Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

1110 U. Weierstall
0.37 nm above the surface compared to 0.7 nm for the gas phase mole-
cule. Upon adsorption at room temperature the molecules diffuse to
step edges and are stabilized with their wire parallel to step edges,
which prevents good electronic contact (Kuntze et al., 2002).
The problem of controlling the electronic contact between the mole-
cule and its electrodes has been addressed in an investigation with the
Lander molecule. Reproducible contact formation was obtained by
lateral manipulation of the Lander on Cu(111) with an LT-STM (Moresco
et al., 2003). The molecules where adsorbed at 70 K instead of room
temperature to avoid postdeposit thermal diffusion. Figure 17–34 (A3)
shows an STM image of a single Lander on a terrace where the four
bumps are attributed to the four legs of the molecule. When a molecule
is pushed to the step with its central wire parallel to it, it reaches a fi nal
conformation imaged in Figure 17–34 (B3). Separated by the legs, the
central wire is not interacting with the step edge and the standing wave
pattern (LDOS oscillations) (B4) on the upper terrace has not changed.
If the molecule is repositioned with its wire oriented perpendicular to
the step edge, a notable modifi cation of the standing wave pattern on
the upper terrace is observed. The amplitude of the standing wave
pattern is reduced at the contact point compared to the clean step edge
case (4C). Simulations of the standing wave pattern indicated that the
perturbation is caused by the terminal part of the molecular wire on
the upper terrace. This contact area is visible in the STM image as an
additional small bump (C3) and its location was confi rmed by elastic-
scattering quantum chemistry STM image calculations (Sautet and
Joachim, 1991) (C2). The molecule could also be decontacted by reverse
lateral manipulation, and the original step edge and molecule image
were recovered.
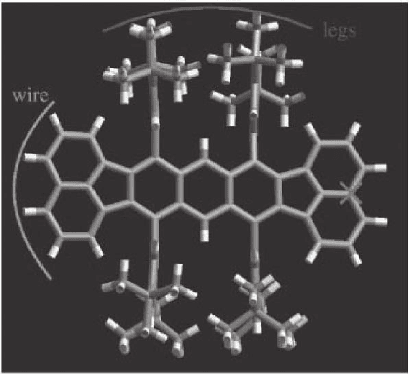
Figure 17–33. Top view of the chemical structure of the Lander molecule,
which was designed to be a model system for a molecular wire. The molecular
wire and the four legs that support the wire are shown. (From Moresco et al.,
2003.)
Chapter 17 Low-Temperature Scanning Tunneling Microscopy 1111
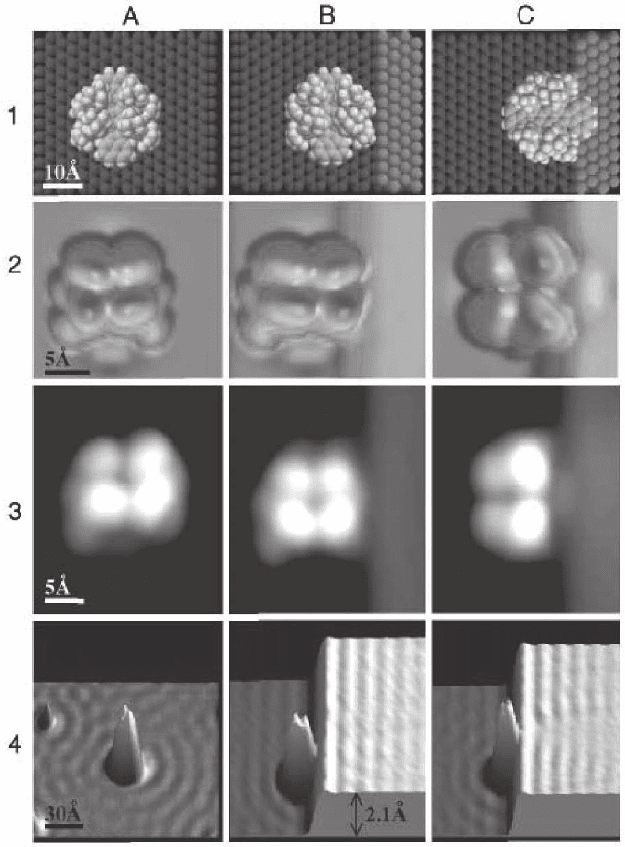
Figure 17–34. Contacting a molecular wire to a step: Lander molecules on a
step-free Cu(111) surface (column A) and contacted to a (100) step. Molecular
wire parallel (column B) and orthogonal (column C) to the step. Row 1: Sphere
models of molecular structures. Row 2: Calculated STM images corresponding
to the sphere models above. Row 3: STM images at 8 K. Row 4: STM measure-
ments showing LDOS oscillations. In (C2) and (C3), an additional bump
appears corresponding to the contact point of the wire to the step. Modifi ca-
tion of the standing wave pattern on the upper terrace is observed only when
the wire is orthogonal to the step (C4). (From Moresco et al., 2003.)
