Egerton R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope
Подождите немного. Документ загружается.

328 5 TEM Applications of EELS
Brown (1990) recorded energy-loss spectra that revealed the helium K-ionization
edge, after subtraction. Edge quantification using a hydrogenic K-shell cross section
led to an estimate of the He concentration in a 20-nm bubble: 2 × 10
28
atoms/m
3
,
corresponding to a He pressure of 2 kbar (0.2 GPa). More recently, Fréchard et al.
(2009) performed a systematic study of He bubbles in martensitic steel. The Fe
plasmon peak was modeled as a Gaussian and subtracted to yield a Gaussian-like
helium signal (Fig. 5.25), which was quantified using Hartree–Slater cross sections.
For bubble diameters less than 5 nm, the He density matched that of liquid He
and was three times as high for a 2-nm bubble. The helium peak blue-shifted by
about 1 eV with decreasing bubble diameter, supposedly a result of Pauli repulsion
(wavefunction overlap between adjacent atoms).
Using energy-loss spectroscopy with a broad electron beam, Fink (1989) esti-
mated the average pressure in He bubbles formed in Al and Ni by ion implantation.
The excitation threshold was shifted from the free-atom value (21.23 eV) to about
24 eV. Taking this blueshift to be proportional to He density, the He pressure P was
found to be inversely proportional to the bubble radius r (Pr = 90 kbar nm) for
bubbles in aluminum. In the case of implanted Ni, the pressure inside the smallest
bubbles exceeded 250 kbar, corresponding to a density 10 times larger than liquid
He, so the helium was assumed to be in solid form. Confirmation by electron diffrac-
tion was hampered by the small scattering amplitude of He, but electron diffraction
peaks have been recorded from bubbles of other rare gases ion-implanted into Al
and have indicated epitaxy with the surrounding matrix.
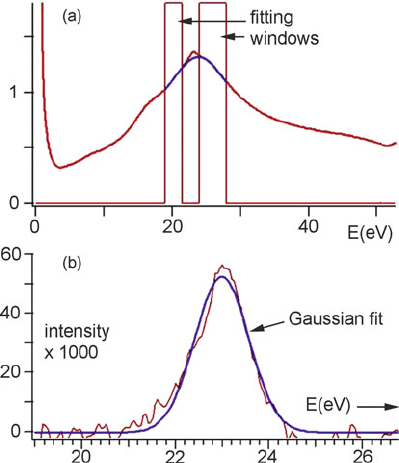
Fig. 5.25 (a) Energy-loss
spectrum recorded from the
center of a He bubble in
EM10 martensitic steel,
showing the plasmon-fitting
windows. (b) Subtracted He
signal and Gaussian fit to the
data. From Fréchard et al.
(2009), copyright Elsevier
5.4 Elemental Analysis from Core-Loss Spectroscopy 329
5.4.2 Measurement of Lithium, Beryllium, and Boron
The elements Li, Be, and B give K-ionization edges in the 30–200 eV region, super-
imposed on a relatively large background. In very thin specimens (t/λ < 0.3), this
background represents the tail of the valence electron plasmon peak; in thicker ones,
plural plasmon s cattering dominates. Hofer and Kothleitner (1993) used Fourier
log deconvolution to improve an AE
−r
fit to the background preceding Li and Be
edges recorded from mineral specimens. In this energy region, a K-edge is likely
to overlap with L-orM-edges of other elements, complicating quantitative analy-
sis. The problem is reduced by using a small energy window (<50 eV), at the
risk of systematic error due to energy-loss near-edge structure (Section 4.5.2). A
more satisfactory solution for Li and Be edges (Hunt and Williams, 1991; Hofer
and Kothleitner, 1993) is to employ MLS fitting to reference edges recorded from
simple compounds that have the same coordination and similar near-edge structure.
Lithium cannot be analyzed with current EDX detectors and is measurable by
WDX spectroscopy only by applying a considerable electron dose, with poten-
tial radiation damage. Chan and Williams (1985) evaluated EELS as a means of
quantitative analysis of Al/Li alloys, used in aerospace applications because of
their high strength/weight ratio. To minimize the Li K-edge background, very thin
(<50 nm) specimens and small collection angles (β < 5 mrad) were necessary.
