate, red is low and probably represents false positives.
The brightness of the colour corresponds to correlation peaks heights. c) Final ribosome atlas after
removal of putative false positives (images courtesy of J. Ortiz, MPI of Biochemistry, Martinsried,
Germany).
Figure 7–25. a) Averaged structure of the 70S ribosome derived from the dataset above (average from
300 individual particles to ∼4 nm resolution). The map highlights the 30S subunit in yellow. b) Docking
of high resolution structures of the 70S ribosome into the map shown in A. c) “Crown view” of B
(images courtesy of J. Ortiz, MPI of Biochemistry, Martinsried, Germany).
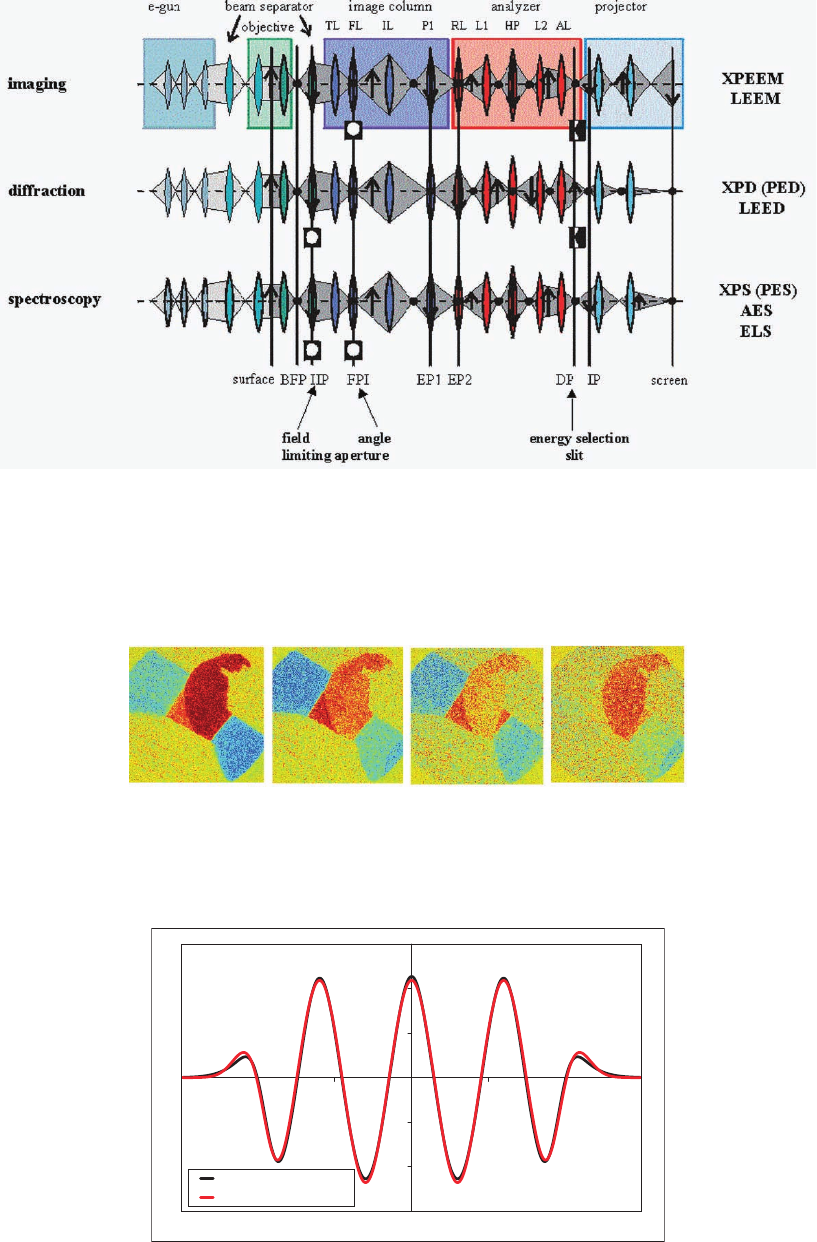
Figure 10–13. Electrostatic correction of chromatic aberration. (a) Rays in a quadrupole quadruplet
or sextuplet. (b) Match between the potentials needed to satisfy Scherzer’s condition and those in the
corrector.
