Hawkes P.W., Spence J.C.H. (Eds.) Science of Microscopy. V.1 and 2
Подождите немного. Документ загружается.

1000 P. Sutter
are immobile on the adsorbate-free surface. Vacancy diffusion is greatly
enhanced by adsorbed O
2
. At temperatures suffi ciently low that the
diffusion of adsorbed O
2
molecules can be captured by STM, the sub-
traction of consecutive frames in time-lapse STM shows that single
oxygen vacancies diffuse along [11
¯
0] from one bridging O row to the
next, always in the presence of neighboring O
2
molecules.
The role of O
2
molecules in the vacancy diffusion process is estab-
lished from detailed investigation of single vacancy hops, again based
on time-lapse STM movies at low temperature. As an O
2
molecule dif-
fusing along a Ti row approaches an oxygen vacancy, it dissociates and
contributes one oxygen atom toward healing the vacancy, thus creating
a metastable intermediate consisting of a single O atom. The O adatom
is highly reactive, as corroborated in separate experiments involving
dosing of atomic oxygen. It rapidly recombines with a bridging O atom
and emerges as an O
2
molecule. If in this process the bridging O atom
is removed from one of the adjacent rows, the net result is a diffusion
jump of an oxygen vacancy by one bridging oxygen row. Given this
O
2
-mediated mechanism of oxygen vacancy diffusion, the rate of dif-
fusion events is expected to scale linearly with O
2
coverage. STM
movies obtained at different O
2
exposure show that this is indeed the
case.
While early imaging of dynamic surface processes was performed
almost invariably in UHV, several applications require STM imaging
in what is seen as more “realistic” environments for those applications.
A prominent example is heterogeneous catalysis. It has been recog-
nized that actual reactions under technologically relevant conditions,
often involving elevated temperatures and pressures at or above atmo-
spheric pressure, can involve surface structures and compositions, and
entire reaction mechanisms that differ substantially, even qualitatively,
from those of “simulated” reactions running in UHV, a situation com-
monly termed the “pressure gap” problem of heterogeneous catalysis.
To address the need for imaging with high spatial and temporal resolu-
tion at elevated pressure, a family of dedicated STM instruments was
developed (Rasmussen et al., 1998; Jensen et al., 1999; Lægsgaard et al.,
2001; Rößler et al., 2005). These instruments allow sample preparation
and surface analysis in UHV, followed by exposure to reactants at high
pressure and simultaneous STM imaging. A particularly elegant imple-
mentation of this concept is the “reactor STM,” allowing dynamic STM
imaging of surfaces exposed to reactants in a compact catalytic fl ow
reactor in combination with the simultaneous analysis of the reaction
products by mass spectrometry.
Figure 15–23 shows an example of a complex dataset obtained during
high-pressure CO oxidation on Pt(110) (Hendriksen and Frenken,
2002). The upper panel traces mass spectrometer signals for O
2
, CO,
and CO
2
, showing initial exposure to CO, followed by the introduction
of molecular oxygen into the reactor. The panel below shows represen-
tative STM images obtained at specifi c stages of the reaction, during
which the sample is kept at a constant temperature of 425 K. Images A,
B, E, F, and H show fl at terraces separated by steps, representing the
metallic, CO-covered Pt(110) surface. Image C shows the change in
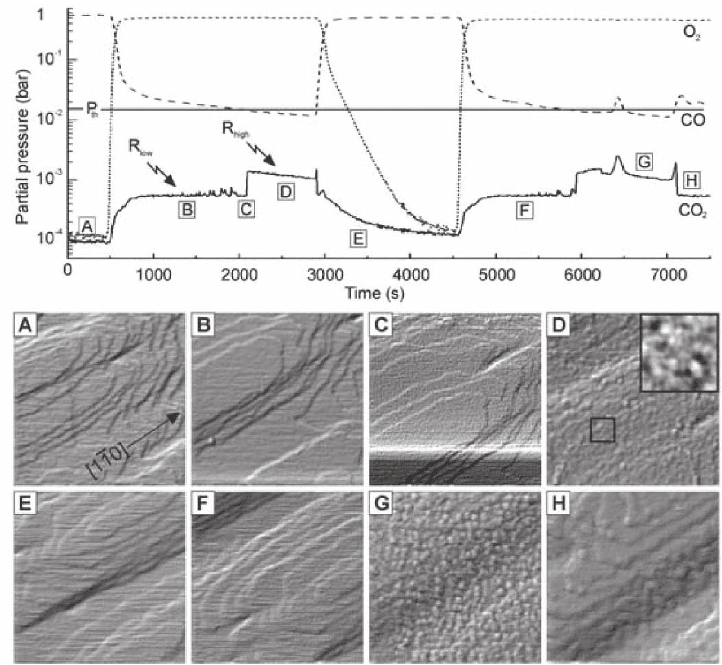
Chapter 15 Scanning Tunneling Microscopy in Surface Science 1001
surface morphology, a pronounced surface roughening, during a step
in activity giving rise to a sudden increase in CO
2
evolution, demon-
strating a direct link between surface roughness and activity for this
surface involving a mechanism that is not observed at low pressure.