The pre-edge background could then be successfully modeled by an AE
−r
func-
tion and extrapolated over a 40-eV interval containing both the Li K- and A1 L
23
edges.
The use of lithium as a battery material has led to EELS being used, along with
TEM imaging and diffraction, to characterize the various phases involved (Muto
et al., 2009; Wang et al., 2010b). The main problems for Li quantification are
double-plasmon excitation, giving low edge/background ratio intensity at the Li K-
edge, and the sensitivity of this element to the electron beam. Radiation damage
occurs through radiolysis and also displacement damage, since the incident-beam
threshold energy is normally below 20 keV. Strong electron–hole interaction and rel-
ativistic effects (in an anisotropic matrix, see Appendix A) complicate fine structure
analysis of the edge. An incident energy of 200 keV or more helps by increasing the
inelastic mean free path, equivalent to a thinner specimen. The Li-K near-edge struc-
ture can be an effective tool for differentiating between lithium compounds such as
LiC
6
,Li
2
CO
3
, and Li
2
O and for comparing measured ELNES with calculations
based on an assumed structure; see Fig. 5.26.
Beryllium forms coherent precipitates in copper, giving high strength through
age-hardening. Using EELS, Strutt and Williams (1993) found that the Be/Cu ratio
of γ -phase precipitates increased with decreasing aging temperature, contrary to
the expected phase diagram. The lowest quantified concentration of Be i n the pre-
cipitates was about 10 at.%. In their analysis of BeO-doped SiC, Liu et al. (1991)
avoided conventional background fitting by recording a spectrum from pure SiC.
After scaling and subtracting this spectrum, weak features at 188 eV indicated a
beryllium content considerably less than 1%. EELS has also been employed to
detect 10-nm Be grains in lung tissue (Jouffrey et al., 1978).
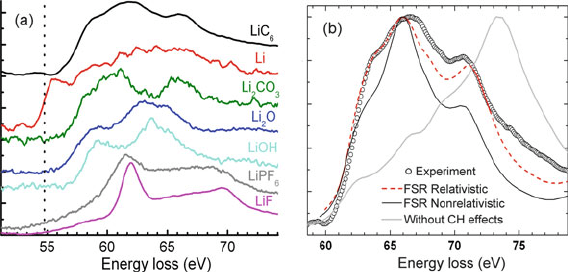
330 5 TEM Applications of EELS
Fig. 5.26 (a) Lithium K-edge structure of fully lithiated graphite (LiC
6
), metallic Li, and several
other compounds. (b) Measured Li-K spectrum of Li-intercalated graphite, compared with FEFF-
9.05 calculations using the final state rule (FSR) approximation, with and without inclusion of core
hole (CH) and relativistic effects. From Wang et al. (2010b), copyright American Chemical Society
Boron is easier to quantify due to its higher K-edge energy (188 eV). Using a
50-nm-diameter probe in a field-emission STEM and second-difference recording,
Leapman (1992) detected 1% of boron in silicon. The edge is partially obscured
by EXELFS modulations from the preceding Si L
23
edge. Boride particles (3–5 nm
diameter) in silicon have been identified in CTEM core-loss images, confirming a
previous HREM interpretation based on the coherency of SiB precipitates (Frabboni
et al., 1991).
Boothroyd et al. ( 1990) detected 0.5 at.% B in Ni
3
Al from parallel EELS data
in which gain variations were reduced to 0.005% by use of iterative averaging
(Section 2.5.5). A second-difference filter was applied to suppress EXELFS modu-
lations from the preceding Ni M- and Al L-edges. Good energy resolution helps in
distinguishing the intrinsically sharp boron edge from the more gradual EXELFS
modulations.
Boron-containing compounds have been investigated for use in neutron capture
cancer therapy (BNCT). Bendayan et al. (1989) used electron spectroscopic imaging
(ESI) to show that B-containing biopolymeric conjugates are absorbed intracel-
lularly by colorectal cells. As a preliminary to BNCT studies, Zhu et al. (2001)
analyzed data recorded from B/C test specimens, finding that concentrations down
to 0.2% could be measured with 10% accuracy by conventional background fitting
but with fitting windows before and after the boron K-edge, so that the background
was derived by interpolation; see Section 4.4.1.