5.2 eV 6.4 eV 9.6 eV 11.4 eV
Figure 8–26. Quantum size contrast between regions with different thickness of an Fe fi lm on W(110),
taken with different electron energies, that is wavelengths. The images in the top row show the inten-
sity and those at the bottom the magnetic signal (exchange asymmetry). Blue and red correspond to
opposite magnetization directions.
88
Figure 8–18. The three fundamental operation modes of a SPELEEM system. The various sections of
the instrument are shown folded into one plane. In imaging and diffraction the energy selection slit
is inserted in the dispersive plane DP and the image/diffraction pattern behind DP is imaged with
the projector. The intermediate lens IL is used to switch between imaging and diffraction, simultane-
ously with the exchange of the contrast aperture in FPI and the fi eld-limiting aperture in IIP. For fast
spectroscopy both apertures are inserted, the energy selection slit is removed, and the dispersive plane
is imaged by the projector.
15
-3E+08
-2E+08
-1E+08
0E+00
1E+08
2E+08
3E+08
-15 -10 -5 0 5 10 15
Z [mm]
Quadrupole field [V/m/m]
Scherzer's condition
3D Quadrupole
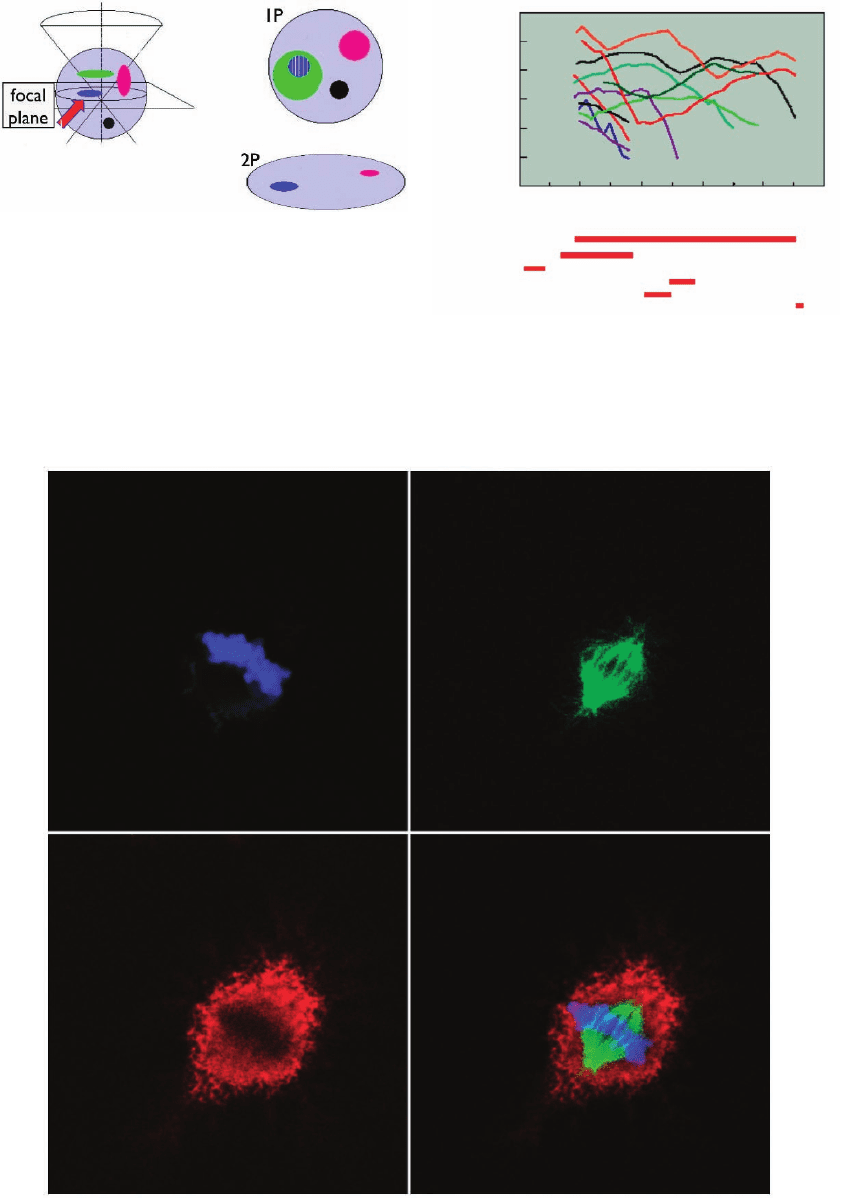
Figure 11–12. Multiple excitation of three fl uorescent dyes using 740 nm under a TPE regime. The
conventional excitation would have required the utilization of 360 or 405 nm, 488 nm, and 543 nm laser
lines. The fi nal image (lower right quadrant) is realized by merging the three subsets. (This image has
been acquired by students of the Biotechnology School during the course of Advanced Microscopy
Techniques activated at the University of Genoa, academic year 2005. Advisors: Grazia Tagliafi erro
and Alberto Diaspro.)