1112 U. Weierstall
In a variable-temperature STM experiment it has been found that the
Lander molecule can act as a template, self-fabricating short metallic nano-
structures at step edges. Lander molecules where adsorbed at room tem-
perature on the Cu(110) surface and their conformation and anchoring at
step edges were studied at 100 K. At room temperature the Cu kink atoms
are highly mobile and the Lander molecule reshapes the fl uctuating Cu
step adatoms into tooth-like nanostructures perpendicular to the step
edges. The dimension and shape of the Lander molecule form a perfect
template for a double row of Cu atoms. Moving the molecule away form
the step edge by lateral manipulation at low temperature revealed the
underlying restructuring of the step edges (Figure 17–35). Upon adsorp-
tion of the Lander molecules at 150 K, no restructuring of the Cu step edges
was observed, since the mobility of the Cu kink atoms at this temperature
is not high enough for the molecular template to be effective.
At low temperatures, the short Cu nanowire acts as a sliding “rail”
for the Lander molecule. By moving the molecule to the end of such a
nanostructure, a model geometry can be obtained where one end of
the central molecular wire is electronically connected to the metallic
wire. A detailed study of the lateral manipulation of the Lander mole-
cule along such an atomic wire has been performed with an LT-STM
at 8 K (Grill et al., 2004). Lateral displacements of the molecule have
been separated into monoatomic steps and it has been shown that
single molecular legs can be rotated reversibly while keeping the
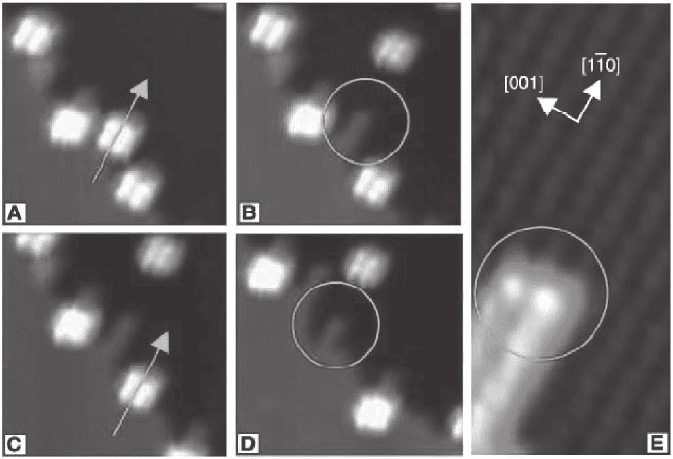
Figure 17–35. Self-assembly of nanowires at step edges initiated by Lander molecule adsorption. (A–
D) Low-temperature STM images of a manipulation sequence of the Lander molecules from a step
edge on Cu(110) The arrows show which molecule is being pushed aside; the circles mark the tooth-
like structures that are visible on the step where the molecule was docked. (E) Zoom-in STM image
showing the characteristic two-row width of the tooth-like structure after removal of a single Lander
molecule. (From Rosei et al., 2002.)
Chapter 17 Low-Temperature Scanning Tunneling Microscopy 1113
central wire fi xed. Comparison with theory confi rmed that the central
wire is contacted to the metallic Cu atomic wire.
In a recent LT-STM study of the Lander molecule, it has been shown
that despite the fact that the legs elevate the molecular wire away from
the surface, there is still an electronic interaction between the central
wire and the surface states of the substrate (Gross et al., 2004). This
was shown by comparing the standing wave patterns of surface-state
electrons scattered off the molecule with calculated patterns taking
into account scattering from different areas of the molecule.