5.4.3 Measurement of Carbon, Nitrogen, and Oxygen
The absence of beam-induced hydrocarbon contamination is an obvious require-
ment for the unambiguous identification and measurement of carbon. Microscope-
induced contamination is reduced by using oil-free pumping and a liquid nitrogen
5.4 Elemental Analysis from Core-Loss Spectroscopy 331
trap or cold finger, giving a low partial pressure of hydrocarbons in the vicinity of the
specimen. Specimen-borne contamination can be minimized by careful attention to
cleanliness during specimen preparation and by liquid nitrogen cooling of the spec-
imen during microscopy, which reduces the mobility of hydrocarbon molecules on
the specimen surface. Surface hydrocarbons are desorbed or rendered immobile (by
polymerization) through mild baking of a specimen, either inside the microscope or
before insertion, or by withdrawing the TEM condenser aperture and defocusing the
illumination in order to strongly irradiate regions surrounding those to be analyzed.
A more recent technique is to oxidize the carbon with oxygen radicals generated by
a plasma source attached to the s ide of the TEM column (Horiuchi et al., 2009).
The identification of carbide and nitride precipitates in steel has been an impor-
tant application of core-loss spectroscopy. Figure 5.27 illustrates how TiC and TiN
precipitates can be more easily distinguished from K-loss spectra than from their
morphology or diffraction pattern. Atomic ratios of transition metals within car-
bides have been estimated from the appropriate L-ionization edges (Fraser, 1978;
Baker et al., 1982).
Extraction replicas can be used to isolate small particles for spectroscopy, but the
usual replicating materials (carbon or polymers) complicate the analysis of carbon.
Garratt-Read (1981) used a 50-nm coating of evaporated aluminum for extraction
and showed that the carbon content of presumed V(C, N) precipitates in vanadium
HSLA steel was less than his detection limit, then about 5 at.%. Silicon extraction
replicas, made by RF sputtering in argon, have also been used (Duckworth et al.,
1984). One potential problem is the loss of carbon from carbide precipitates when
irradiated by a small probe. However, VC precipitates down to 1 nm diameter have
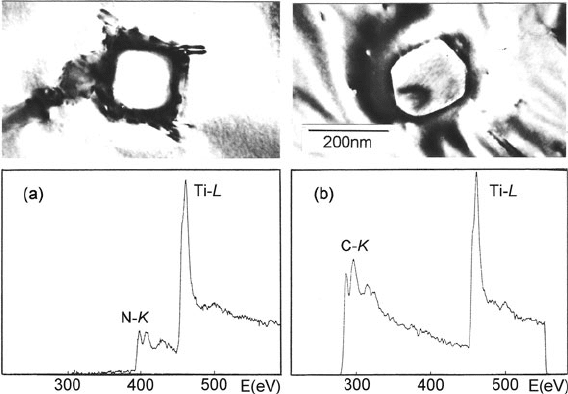
Fig. 5.27 Micrographs and core-loss spectra of (a)TiNand(b) TiC precipitates within a Ti-rich
phase in stainless steel. From Zaluzec (1980), copyright Claitor’s Publishing, Baton Rouge, LA

332 5 TEM Applications of EELS
been analyzed with parallel-recording EELS, allowing changes in composition to be
monitored during their growth sequence (Craven et al., 1989). More recently, Craven
et al. (2008) used EELS spectrum imaging and field evaporation atom probe tech-
niques to study particles in high-strength low-alloy steel. These particles were found
to be nitrogen-rich vanadium and chromium carbonitrides surrounded by an atmo-
sphere of segregated atoms. The authors remark that the use of a dual-readout EELS
system, giving core-loss and low-loss spectra at each pixel, would have allowed the
vanadium content of each particle to be determined.
Energy-loss spectroscopy of various forms of elemental carbon (C
60
, nanotubes,
graphene, etc.) is discussed in Section 5.7.3.