Although capable of mapping dynamic surface phenomena, STM
movies have obvious limitations in imaging fast dynamic processes,
such as surface diffusion at room temperature or above. A possible
solution is the development of novel approaches and instruments for
very high-speed STM imaging. As an alternative, frame-by-frame
imaging can be abandoned altogether if only a map of the trajectory
of the diffusing species is desired. The recognition of this fact led to
the development of atom tracking STM (Swartzentruber, 1996). In atom
tracking, the STM tip is locked onto a diffusing surface species using
a 2D lateral feedback mechanism. Once locked, the feedback maintains
Figure 15–23. Simultaneous mass spectrometry and STM imaging in a catalytic fl ow reactor: CO
oxidation on Pt(110). (Top) Mass spectrometer signals of O
2
, CO, and CO
2
measured at the output of
the fl ow reactor cell. (Bottom) STM images on the Pt(110) catalyst surface acquired at selected stages
of the process: CO adsorption (A), O
2
fl ow (B–D), and repetition of the sequence (E–H). Note the strong
surface roughening in (D), associated with increased CO
2
evolution. (Reprinted with permission from
Hendriksen and Frenken, © 2002 by the American Physical Society.)

1002 P. Sutter
the tip over that species and tracks its coordinates as it diffuses over
the substrate. In the atom-tracking mode, the STM spends all of its time
measuring the diffusion trajectory, which results in substantially
improved time-resolution compared to time-lapse STM movies.
Atom tracking STM has been used to measure the diffusion kinetics
of Si (Swartzentruber, 1996) and SiGe (Qin et al., 2000) dimers on
Si(001) above room temperatures, of water molecules on Pd(111) (Mitsui
et al., 2002), and of Pd atoms in a Pd/Cu(001) surface alloy (Grant
et al., 2001). The latter example is illustrated in Figure 15–24. Pd atoms
in the surface alloy are imaged as protrusions in STM. Locking the
STM tip onto individual Pd atoms, their diffusion pathway can be
tracked. Analyzing the residence time (the time between hops) and
jump length leads to the conclusion that there is no time correlation
between individual Pd diffusion events, and that the diffusion is medi-
ated by surface vacancies rather than Cu adatoms, i.e., involves rapidly
diffusing vacancies visiting Pd atoms in the surface layer. From
the temperature dependence of the Pd hop rate—determined from
temperature-dependent tracking experiments—the activation energy
of the overall process, in this case equal to the sum of the vacancy for-
mation energy and the energy barrier for lateral Pd-vacancy exchange,
can be measured.
In contrast to time-lapse STM, in which the tip is scanned rapidly
across a larger fi eld of view, the tip maintains close contact with the
diffusing entity in atom tracking. Hence, the observed diffusion process
could be affected by tip–sample interactions. While this question has
to be studied on a case-by-case basis, at least one system [SiGe dimer
Figure 15–24. Diffusion kinetics of Pd atoms in the Pd/Cu(001) surface alloy.