Chemical bond formation was studied with an LT-STM (Lee and Ho,
1999). Individual iron atoms were evaporated and coadsorbed with CO
molecules on an Ag(110) surface at 13 K. A CO molecule was trans-
ferred from the surface to the STM tip and bonded with an Fe atom on
the surface to form Fe(CO). A second CO molecule could then be added
to form Fe(Co)
2
. This is shown in Figure 17–36. The adsorption sites of
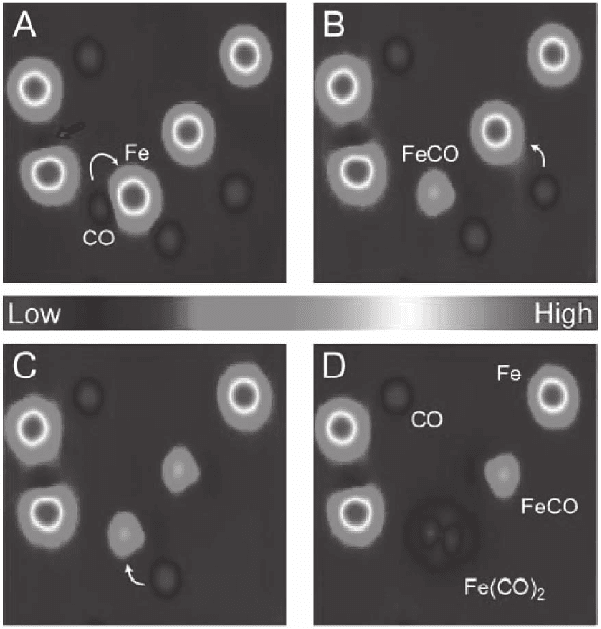
Figure 17–36. Bond formation induced with STM tip. A sequence of STM
constant-current images at 13 K showing the formation of Fe–CO bonds by
vertical manipulation. Fe atoms are imaged as protrusions and CO molecules
as depressions. The white arrows indicate the pair of adsorbates involved in
each bond formation step. In (B) and (C) a CO molecule has been picked up
and bonded to an Fe atom to form Fe(CO). In (D) a second CO molecule has
been bonded to Fe(CO) to form Fe(CO)
2
. (From Lee and Ho, 1999.) (See color
plate.)
1114 U. Weierstall
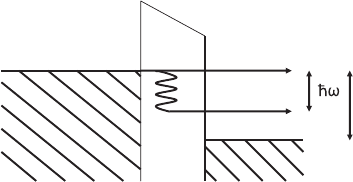
Fermi level
eU
Metal A Metal B
Figure 17–37. Elastic and inelastic tunneling channels. Tunneling electrons
can excite a molecular vibration of energy
-
hω only if eU >
-
hω. For smaller
energies, there is no fi nal state into which the electron can tunnel. Therefore
the inelastic current has a threshold at
-
hω/e. The increase in conductance at
the threshold is typically 1–10% in an STM experiment.
the reactants could be determined by resolving the underlying Ag
lattice with a CO molecule attached to the tip, which leads to increased
resolution in the constant-current image. This increase in spatial reso-
lution can be attributed to the more localized wavefunction of the
molecule-terminated tip. Analysis with inelastic tunneling spectros-
copy provided spectroscopic support for the identifi cation of the created
single molecule products with Fe(CO) and Fe(CO)
2
.
Assembly of an artifi cial nanostructure composed of a copper(II)
phthalocyanine (CuPc) molecule bonded to two gold atomic chains on
NiAl(110) has been realized with an LT-STM (Nazin et al., 2003b). The
electronic structure of this model metal–molecule–metal junction was
studied by spatially resolved STS and systematically tuned by varying
the number of gold atoms in the chains. Splitting and shifting of
molecular orbital energies and modifi cation of the local electronic
structure of the electrodes were observed. These effects determine the
alignment of the molecular orbital energies with respect to the Fermi
energy of the metal and affect the conductivity of the junction.