Nitrogen in solution within the γ -phase of duplex stainless steel was measured
by Yamada et al. (1992)as0.26± 0.04 wt.%. They used a 120-kV TEM in diffrac-
tion mode and 15-mrad collection semi-angle. The nitrogen content of the α-phase
was at or below the detection limit, about 0.2 at.%. This low limit was achieved
by iterative averaging of the spectra and by employing a top-hat filter to give
second-differential spectra. Quantification involved the use of narrow (2.5-eV) inte-
gration windows around the N and Fe second-differential peaks, together with a
calibration curve of N/Cr intensity ratio against N/Cr concentration, measured using
high-nitrogen alloys.
Some types of natural diamond contain octahedral-faceted inclusions a few
nanometers in size, known as voidites. EELS measurements r eveal a sharply peaked
K-edge at 400 eV, indicating the presence of nitrogen. Analysis of 20 voidites
(Bruley and Brown, 1989) gave an average nitrogen concentration about half the
carbon concentration and independent of voidite size; see also Fig. 5.64. The shape
of the nitrogen K-edge was consistent with the presence of N
2
rather than NH
3
(proposed as an explanation for previous lattice images). Bruley (1992) found that
nitrogen may be present at platelet defects in diamond, but only at a level of the
order of a tenth of a monolayer.
Oxygen can also occur in voidites, including those present in interplanetary dust
particles. Erni et al. (2005) found their nanometer-sized vesicles to contain molec-
ular oxygen (and a small fraction of H
2
O) as evidenced by the appearance of a π
∗
peak at 531 eV; see Fig. 5.28. After subtraction of the K-edge of the matrix, the
difference spectrum showed close agreement with that of O
2
gas available from a
core-loss database
2
; see Fig. 5.28b.
Using a field-emission STEM and serial-recording spectrometer, Bourret and
Colliex (1982) reported evidence for the segregation of oxygen at dislocation cores
in germanium. Background fitting to the oxygen K-edge and extrapolation over
100 eV revealed an oxygen signal ≈1% of the background. During subsequent
HREM imaging of the dislocations, oxygen was removed by the electron irradiation,
with a characteristic dose estimated to be 10
4
C/cm
2
. Subsequent studies have taken
advantage of the improved collection efficiency of a parallel-recording spectrometer
and second-difference techniques (Yamada et al., 1992).
2
A. Hitchcock and colleagues: http://unicorn.mcmaster.ca/corex/name-list.html
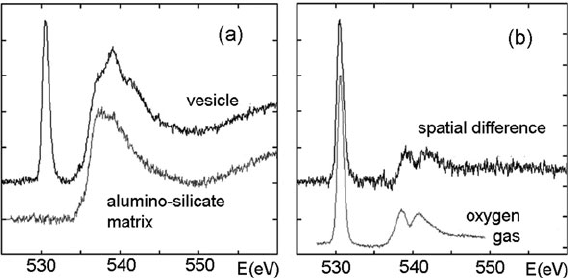
5.4 Elemental Analysis from Core-Loss Spectroscopy 333
Fig. 5.28 (a) Oxygen K-edge recorded by monochromated EELS from a voidite and from the
surrounding silicate matrix of an interplanetary dust particle, collected from the stratosphere. (b)
Difference spectrum from (a) compared with the core-loss spectrum recorded from O
2
gas, both
spectra showing a π
∗
peak at 531 eV and σ
∗
peaks at 539 and 542 eV. From Erni et al. (2005),
copyright Elsevier
Disko et al. (1991) utilized the fact that the Al–L
23
thresholdinAl
2
O
3
is shifted
upward in energy by 4.5 eV (relative to Al metal) to distinguish regions of Al–Al and
Al–O bonding in oxide-strengthened aluminum alloys formed by cryomilling in liq-
uid nitrogen. Their procedure provided an indication of the oxide/metal fraction in
different regions. The spectrometer was also used to acquire pairs of core-loss spec-
tra shifted by 7 eV; a peak at 400 eV in the ratio (log-derivative spectrum) provided
a qualitative but sensitive test for small percentages of nitrogen. Nitrogen quantifi-
cation was achieved by careful AE
−r
background fitting and gave N/O ratio ≈ 1in
some of the particles.