Rhodamine B
Bodipy Fl
Dil
Coumarin
Lucifer Yellow
Fluorescein
Cascade Blue
Pyrene
Bis-MSB
Dansyl
DAPI
600 700 800
10
3
Two photon Excitation
Cross Sections (GM)
Wavelength (nm)
Ti:Sapphlie
SHG of Cr:YAG
SHG of Cr:Forsterite
Cr:LiSGAF
Cr:LiSAF
Nd:YLF or Nd:glass
10
2
10
0
10
–1
10
–2
10
–3
10
1
900 1000 1100
Figure 11– 6. Two-photon cross-sections for popular
fl uorescent molecules as a function of the excitation
wavelength. Red bars indicate the emission range of
some common laser sources utilized in TPE micro-
scopy and spectroscopy.
Figure 11–2. Comparison between conventional
(1P) and two-photon (2P) excitation with respect to
image formation. When focusing on the actual
focal plane under 1P, a contribution from adjacent
planes that are physically excluded in the 2P process
is obtained, as happens in a confocal setup. (From
Giuseppe Vicidomini, LAMBS, MicroScoBio, Uni-
versity of Genoa.)
z
r
k
z
k
r
2%
4% 100%
PSF OTFPSF OTF
Widefield Confocal SWM I
5
M 4Pi C
TPE 4Pi A TPE 4Pi C
PSF OTF
o
det
o
o
ex
=
h
det
h
h
ex
=
x
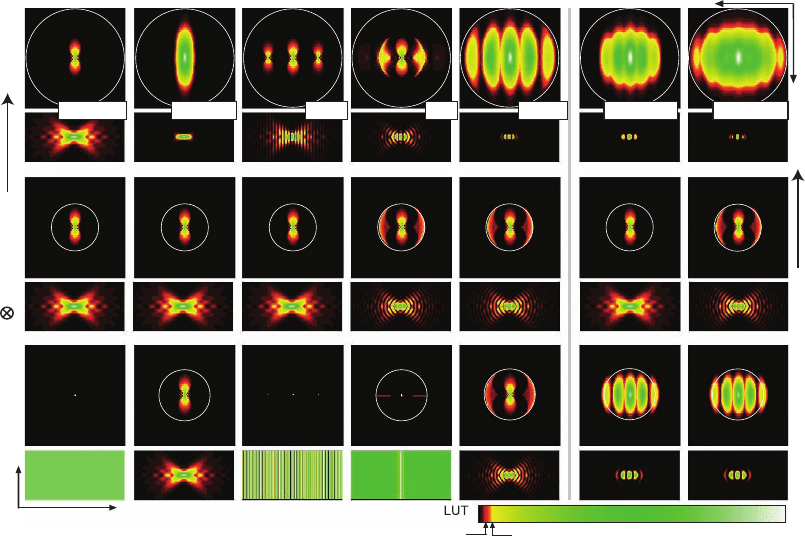
Figure 12–4. Overview of the excitation (bottom), detection (center), and effective (top) OTFs’ modulus
and the corresponding PSFs of the wide-fi eld, confocal, standing wave (SWM), I
5
M, 4Pi confocal type
C, TPE 4Pi confocal type A, and TPE 4Pi confocal type C microscopes. The color look-up table (LUT)
has been designed to emphasize the important weak OTF regions. The OTFs are shown in the squares
above the corresponding PSFs; the zero frequency point is in the center and the largest frequency
displayed is 2π/80 nm
−1
. The circles represent the maximum possible carrier as explained in Figure
12–3. For TPE, the excitation OTFs slightly extend over these circles because the excitation wavelength
of 800 nm is less than double the one-photon excitation wavelength of 488 nm. While all these methods
extend the OTF along the axial direction, they fundamentally differ in contiguity and absolute strength
within the support region. For example, there are pronounced frequency gaps for the SWM and
depressions for the I
5
M. The rectangular images of the PSFs represent a region of 5 × 2.5 µm with the
geometric focus in the center.