3.3 Local Inelastic Electron Tunneling Spectroscopy
Besides the dominant elastic electron tunneling process, for which the
electron energy is equal in the initial and fi nal state, inelastic tunneling
can occur if the tunneling electrons couple to some modes ω in the
tunneling junction. Figure 17–37 shows an energy diagram for T = 0,
illustrating elastic and inelastic tunneling processes. In the case of
inelastic tunneling the electron loses energy
-
hω to a mode in the tun-
neling barrier. According to the Pauli exclusion principle, tunneling is
possible only if the fi nal state after the inelastic tunneling event is ini-
tially unoccupied. The bias voltage dependence of the tunneling current
with inelastic tunneling is shown schematically in Figure 17–38. The
elastic tunneling current increases linearly, proportional to V. As long
as the bias voltage is smaller than the lowest energy mode that can be
excited in the gap, inelastic tunneling processes cannot occur. At the
threshold bias V =
-
hω/e, the inelastic channel opens up, and the number
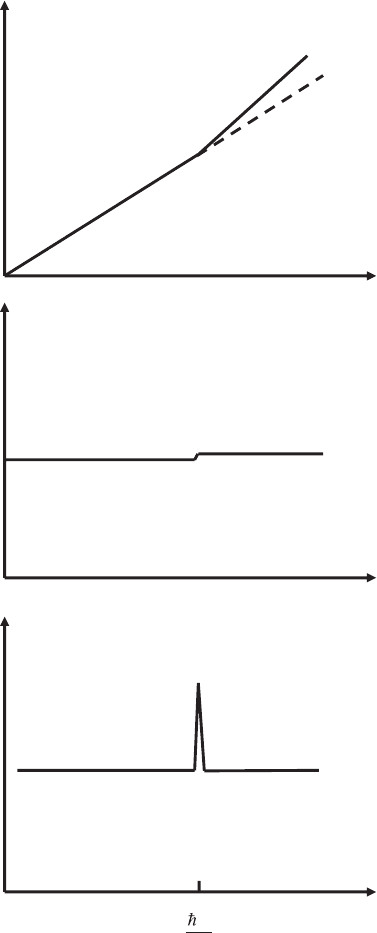
Chapter 17 Low-Temperature Scanning Tunneling Microscopy 1115
of electrons using this channel increases linearly with V. Therefore the
total current has a kink at the threshold bias voltage. In the differential
conductance curve dI/dV, the kink becomes a step and the second
derivative d
2
I/dV
2
exhibits a peak at the threshold. If several modes ω
i
can be excited in the tunneling process, each mode leads to a peak in
the differential conductance at the corresponding voltage V
i
=
-
hω
i
/e.
Inelastic electron tunneling spectroscopy (IETS) can therefore be
regarded as a special form of electron energy loss spectroscopy.
e
ω
I
total
elastic
V
dI/dV
V
d
2
I/dV
2
V
Figure 17–38. Sche-
matic current versus
voltage curves with
elastic and inelastic
tunneling. A kink is
observed when the
inelastic tunneling
channel opens up. The
kink becomes a step in
the fi rst derivative and
a peak in the second
derivative.
1116 U. Weierstall
IETS has been shown to be a powerful technique for measuring the
vibrational spectra of molecules that have been intentionally incorpo-
rated into a metal–oxide–metal tunneling junction (Jaklevic and Lambe,
1966). Vibrational spectroscopy can be performed with a variety of
other techniques including electron energy loss spectroscopy, infrared
absorption spectroscopy, Raman spectroscopy, inelastic neutron scat-
tering, and helium atom scattering. All of these techniques have in
common with IETS that they rely on macroscopic numbers of mole-
cules to achieve detectable signal levels. The signal is therefore an
average over molecules whose local environment can vary. The major
drawback of traditional IETS with planar metal–oxide–metal junctions
is that the molecules are buried within the junction, which is diffi cult
to characterize microscopically.
Replacing the oxide layer by vacuum and the top planar electrode
by a sharp STM metal tip has made it possible to extend IETS to single
adsorbed molecules. One great advantage of performing vibrational
spectroscopy with the STM is that the high spatial resolution of STM
images permits changes in molecular spectra to be correlated with
variations in the local environment on an atomic scale. STM-IETS was
proposed as early as 1985 (Binnig et al., 1985b). Since the changes in
tunneling conductance resulting from opening of additional inelastic
tunneling channels are typically 0.1–1% for planar junctions and 1–10%
for STM junctions, the relative stability of the tunneling current has to
be better than 1% to obtain reasonable IET spectra with the STM. The
physics of tunneling then dictates a tunneling gap stability of better
than ∼0.005 Å
´
over the time it takes to complete one scan of the spec-
trum (Lauhon and Ho, 2001). Because the vibrational features are very
sharp, liquid helium temperatures are required to avoid thermal broad-
ening of the Fermi levels. Hansma (1982) estimated an effective resolu-
tion of 5.4k
B
T (∼140 mV at room temperature) for inelastic tunneling,