Oxides and nitrides of silicon have been analyzed by examining the shape of
the Si L-edge. By comparing the fine structure with that recorded from several can-
didates, Skiff et al. (1986) showed that oxygen precipitation in Si produces SiO,
not SiO
2
. They also established that precipitates in damaged regions of N
+
ion-
implanted Si were Si
3
N
4
.UsingtheSiL- and O K-edges of SiO
2
as standards, an
analysis of semi-insulating polycrystalline oxygen-doped silicon (SIPOS) revealed
only 15 at.% of oxygen (Catalano et al., 1993), but after annealing at 900
◦
Cthe
material decomposed into silicon nanocrystals and a matrix containing 36 at.% oxy-
gen. Kim and Carpenter (1990) have shown that the native oxide formed on silicon
at room temperature has a composition close to SiO, suggesting that metastable
amorphous solid solutions of Si and O can exist as a single phase over the whole
range from Si to SiO
2
.
5.4.4 Measurement of Fluorine and Heavier Elements
Some fluorinated organic compounds have normal biological activity and can be
used as molecular markers for specific sites in cells. By forming energy-selected
334 5 TEM Applications of EELS
images with the fluorine K-edge, the segregation of (difluoro)serotonin was demon-
strated (Costa et al., 1978). Fortunately, compounds in which fluorine is directly
attached to an aromatic ring are relatively stable under electron irradiation, some-
times withstanding doses as high as 10
4
C/cm
2
if the specimen is cooled to −160
◦
C
(Ciliax et al., 1993).
The distribution of sulfur, phosphorus, and calcium is of interest in biological
systems but the dose required for mapping these elements by x-ray K-emission spec-
troscopy is often destructive. EELS offers the option of using L-shell ionization, for
which the cross section is relatively large, allowing higher detection sensitivity (see
later, Fig. 5.32). Because of the low L-edge energies of S and P (135, 165 eV) the
spectral background is high, even for very thin specimens. This background can be
suppressed by using first- or second-difference techniques (Section 4.4.5), the sensi-
tivity then depending on the noise level and to some extent on the energy resolution.
Detection of Ca, Ti, and transition elements is helped by the fact that these ele-
ments display sharp white-line peaks at the ionization threshold (Fig. 3.45). These
peaks become amplified in difference spectra, so that less than 100 ppm of alkaline
earths, transition metals, and lanthanides could be detected in a glass test specimen
(Leapman and Newbury, 1993).
Based on calculations and experimental results, Wang et al. (1992) prescribed
optimum conditions for the detection of phosphorus in biological tissue with a
parallel-recording spectrometer: t/λ ≈ 0.3 and a 15-eV shift between spectra if
first-difference recording is used. At that time, EELS was estimated to be 15 times
more sensitive than EDX spectroscopy: with 0.5-nm beam current and 100-s record-
ing time, the minimum detectable concentration was calculated as 8.4 mmol/kg
(≈100 ppm), equivalent to 34 phosphorus atoms in a 15-nm probe.
Electron spectroscopic imaging (ESI) in a conventional TEM has been used
extensively to provide qualitative or semiquantitative elemental maps of biologi-
cal specimens. Since phosphorus is a constituent of DNA, P–L
23
images have been
employed to investigate DNA configurations within 80 s ribosomes (Shuman et al.,
1982) and chromatin nucleosomes (Ottensmeyer, 1984). The latter contain about
300 atoms of phosphorus and the signal/noise ratio of the corresponding phosphorus
signal was about 30. Köpf-Maier (1990) employed 80-keV energy-selected imag-
ing to analyze the distribution of titanium and phosphorus in human tumors as a
function of time after therapeutic doses of titanocene dichloride. The maximum Ti
concentration in cell nuclei and nucleoli occurred after 48 h and was accompanied
by an enrichment of phosphorus, confirming that the primary interaction occurs with
nucleic acids, particularly DNA.
ESI has also been used to image the distribution of heavier elements such as tho-
rium, cerium, and barium (formed as cytochemical reaction products) in order to
detect enzyme activities within a cell (Sorber et al., 1990). In some cases, K-edges
were used, e.g., to detect aluminum in newt larvae (Böhmer and Rahmann, 1990).
