Egerton R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope
Подождите немного. Документ загружается.

x Contents
4.3.3 Bayesian Deconvolution ................. 255
4.3.4 Other Methods ...................... 256
4.4 Separation of Spectral Components . . ............. 257
4.4.1 Least-Squares Fitting . .................. 258
4.4.2 Two-Area Fitting . . . .................. 260
4.4.3 Background-Fitting Errors . . . ............. 261
4.4.4 Multiple Least-Squares Fitting . ............. 265
4.4.5 MultivariateStatisticalAnalysis............. 265
4.4.6 Energy- and Spatial-Difference Techniques . . . .... 269
4.5 ElementalQuantification..................... 270
4.5.1 IntegrationMethod.................... 270
4.5.2 CalculationofPartialCrossSections .......... 273
4.5.3 Correction for Incident Beam Convergence . . . .... 274
4.5.4 Quantification from MLS Fitting ............ 276
4.6 Analysis of Extended Energy-Loss Fine Structure . . . .... 277
4.6.1 FourierTransformMethod................ 277
4.6.2 Curve-Fitting Procedure ................. 284
4.7 Simulation of Energy-Loss Near-Edge Structure (ELNES) . . . 286
4.7.1 Multiple Scattering Calculations ............. 286
4.7.2 BandStructureCalculations............... 288
5 TEM Applications of EELS ...................... 293
5.1 Measurement of Specimen Thickness . ............. 293
5.1.1 Log-RatioMethod.................... 294
5.1.2 Absolute Thickness from the K–K Sum Rule . . .... 302
5.1.3 Mass Thickness from the Bethe Sum Rule . . . .... 304
5.2 Low-Loss Spectroscopy . . . .................. 306
5.2.1 IdentificationfromLow-LossFineStructure ...... 306
5.2.2 Measurement of Plasmon Energy
and Alloy Composition ................. 309
5.2.3 CharacterizationofSmallParticles ........... 310
5.3 Energy-Filtered Images and Diffraction Patterns ........ 314
5.3.1 Zero-Loss Images . . .................. 315
5.3.2 Zero-LossDiffractionPatterns.............. 317
5.3.3 Low-Loss Images . . .................. 318
5.3.4 Z-Ratio Images ...................... 319
5.3.5 ContrastTuningandMPLImaging ........... 320
5.3.6 Core-Loss Images and Elemental Mapping . . . .... 321
5.4 Elemental Analysis from Core-Loss Spectroscopy . . . .... 324
5.4.1 Measurement of Hydrogen and Helium . ........ 327
5.4.2 Measurement of Lithium, Beryllium, and Boron .... 329
5.4.3 Measurement of Carbon, Nitrogen, and Oxygen .... 330
5.4.4 MeasurementofFluorineandHeavierElements .... 333
5.5 SpatialResolutionandDetectionLimits............. 335
5.5.1 Electron-OpticalConsiderations............. 335
Contents xi
5.5.2 LossofResolutionDuetoElasticScattering ...... 336
5.5.3 DelocalizationofInelasticScattering .......... 337
5.5.4 StatisticalLimitationsandRadiationDamage...... 340
5.6 Structural Information from EELS . . . ............. 346
5.6.1 Orientation Dependence of Ionization Edges . . .... 346
5.6.2 Core-LossDiffractionPatterns.............. 350
5.6.3 ELNES Fingerprinting .................. 352
5.6.4 Valency and Magnetic Measurements
fromWhite-LineRatios ................. 357
5.6.5 UseofChemicalShifts.................. 361
5.6.6 Use of Extended Fine Structure ............. 362
5.6.7 Electron–Compton (ECOSS) Measurements . . .... 366
5.7 Application to Specific Materials . . . ............. 368
5.7.1 Semiconductors and Electronic Devices . ........ 368
5.7.2 Ceramics and High-Temperature Superconductors . . . 374
5.7.3 Carbon-Based Materials ................. 378
5.7.4 Polymers and Biological Specimens . . . ........ 386
5.7.5 Radiation Damage and Hole Drilling . . ........ 389
Appendix A Bethe Theory for High Incident Energies
and Anisotropic Materials ................. 399
A.1 Anisotropic Specimens . . . ............. 402
Appendix B Computer Programs ..................... 405
B.1 First-Order Spectrometer Focusing . . ........ 405
B.2 Cross Sections for Atomic Displacement
andHigh-AngleElasticScattering .......... 406
B.3 Lenz-Model Elastic and Inelastic Cross Sections . . . 406
B.4 SimulationofaPlural-ScatteringDistribution .... 407
B.5 Fourier-Log Deconvolution . ............. 408
B.6 Maximum-Likelihood Deconvolution ........ 409
B.7 Drude Simulation of a Low-Loss Spectrum . .... 409
B.8 Kramers–KronigAnalysis .............. 410