e) f)
1 µm
a) b) c)
z
k
z
d)
1)
l(k
z
)
l(k
z
)
-1
3)
FFT
z
4)
confocal
4pi (raw)
4pi
FFT
z
2)
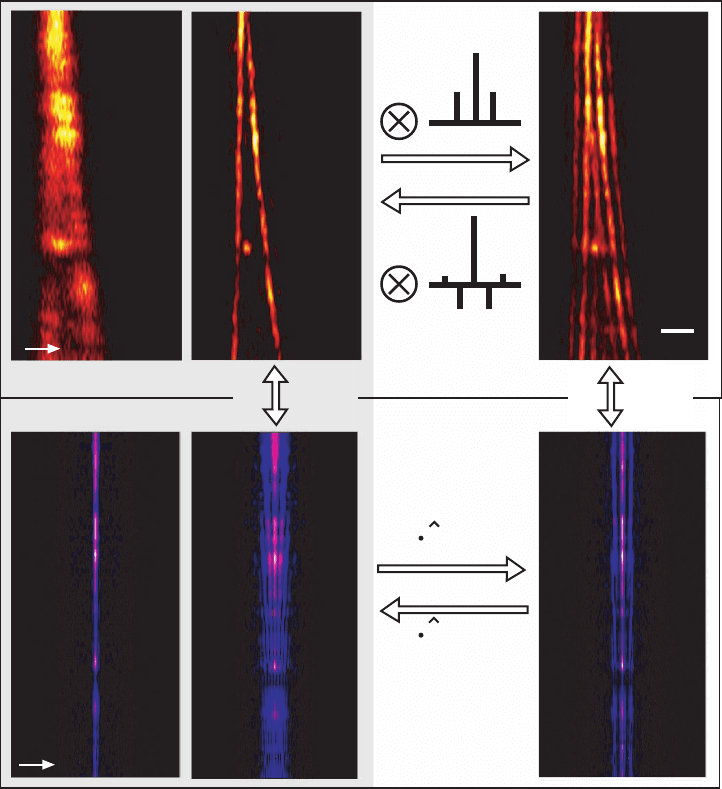
Figure 12–5. Lobe removal and deconvolution in 4Pi microscopy. The fi gure shows images of the same
pair of actin fi bers in a fi xed mouse fi broblast cell recorded in the TPE confocal (a) and TPE 4Pi type
A (b and c) mode. The corresponding Fourier transform along the optic axis is also shown (d, e, and
f). The fi ve-fold axial resolution increase (a vs. b) and the correspondingly extended OTF (d vs. e) are
immediately visible. The side lobes are well below 50% and the factorization of the PSF’s axial and
lateral dependence is possible in 4Pi microscopy. Therefore, an inverse discrete fi lter can be found and
its application to the raw data (c) yields a valid and almost artifact-free image (b). Alternatively, lobe
removal can be performed in the frequency domain. Equation (16) indicates that the Fourier transforms
of the raw data (f) is given by the product of the Fourier transform of the lobe-free image (e) and the
lobe function
l
(k
z
). Thus, (2) Fourier transforming the raw data, (3) multiplying with the inverse of
the lobe function’s Fourier transform,
l
−1
(k
z
), and (4) Fourier backtransforming lead to almost the
same lobe-free image. This method can be applied even if the separation of axial and lateral depen-
dence is impossible for the PSF. The Fourier transforms along the axial direction (3 and 4) merely have
to be replaced by their 3D counterparts.