while vibrational features are typically only a few millivolts wide. For
those reasons, vibrational spectroscopy with the STM has proved dif-
fi cult. First experiments probing a cluster of sorbic acid molecules
adsorbed on graphite at 4 K reported large jumps in the fi rst derivative
spectrum instead of the expected second derivative spectrum (Smith
et al., 1987). The peaks where attributed to characteristic vibrations of
molecules. However, due to molecular diffusion events during the
measurements, the spectra were not very reproducible and the energies
of the peaks were different form those measured in bulk tunnel junc-
tions. Reproducible single-molecule vibrational spectroscopy has been
achieved only recently with an LT-STM (Stipe et al., 1998). In these
landmark experiments, a Cu(100) surface was dosed with acetylene
(C
2
H
2
) and deuterated acetylene (C
2
D
2
). Vibrational spectra where
aquired at 8 K above single molecules with the use of a tracking scheme
to position the tip at the center of the molecule, with lateral and vertical
resolution of better than 0.1 and 0.01 Å
´
, respectively. Contributions
from the electronic spectrum of the tip and the substrate could be
minimized by subtracting spectra taken over a clean area of the surface
from the molecular spectra. The I–V curves from a single molecule and
the clean surface (Figure 17–39A) show the expected linear dependence
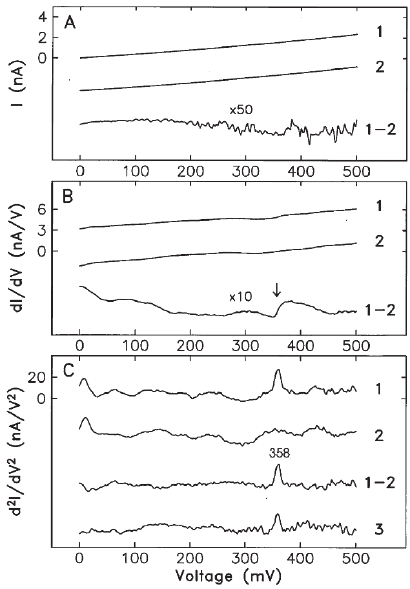
Chapter 17 Low-Temperature Scanning Tunneling Microscopy 1117
for metallic junctions. The differential conductance dI/dV shows an
increase of 4.2% at 358 mV, resulting from the excitation of the C—H
stretch mode (Figure 17–39B). The second derivative d
2
I/dV
2
reveals a
distinct peak at 358 mV (Figure 17–39C, compare with the idealized
view of Figure 17–38). An isotopic shift to 266 mV was observed for
deuterated acetylene and the C—D stretch mode. These values are in
close agreement with those obtained by EELS for the same molecules
on Cu(100). The ability to spectroscopically identify molecules with the
STM makes it possible to implement chemical-sensitive STM imaging.
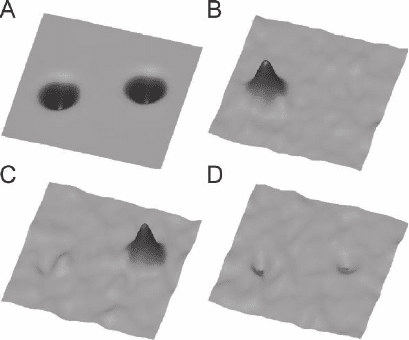
This has been demonstrated by recording a d
2
I/dV
2
map above both
acetylene isotopes. When the dc voltage was fi xed at 358 mV, only one
of the two molecules (C
2
H
2
) was imaged, whereas at 266 mV, the other
molecule (C
2
D
2
) was imaged (Figure 17–40). Thus, individual adsorbed
molecules can be identifi ed by their vibrational spectra and inelastic
images. In contrast, identifi cation and characterization of adatoms and
Figure 17–39. Molecular vibrational spectra observed with the STM at 8 K. (A)
I–V curves recorded with the STM tip directly over the center of an acetylene
molecule (1) and over the bar Cu(100) surface (2). (8) dI/dV on the molecule
(1) and on the substrate (2). (C) d
2
I/dV
2
on the molecule (1) and on the substrate
(2). A peak at 358 mV is visible in the difference spectrum. (3) An average over
279 scans of 2 min each (10 h total data acquisition time directly above the
molecule) with a different tip. The conductance change due to inelastic tun-
neling was 3–4% with different tips. (From Stipe et al., 1998.)
1118 U. Weierstall
molecules by electronic spectroscopy with the STM are problematic
because the electronic energy levels are broadened and shifted upon
adsorption and the adsorbed molecule’s spectrum becomes convolved
with the STM tip’s electronic spectrum (Crommie et al., 1993c).
STM-IETS has been used to determine the orientation of individual
C
2
HD molecules (deuterated acetylene) adsorbed on the Cu(100) surface
at 8 K (Stipe et al., 1999a). By setting the bias voltage to the vibrational
energy of the C—D stretch mode in C
2
HD, and simultaneous recording
the constant-current image and the vibrational image (d
2
I/dV
2
map),
the spatial distribution of the C—D stretch signal within the molecule
could be determined. Since the inelastic image has its maximum near
the midpoint of the C—D bond, it locates the position of the bond in
this case.