B.9 Kröger Simulation of a Low-Loss Spectrum . .... 412
B.10 Core-LossSimulation................. 412
B.11 Fourier Ratio Deconvolution ............. 413
B.12 Incident-Convergence Correction . . . ........ 414
B.13 Hydrogenic K-Shell Cross Sections . ........ 414
B.14 Modified-Hydrogenic L-Shell Cross Sections .... 415
B.15 Parameterized K-, L-, M-, N-, and O-Shell
CrossSections..................... 416
B.16 Measurement of Absolute Specimen Thickness . . . 416
B.17 TotalInelasticandPlasmonMeanFreePaths .... 417
B.18 Constrained Power-Law Background Fitting . .... 417
Appendix C Plasmon Energies and Inelastic Mean Free Paths ..... 419
xii Contents
Appendix D Inner-Shell Energies and Edge Shapes ........... 423
Appendix E Electron Wavelengths, Relativistic Factors,
and Physical Constants ................... 427
Appendix F Options for Energy-Loss Data Acquisition ........ 429
References .................................. 433
Index ..................................... 485
Chapter 1
An Introduction to EELS
Electron energy-loss spectroscopy (EELS) involves analyzing the energy distribu-
tion of initially monoenergetic electrons after they have interacted with a specimen.
This interaction is sometimes confined to a few atomic layers, as when a beam of
low-energy (100–1000 eV) electrons is “reflected” from a solid surface. Because
high voltages are not involved, the apparatus is relatively compact but the low
penetration depth implies the use of ultrahigh vacuum. Otherwise information is
obtained mainly from the carbonaceous or oxide layers on the specimen’s surface.
At these low primary energies, a monochromator can reduce the energy spread of
the primary beam to a few millielectron volts (Ibach, 1991) and if the spectrometer
has comparable resolution, the spectrum contains features characteristic of energy
exchange with the vibrational modes of surface atoms, as well as valence electron
excitation in these atoms. The technique is therefore referred to as high-resolution
electron energy-loss spectroscopy (HREELS) and is used to study the physics and
chemistry of surfaces and of adsorbed atoms or molecules. Although it is an impor-
tant tool of surface science, HREELS uses concepts that are somewhat different to
those i nvolved in electron microscope studies and will not be discussed further in
the present volume. The instrumentation, theory, and applications of HREELS are
described by Ibach and Mills (1982) and by Kesmodel (2006).
Surface sensitivity is also achieved at higher electron energies if the electrons
arrive at a glancing angle to the surface, so that they penetrate only a shallow
depth before being scattered out. Reflection energy-loss spectroscopy (REELS) has
been carried out with 30-keV electrons in a molecular beam epitaxy (MBE) cham-
ber, allowing elemental and structural analysis of the surface during crystal growth
(Atwater et al., 1993). Glancing angle REELS is also possible in a transmission
electron microscope (TEM) at a primary energy of 100 keV or more, as discussed
in Section 3.3.5.
If the electrons arrive perpendicular to a sufficiently thin specimen and their
kinetic energy is high enough, practically all of them are transmitted without reflec-
tion or absorption. Interaction then takes place inside the specimen, and information
about its internal structure can be obtained by passing the transmitted beam into a
spectrometer. For 100-keV incident energy, the specimen must be less than 1 μm
thick and preferably below 100 nm.
1
R.F. Egerton, Electron Energy-Loss Spectroscopy in the Electron Microscope,
DOI 10.1007/978-1-4419-9583-4_1,
C
Springer Science+Business Media, LLC 2011
2 1 An Introduction to EELS
Such specimens are s elf-supporting only over limited areas, so the incident elec-
trons must be focused into a small diameter, but in doing so we gain the advantage
of analyzing small volumes. Electron lenses that can focus the electrons and guide
them into an electron spectrometer are already present in a transmission electron
microscope, so transmission EELS is usually carried out using a TEM, taking
advantage of its imaging and diffraction capabilities to identify the structure of the
material being analyzed.