(a) Site visitation map of an individual Pd atom, obtained by atom-tracking
STM at a temperature of 62°C. The square array marks the position of the
Cu(001) unit mesh. In this dataset the atom hopped 853 times in a time interval
of 5557 s. (b) Temperature dependence between 31 and 69°C of the average hop
rate of an incorporated Pd atom. The average residence time of Pd atoms
decreases from 145.3 to 5.0 s in this temperature range. The data follow an
Arrhenius form with an activation energy of 0.88 eV and measured prefactor
of 10
12.4±0.4
Hz. (Reprinted with Permission from Grant et al., © 2001 by the
American Physical Society.)
Chapter 15 Scanning Tunneling Microscopy in Surface Science 1003
diffusion on Si(001)] has been identifi ed in which the diffusion mecha-
nism depends on the sign of the electric fi eld between tip and sample
(Sanders et al., 2003), indicating that the presence of the probe tip can
indeed affect the measurement.
4.3 Atom and Molecule Manipulation
Atom and molecule manipulation (Hla and Rieder, 2003) experiments
utilize the sharp STM probe tip and the ability to control it laterally
and vertically with picometer resolution to build and modify
nanometer-scale structures, typically in combination with their analy-
sis by STM imaging and tunneling spectroscopy. While conceptually
related to atom tracking, manipulation experiments are performed in
a different regime: the sample is cooled to temperatures low enough
that all thermal diffusion is frozen out, and the tip is typically brought
into close contact to induce strong tip–adatom/molecule interactions.
Since the fi rst demonstration of atomic manipulation of Xe/Ni(110)
by Eigler and Schweizer (1990), manipulation experiments have
branched out considerably, and now encompass scenarios as diverse as
controlled chemical reactions of individual molecules (Lee and Ho,
1999), contacting single molecules with atomic metal wires (Nazin et
al., 2003), construction of mechanical logic gates from adsorbed mole-
cules (Heinrich et al., 2002), and control of excess charge on individual
atoms at insulator surfaces (Repp et al., 2004). While generally based
on some form of tip–sample interaction, atom or molecule manipula-
tion can involve a number of different physical mechanisms: close
proximity and short-range chemical interaction between a tip atom and
adatom to affect the potential landscape seen by the adatom on the
surface, vibrational excitation within adsorbed molecules or between
substrate and adatom, electric fi eld, or direct charging by tunneling
electrons. Different patterns of adsorbate motion can be induced,
including lateral hopping between adsorption sites on the substrate,
vertical transfer between sample and tip, rotation, as well as the con-
trolled making and breaking of individual bonds. Substrate surfaces
with relatively low symmetry, e.g., (110) surface orientations or stepped
surfaces, are often used to establish one-dimensional diffusion path-
ways between substrate adsorption sites that help guide the manipula-
tion process.
Lateral manipulation is based on establishing close proximity
between an adatom and the STM tip to increase the interaction between
them. The approach process is controlled via the tunneling resistance,
which is lowered from typically several hundred MΩ during imaging
to values of the order of 100 kΩ for manipulation. Depending on the
particular system, the lateral force needed to move an adsorbate
between adjacent adsorption sites can be either repulsive or attractive,
giving rise to manipulation by pushing and pulling, respectively. The
manipulation process itself is monitored by measuring the tip height
(or z-piezo voltage) during the lateral motion of the tip, as illustrated
in Figure 15–25 for Pb/Cu(211) (pulling) and CO/Cu(211) (pushing)
(Meyer and Rieder, 1998).
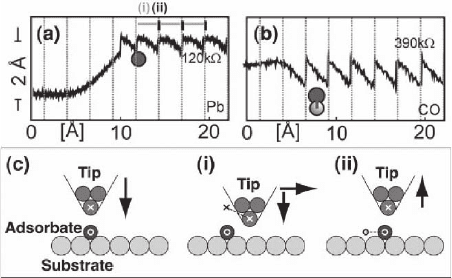
1004 P. Sutter
The adsorbate motion in both pushing and pulling modes is strongly
infl uenced by the preferred adsorption sites defi ned by the substrate.