Here the advantages of EELS over EDX spectroscopy are less obvious. However,
edges in the 1000–3000 eV region can have good signal/background ratios, rel-
atively unaffected by plural scattering, so specimens can be as thick as 0.5 μm
(Egerton et al., 1991).
5.5 Spatial Resolution and Detection Limits 335
5.5 Spatial Resolution and Detection Limits
The spatial resolution obtainable in energy-loss spectroscopy or energy-filtered
imaging depends on several factors, now to be discussed. In the case of core-loss
microanalysis, s patial resolution is closely connected with the concept of elemental
detection limits, as explained in Section 5.5.4.
5.5.1 Electron-Optical Considerations
In a scanning transmission electron microscope (STEM), the s patial resolution
of the image (or of point analysis) depends on the diameter d of the incident
probe. The latter can be made small by demagnifying the source with condenser
lenses and probe-forming lenses but the electron optical brightness B (i.e., cur-
rent/area/steradian) at the plane of focus remains the same as at the source. Source
brightness is roughly proportional to the accelerating voltage; for V
0
= 100 kV,
B ≈ 3 × 10
12
Am
–2
sr
–1
for a Schottky source and ≈10
13
Am
–2
sr
–1
for a
cold field-emission source. The smallest obtainable probe diameter can therefore be
written as
d = 2I
1/2
/(παB
1/2
) (5.16)
where I is the probe current and α the probe convergence semi-angle. As seen from
Eq. (5.16), the product (dα) of beam diameter and angular spread is constant within
the optical system. Large demagnification gives small d but large α, resulting in
spherical aberration tails (extending to a radius ≈ C
s
α
3
) unless an aberration cor-
rector is used. A further result of large α is a small depth of focus: if the probe is
focused at the mid-plane of a specimen of thickness t, its geometrical diameter is αt
at the beam entrance and exit surfaces.
In a thermionic source TEM, a sub-nanometer probe can be formed but with
relatively low current (a few picoamperes). The current–density profile contains a
sharp central peak surrounded by electron-beam tails that contain an appreciable
fraction of the incident current and may extend for many nanometers (Cliff and
Kenway, 1982). Deconvolution techniques have been used to correct concentration
profiles for the effect of these aberration tails, based on a measured or calculated
incident beam profile (Thomas, 1982; Weiss and Carpenter, 1992).
If a TEM is operated in broad-beam imaging mode, a small region of specimen
can be chosen for EELS by a selected-area diffraction aperture or (in TEM imaging
mode) the spectrometer entrance aperture. However, the imaging lenses suffer from
both spherical and chromatic aberration, the latter being severe at high energy loss
(Section 2.3.2) unless the high voltage is raised by an equal amount or the objec-
tive lens refocused (Schenner and Schattschneider, 1994). Spherical aberration can
seriously degrade the resolution in thicker specimens, but can be limited by means
of an objective aperture or eliminated by a post-specimen aberration corrector. The
336 5 TEM Applications of EELS
spatial resolution of an energy-filtered image may also be limited by the pixel size
of the recording camera, if the image magnification is not sufficiently high.
5.5.2 Loss of Resolution Due to Elastic Scattering
When an electron beam enters a specimen, it spreads laterally. Most of this spread-
ing comes from elastic scattering, whose average deflection angle is larger than
that of the inelastic scattering (Fig. 3.7). As depicted in Fig. 1.12, beam broaden-
ing degrades the spatial resolution of x-ray emission spectroscopy; simple models
suggest a broadening proportional to t
3/2
, amounting to 10 nm or more for a
100-nm-thick foil and 100-keV incident electrons (Goldstein et al., 1977).
In the case of EELS, the angular divergence of the beam entering the spectrom-
eter can be limited to some chosen value β by means of a collection aperture. This
aperture also tends to exclude electrons present in incident probe aberration tails
and stray electrons produced in the TEM illumination system. The EELS signal is
unaffected by secondary electrons generated by the electron probe, which can pro-
duce x-rays away from the incident beam. These various factors imply somewhat
better spatial resolution than is obtainable from EDX spectroscopy, as confirmed
experimentally (Collett et al., 1984; Titchmarsh, 1989; Genç et al., 2009).