The previous two examples show the ability of the LT-STM to resolve
internal vibrations of molecules adsorbed on surfaces. The internal
modes can be used in surface chemical analysis for the identifi cation
of adsorbed species. External vibrations, i.e., vibrations of adsorbed
molecules with respect to the substrate, are more sensitive to the inter-
action of the adsorbed molecule with the substrate. These external
modes generally have lower energy than the internal ones, and are not
easily accessed by some of the averaging vibrational spectroscopy
methods mentioned above. External vibrational modes of benzene
molecules on an Ag(110) surface have been detected with an LT-STM
at 4 K (Pascual et al., 2001a). These measurements confi rmed that the
Figure 17–40. Chemical sensitive imaging. Spectroscopic spatial images of the
inelastic channels for C
2
H
2
and C
2
D
2
. (A) Constant-current image of a C
2
H
2
(left) and a C
2
D
2
(right) molecule. This image is an average of STM images
recorded simultaneously with the vibrational images. The molecules appear
identical in this normal imaging mode. d
2
I/dV
2
maps (vibrational images) of
the same area were recorded at (B) 358 mV, (C) 266 mV, and (D) 311 mV. In (B)
only C
2
H
2
is visible, whereas in (C) C
2
D
2
is visible. The symmetric, round
appearance of the molecules is attributed to the rotation of the molecule
between two equivalent orientations during the experiment. (From Stipe et al.,
1998.)
Chapter 17 Low-Temperature Scanning Tunneling Microscopy 1119
external vibrations are strongly sensitive to the nature of the molecule–
substrate bond. For internal vibration modes, the inelastic signal has
been shown to be very localized at the position of the particular bond
excited (Stipe et al., 1999a). In contrast the spatial distribution of the
inelastic tunneling, signal for external modes extends over the whole
area of the molecule (Pascual et al., 2001a).
In a detailed study of temperature effects, electronic structure
contributions, and tip effects on STM-IETS, Lauhon and Ho (2001)
suggested that functionalization of the tip by transfer of a known mol-
ecule to the tip offers a means of accessing different vibrational modes.
This has been confi rmed in later experiments (Moresco et al., 1999;
Hahn and Ho, 2001) where CO and C
2
H
4
molecules have been trans-
ferred to the tip and single CO and O
2
molecules were probed with
this tip.
There are a few caveats regarding the application and interpretation
of STM-IETS spectra. In STM-IETS as in traditional IETS, there are no
strict selection rules. Modes involving motion parallel and perpendicu-
lar to the surface can be excited. Modes are not observed for all mole-
cules and not all modes are necessarily observed for any particular
molecule. The symmetry of vibrational modes and electronic reso-
nances of an adsorbate seem to give rise to selection rules for vibra-
tional mode detection in STM-IETS (Lorente et al., 2001). A dependence
of the STM-IETS signal on the molecular orientation on the surface has
been shown for C
60
on Ag(110) (Pascual et al., 2002). The spectroscopic
maps showed a correlation between the enhanced vibrational signal
and orientational symmetry of the adsorbed molecule observed in
constant-current mode.
To complicate matters more, it has been shown that vibrational exci-
tation can lead to suppression of elastic tunneling and produce dips
instead of peaks in the differential conductance spectrum (Hahn et al.,
2000). This has been recently explained theoretically by Lorente (2004).
Despite this complexity, there are many advantages of STM-IETS, i.e.,
that the adsorbate geometry is well defi ned and the effect of adsorbate
orientation can be studied systematically. Recent progress in the theo-
retical analysis of STM-IETS may greatly enhance its ability to probe
chemistry at the spatial limit (Mingo and Makoshi, 2000; Makoshi and
Mingo, 2002; Lorente and Persson, 2000; Lorente, 2004).
3.4 STM-Induced Photon Emission
Injection of electrons or holes form the tip of an STM into the surface
leads to the emission of light for many materials. The fi rst observation
of light emitted from the tunneling junction of an STM in the low-bias
tunneling regime (eV < Φ where Φ is the work function) goes back to
Coombs et al. (1988). The highly localized tunneling current allows
high spatial resolution that enables experiments with single nanostruc-
tures and molecules. Photon emission from the tunneling junction of
an STM can be used to measure the optical properties of the sample
surface in the nanometer regime. Photon emission from metals involves
surface plasmons, which are inelastically excited by the tunneling elec-