A sawtooth-like vertical motion of the tip accompanies each jump of
the adsorbate between neighboring adsorption sites (Figure 15–25a and
b). The origin of this tip motion is illustrated in Figure 15–25c for
the example of an attractive tip–adsorbate interaction. Following the
approach to the adsorbate, the tip is moved laterally. Initially, the adsor-
bate, held in the potential well of a substrate adsorption site, does not
follow. The tunneling gap thus increases, and the tip moves forward
to maintain a constant tunneling current (i). Ultimately, the interaction
with the tip induces a lateral jump of the adsorbate into a neighboring
potential minimum. The tunneling gap closes abruptly and the feed-
back loop causes the tip to retract.
The precision and complexity of structures achievable by lateral
manipulation is illustrated in Figure 15–26 (Heinrich et al., 2002). CO
molecules adsorbed in monomer, dimer, or trimer confi gurations on
Cu(111) give rise to distinct contrast in constant current STM. While
monomers and dimers are stable at low temperature (5 K in this
example), there are three distinct confi gurations for trimers: a stable
three-fold symmetric “close-packed” arrangement, and metastable
“straight-line” and “bent-line” (“chevron”) confi gurations, which relax
with time constants of the order of seconds into the stable state. Complex
structures consisting of up to 545 CO molecules were built with atomic
precision such that any neighboring molecules were initially in stable
dimer confi gurations, but could be changed to an unstable “chevron”
confi guration with a place exchange of a single molecule in the vicinity.
In this way, molecular cascades, similar to toppling rows of standing
Figure 15–25. Mechanisms of lateral atom manipulation. (a) “Pulling” manip-
ulation via attractive interaction between a Pb atom on Cu(211) and the W tip.
(b) “Pushing” manipulation via repulsive interaction between a CO adsorbate
and the W tip. In both cases a sawtooth-like tip height profi le with the peri-
odicity of the substrate adsorption sites is observed (Reprinted with permis-
sion from Meyer and Rieder, © 1998 The Materials Research Society). (c)
Illustration of the tip and adsorbate motion during manipulation via attractive
interaction. (i) Lateral motion of the tip, accompanied by an approach toward
the substrate. (ii) Hopping of the adsorbate, followed by a sharp retraction of
the tip.
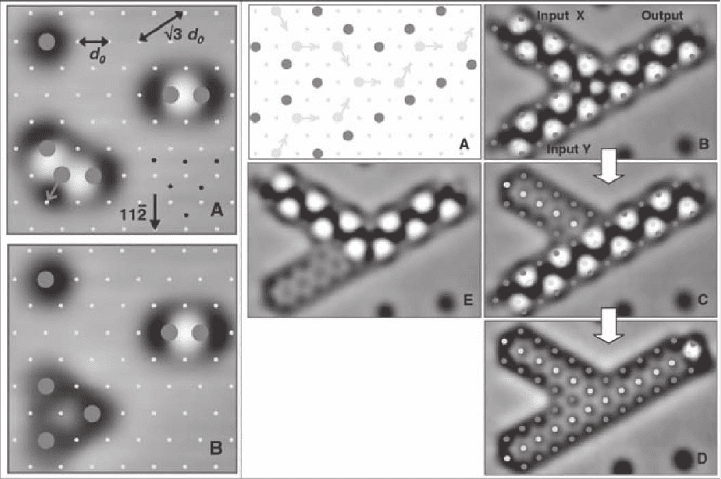
Chapter 15 Scanning Tunneling Microscopy in Surface Science 1005
dominoes, could be constructed. Mechanical computation was demon-
strated by molecular cascades set up as logic AND gates, two-input,
and three-input sorters.