For an amorphous specimen, the collimation effect of the angle-limiting aperture
can be estimated from simple geometry, as shown in Fig. 5.29a. If scattering occurs
at the top of the foil, the volume of specimen sampled by the recorded electrons
(contained within a cone of semi-angle β) is largest; if it occurs at the bottom, this
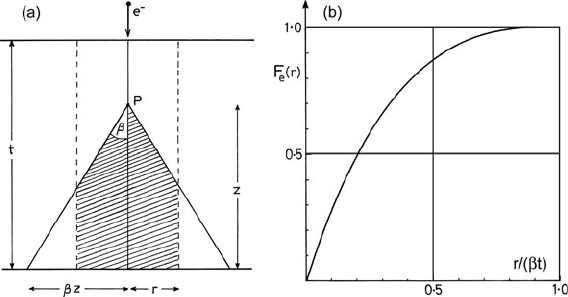
Fig. 5.29 (a) Beam broadening due to scattering at a point P, a distance z from the exit surface
of a specimen. The shaded area represents the excited volume that lies within a distance r of the
optic axis and gives rise to inelastic scattering within the collection aperture. (b) Fraction F
e
(r)of
the elastically scattered electrons (scattering angle < β) contained within a radius r of the incident
beam axis. This estimate assumes that the angular width of elastic scattering is large compared to
that of inelastic scattering and large compared to the collection semi-angle β
5.5 Spatial Resolution and Detection Limits 337
volume is zero. Averaging over the thickness of the specimen, the fraction F
e
(r)of
electrons that have traveled radial distances up to r within the specimen is given
by Fig. 5.29b. Almost 90% of the electrons collected by the aperture are contained
within a specimen volume of diameter 2r ≈ βt, which is below 1 nm for t = 50 nm
and β < 20 mrad.
In the case of a crystalline specimen, the collection angle may be chosen to
exclude Bragg beams, suggesting a radial spread (less than βt) that arises mainly
from inelastic scattering and incident beam convergence. Even in the absence of
an aperture, electron channeling can reduce the radial broadening (Browning and
Pennycook, 1993). In effect, beam broadening is delayed up to a depth (below the
entrance surface) at which s-type Bloch waves (more localized at the atomic cen-
ters) are dispersed by inelastic scattering. STEM imaging of atomic columns in a
thin crystal relies on this principle (Pennycook and Jesson, 1991). A practical way
of minimizing beam spreading is to orient the specimen so as to avoid strongly
diffracting conditions.
5.5.3 Delocalization of Inelastic Scattering
In Section 3.11, we discussed the delocalization of inelastic scattering as a conse-
quence of the long-range nature of the electrostatic interaction between an incident
electron and the atomic electrons in a solid. Delocalization was represented by a
point-spread or object function, of width inversely related to the angular width of
inelastic scattering. Here we attempt to represent the delocalization in terms of a
single number, in order to roughly estimate its contribution to the spatial resolution
of EELS or EFTEM imaging and show how it depends on experimental parameters
such as energy loss, incident energy, and collection angle.
A common wave-optical measure of resolution is the Rayleigh diffraction limit:
x = 0.6λ/ sin β ≈ 0.6λ/β where β (<<1 rad) is the aperture semi-angle of the
optical system used to form an image. This formula applies to a situation where
scattering uniformly fills the aperture, whereas the angular width of inelastic scat-
tering is relatively small. In the absence of any angle-limiting aperture, half of the
inelastic scattering is contained within the median scattering angle
θ
, and for a
Lorentzian angular distribution with a cutoff at θ
c
,
θ
≈ (θ
E
θ
c
)
1/2
(Section 3.3).
Taking θ
c
as the Bethe-ridge angle (2θ
E
)
1/2
gives
θ
≈ 1.2(θ
E
)
3/4
, and the object
width containing 50% of the scattered electrons can be estimated as
L
50
≈ 0.6λ/
θ
≈ 0.5λ/θ
E
3/4
(5.17)
If the imaging system contains an aperture (semi-angle β), we can roughly estimate
its effect by combining the lateral broadenings in quadrature:
(d
50
)
2
≈ (0.5λ/θ
E
3/4
)
2
+(0.6λ/β)
2
(5.18)