490nm
244nm
STED
source
EXCT.
source
DET
DC
DC
PP
97nm
104nm
x
z
c)
d) e)
fluorescent
AA
BB
non-fluorescent
450 650
λ[nm]
Excitation
STED
Detection
a)
b)
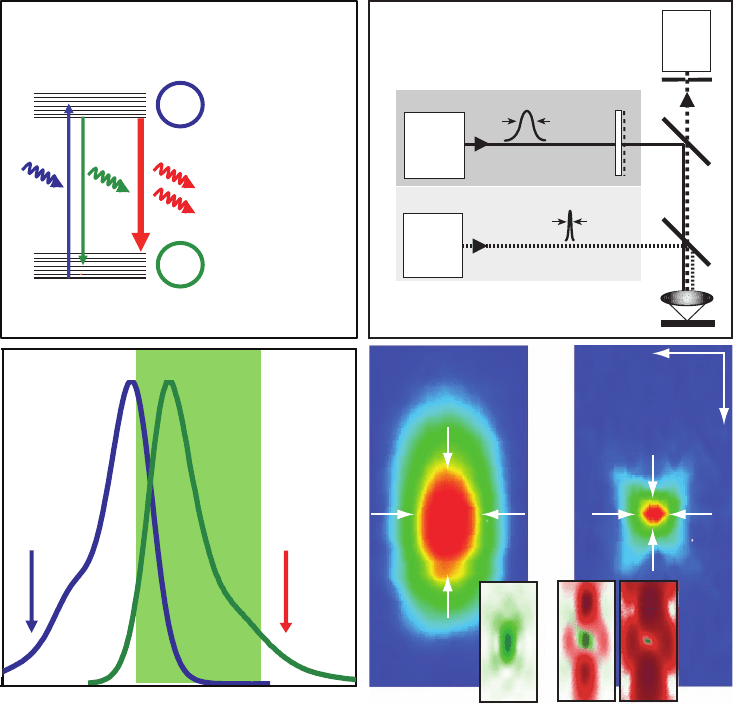
Figure 12–7. Stimulated emission depletion (STED) was the fi rst implementation of the RESOLFT
principle. (a) Dye molecules are excited into the S
1
(state A) by an excitation laser pulse. (b) Fluorescence
is detected over most of the emission spectrum. How ever, molecules can be quenched back into the
ground state S
0
(state B) using stimulated emission before they fl uoresce by irradiating them with a light
pulse at the edge of the emission spectrum shortly after the excitation pulse and before they are able to
emit a fl uorescence photon. Saturation is realized by increasing the intensity of the depletion pulse and
consequently inhibiting fl uorescence everywhere except at the “zero points” of the focal distribution of
the depletion light. (c) Schematic of a point-scanning STED microscope. Excitation and depletion beams
are combined using appropriate dichroic mirrors (DC). The excitation beam forms a diffraction-limited
excitation spot in the sample (inset in d) while the depletion beam is manipulated using a phase-plate
(PP) or any other device to tailor the wavefront in such a way that it forms an intensity distribution with
a nodal point in the excitation maximum (left inset in e). The third inlay shows the resulting quenching
probability when saturating the depletion process. (d) and (e) show an experimental comparison
between the confocal PSF and the effective PSF after switching on the depleting beam. Note the doubled
lateral and fi ve-fold improved axial resolution. The reduction in dimensions (x, y, z) yields ultrasmall
volumes of subdiffraction size, here 0.67 al (Klar et al., 2000), corresponding to an 18-fold reduction
compared to its confocal counterpart. The spot size is not limited on principle grounds but by practical
circumstances such as the quality of the zero and the saturation factor of depletion.