Apart from lateral manipulation, vertical manipulation, i.e., transfer
of adsorbates between sample and tip, is possible. Vertical manipula-
tion of CO molecules, for example, has been used for controlled modi-
fi cation of the STM tip and to induce single molecule reactions (Lee
and Ho, 1999). Vertical transfer is also important for moving adatoms
or molecules across obstactles such as step edges, if they cannot be
surmounted by controlled lateral hops. Manipulation mechanisms
other than direct chemical interaction between the STM tip and adsor-
bates have received increased interest recently. Notably the different
roles of vibrational excitations are actively investigated (Komeda et al.,
2002; Stroscio and Celotta, 2004). Inelastic tunneling can, for instance,
drive controlled rotations of adsorbed molecules (Stipe et al., 1998b).
An intriguing example of atomic manipulation based on inelastic tun-
neling, the controlled manipulation of excess charge on single Au
atoms, is illustrated in Figure 15–27 (Repp et al., 2004). Individual Au
Figure 15–26. Molecule cascades. (Left) (A) Confi gurations of neighboring CO molecules on Cu(111):
isolated CO molecule, dimer, and trimer in a “chevron” confi guration. Large circles mark the positions
of the molecules and small dots indicate the surface layer Cu atoms (1.9 nm scans; T = 5 K). (B) The
same area after one CO molecule in the trimer has hopped to generate a stable close-packed trimer.
(Right) Demonstration of a molecular mechanical AND gate, implemented via cascades of “chevron”-
type CO trimers. (A) Model of the AND gate. (B–D) Sequence of STM images (5.1 nm by 3.4 nm)
showing the operation of the AND gate: (B) Initialization. (C) Result after input X was triggered with
the STM tip. (D) When input Y was triggered, the cascade propagated all the way to the output. (E)
Result of triggering only input Y. (Reprinted with permission from Heinrich et al., © 2002 AAAS.)
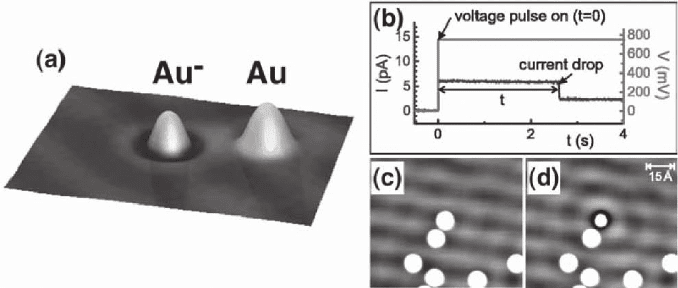
1006 P. Sutter
atoms are deposited on ultrathin, insulating NaCl supported by Cu(111).
Electrons can tunnel between the metal substrate and the STM tip
through the ultrathin insulator, i.e., STM imaging, spectroscopy, and
manipulation are possible for this system. However, the coupling of
the Au adsorbate to the substrate is affected profoundly by the insulat-
ing support. Voltage pulses can be used as a means to reversibly deposit
an excess electron on individual Au atoms. The charging occurs via an
inelastic electron tunneling mechanism enabled by a weak coupling of
the adatom and Cu substrate electronic states. The data suggest that
the coupling is so weak that the lifetime of a negative ion resonance
state of the adatom is in the range of ionic vibrational periods for the
NaCl interlayer, allowing the relaxation of the NaCl lattice, shift of the
negative ion resonance state below the Fermi energy, and capture of
the electron. The excess charge is maintained due to the substantial
relaxation of the underlying NaCl lattice. This stabilization prevents
discharging by tunneling into the metal, and the electron resides on
the Au atoms until removed by a voltage pulse of opposite sign. The
charged Au atom has a distinct signature in constant-current STM
imaging, and the long-range electric fi eld due to the charged Au atom
strongly scatters electrons in interface states at the Cu/NaCl interface.
Charging individual adsorbed atoms is a potentially powerful approach
to tuning their physical properties. For Au/NaCl, signifi cant differ-
ences in surface diffusivity were identifi ed, with the onset temperature
of signifi cant surface diffusion lowered from 60 K (Au) to 40 K (Au
−
).
Other possible modifi cations due to controlled charging at the atomic
level include changes in catalytic activity and the controlled manipula-
tion of the net spin magnetic moment of individual atoms.
Figure 15–27. Charging of individual Au atoms on ultrathin NaCl/Cu(111). (a) Constant-current STM
on a pair of Au atoms, one of which was charged negatively with the other remaining neutral. (b)
Signature of the charging event. Successful charging, achieved by a voltage pulse while maintaining
the tip above a single Au atom, is indicated by a sudden drop in tunneling current. (c and d) Quasi-
particle scattering: neutral Au adatoms do not scatter NaCl/Cu(111) interface-state electrons (c),
whereas the negatively charged adatom acts as a scatterer (d). (Reprinted with permission from Repp
et al., © 2004 AAAS.)
Chapter 15 Scanning Tunneling Microscopy in Surface Science 1007
5 Heterostructures and Buried Interfaces: BEEM,
Quantum Size Effects, and Cross-Sectional STM
The exponential dependence of the tunneling current on the tip–sample
separation makes STM a versatile tool for atomic-resolution micros-
copy and spectroscopy at surfaces. As a result of its unique resolution
and surface sensitivity STM has brought important advances in fi elds
such as catalyis or epitaxial growth, in which surface processes play a
key role. For many technological applications of semiconductor materi-
als, e.g., in device structures such as transistors, detectors, or lasers, the
active regions encompass complex heterostructures, and device per-
formance is affected much less by the free surface than by buried
interfaces. Therefore, a need arises for a technique capable of probing
subsurface structures, electronic properties, and carrier transport. The
mapping of interfacial and transport properties should occur with
nanometer spatial resolution to provide data that are relevant for semi-
conductor devices with progressively reduced dimensions. Since the
early 1990s, several STM-based techniques have been developed to
provide high-resolution imaging and spectroscopy of buried interfaces
and heterostructures. Ballistic electron emission microscopy (BEEM)
operates in a three-terminal confi guration, in which the STM tip, whose
height is controlled by a constant-current feedback loop, injects hot
carriers into a thin fi lm or heterostructure while an additional collector
contact measures the current of carriers that are transmitted through
buried interfaces. Electron interference in a thin metal fi lm, again
with carriers injected from an STM tip and traveling ballistically in
the metal, provides detailed thickness maps of metallic overlayers,
and can—under favorable circumstances—even map the atomic struc-
ture of a buried metal–semiconductor interface. Cross-sectional STM,
fi nally, is used to image complex heterostructures such as superlat-
tices, embedded self-assembled quantum dots, or substitutional mag-
netic impurities, on nonpolar (110) cleavage planes of III–V compound
semiconductors.
5.1 Probing Buried Interfaces (I)—BEEM
BEEM was invented by Kaiser and Bell (1988) to probe Schottky con-
tacts, i.e., metal–semiconductor interfaces with high spatial resolution
(for a recent review, see Narayanamurti and Kozhevnikov, 2001).
Beyond Schottky barriers, the technique can determine the height of
other subsurface potential barriers and it has also been applied to
measure band offsets between different semiconductors, the energies
of transmission resonances in semiconductor quantum wells and
superlattices, and bound states in buried quantum wires and dots. In
addition, electron scattering at subsurface linear and point defects has
been characterized.
We illustrate the operating principle of BEEM using the example of
a metal–semiconductor junction. The technique operates in a three-
terminal confi guration, i.e., adds an additional collector electrode to the
STM tip and sample contact, as shown schematically in Figure 15–28.

1008 P. Sutter
The STM tip is scanned at constant current I
t
, measured between the
tip and base electrode, over the surface of the base layer. The tip serves
as a point source of hot carriers, electrons or holes, injected into the
metal base. If the metal thickness is small compared to the mean free
path for electron–phonon, electron–electron, or impurity scattering,
most carriers reach the metal–semiconductor interface ballistically, i.e.,
with their original energy and momentum distribution. In metals,
the mean free path for electron–phonon scattering of electrons with
energies of a few eV is typically of the order of 10 nm. In signifi cantly
thicker fi lms, electron–phonon scattering would broaden the momen-
tum distribution of the injected carriers, i.e., mostly affect the spatial
resolution of BEEM, while having little effect on the energy distribu-
tion. Hence, the injected ballistic carriers generally have a well-defi ned
energy distribution and can be used to perform spectroscopic mea-
surements on buried potential barriers.
At the metal–semiconductor interface, ballistic carriers with energies
below a threshold equal to the Schottky barrier height eφ
B
are refl ected
back into the metal base, while carriers with energy above the thresh-
old are transmitted into the semiconductor collector (Figure 15–29). If
Figure 15–28. Schematic setup
of a typical BEEM experiment
on a metal (M)–semiconductor
(S) heterostructure.
Figure 15–29. Band diagrams for (a)
ballistic electron and (b) ballistic hole
injection into the metal base on an
n- and p-type semiconductor, respec-
tively. The dark shaded areas indicate
the energy distribution of the injected
carriers, as well as of those transmitted
into the collector. The inset in (a) illus-
trates the onset of the collector current
at a threshold bias corresponding to
the Schottky barrier height, eφ
B
. (After
Bell et al., 1991.)
Chapter 15 Scanning Tunneling Microscopy in Surface Science 1009
other barriers exist in the collector layer, the transport through that
material can involve additional interfacial refl ections. Carriers that are
transmitted through the entire collector layer contribute to the collector
current (or BEEM current), which is measured between base and col-
lector contacts.
The energy of the injected carriers is varied by changing the voltage
applied between the STM tip and metal base. For low bias, none of the
injected carriers is transferred across the metal–semiconductor inter-
face. At voltages above the threshold, some carriers have energy above
the Schottky barrier and can cross the interface, causing an increase in
collector current with increasing tip–sample bias above threshold.
From the onset of the BEEM current, the barrier height eφ
B
can be
determined, e.g., by fi tting the measured I
c
(V) to a theoretical expres-
sion. In a simple one-dimensional theory,
IV RI dEfE fE eV E E eV e
ct F B
(
)
=
(
)
−−
(
)
[]
×−−+
(
)
[]
∫
θφ (6)
where R is a bias independent parameter and f(E) is the Fermi
function.
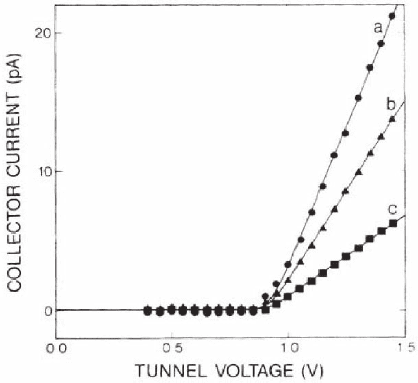
Figure 15–30 shows experimental BEEM spectra on an Au/n-Si(001)
junction, measured at room temperature in N
2
atmosphere, fi tted using
Eq. (6) to determine a Schottky barrier eφ
B
= 0.92 eV. Due to its low
reactivity and oxidation resistance, Au was used in many of the early
BEEM experiments as a nonepitaxial metal base on a variety of other
semiconductors. Schottky barrier heights were determined, for example,
for Au/n-GaAs(001) (eφ
B
= 0.70 eV at 77 K) (Bell et al., 1990) and Au/n-
GaP(110) (eφ
B
= 1.41 eV) (Prietsch and Ludeke, 1991). For Au/n-CdTe(001)
(eφ
B
= 0.69–1.07 eV) (Fowell et al., 1990), strong lateral variations in the
Figure 15–30. BEEM spectra obtained on a polycrystalline Au/n-Si(001) junc-
tion. Spectra a–c (symbols) are measured at different tunneling currents. The
calculated spectra (solid lines) correspond to a common Schottky barrier
height value φ
B
= 0.92 eV and R value of 0.045 eV
−1
. (Reprinted with permission
from Kaiser and Bell, © 1988 by the American Physical Society.)