In this introductory chapter, we present a simplified account of the physical pro-
cesses that occur while “fast” electrons are passing through a specimen, followed
by an overview of energy-loss spectra and the instruments that have been developed
to record such spectra. To identify the strengths and limitations of EELS, we con-
clude by considering the alternative techniques that are available for analyzing the
chemical and physical properties of a solid specimen.
1.1 Interaction of Fast Electrons with a Solid
When electrons enter a solid, they interact with the constituent atoms through elec-
trostatic (Coulomb) forces. As a result of these forces, some of the electrons are
scattered: the direction of their momentum is changed and in many cases they trans-
fer an appreciable amount of energy to the specimen. It is convenient to divide the
scattering into two broad categories: elastic and inelastic.
Elastic scattering involves Coulomb interaction with atomic nuclei. Each nucleus
represents a high concentration of charge; the electric field in its immediate vicinity
is intense and an incident electron that approaches close enough is deflected through
a large angle. Such high-angle deflection is referred to as Rutherford scattering,
since its angular distribution is the same as that calculated by Rutherford for the
scattering of alpha particles. If the deflection angle exceeds 90
◦
, the electron is said
to be backscattered and may emerge from the specimen at the same surface that it
entered (Fig. 1.1a).
The majority of electrons travel further from the center of an atom, where the
electrostatic field of the nucleus is weaker because of the inverse square law and
the fact that the nucleus is partially shielded (screened) by atomic electrons. Most
incident electrons are therefore scattered through small angles, typically a few
degrees (10–100 mrad) in the case of 100-keV incident energy. In a gas or (to a
first approximation) an amorphous solid, each atom or molecule scatters electrons
independently. In a crystalline solid, the wave nature of the incident electrons cannot
be ignored and interference between scattered electron waves changes the contin-
uous distribution of scattered intensity into one that is sharply peaked at angles
characteristic of the atomic spacing. The elastic scattering is then referred to as
diffraction.
Although the term elastic usually implies negligible exchange of energy, this
condition holds only when the scattering angle is small. For a head-on colli-
sion (scattering angle = 180
◦
), the energy transfer is given (in electron volt) by
E
max
= 2148 (E
0
+ 1.002)E
0
/A, where E
0
is the incident energy in megaelectron
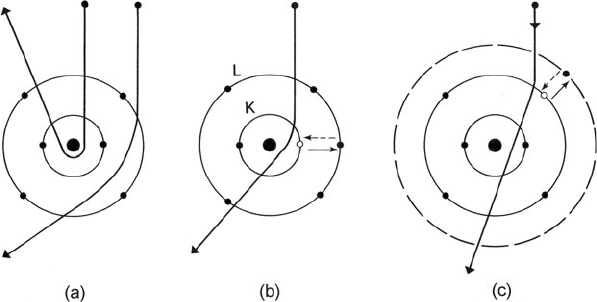
1.1 Interaction of Fast Electrons with a Solid 3
Fig. 1.1 A classical (particle) view of electron scattering by a single atom (carbon). (a) Elastic
scattering is caused by Coulomb attraction by the nucleus. Inelastic scattering results from
Coulomb repulsion by ( b)inner-,or(c) outer-shell electrons, which are excited to a higher energy
state. The reverse transitions (de-excitation) are shown by broken arrows
volt and A is the atomic weight of the target nucleus. For E
0
=100 keV, E
max
> 1eV
and, in the case of a light element, may exceed the energy needed to displace the
atom from its lattice site, resulting in displacement damage within a crystalline sam-
ple, or removal of atoms by sputtering from its surface. However, such high-angle
collisions are rare; for the majority of elastic interactions, the energy transfer is
limited to a small fraction of an electron volt, and in crystalline materials is best
described in terms of phonon excitation (vibration of the whole array of atoms).
Inelastic scattering occurs as a result of Coulomb interaction between a fast inci-
dent electron and the atomic electrons that surround each nucleus. Some inelastic
processes can be understood in terms of t he excitation of a single atomic electron
into a Bohr orbit (orbital) of higher quantum number (Fig. 1.1b) or, in terms of
energy band theory, to a higher energy level (Fig. 1.2).
Consider first the interaction of a fast electron with an inner-shell electron, whose
ground-state energy lies typically some hundreds or thousands of electron volts
below the Fermi level of the solid. Unoccupied electron states exist only above
the Fermi level, so the inner-shell electron can make an upward transition only
if it absorbs an amount of energy similar to or greater than its original binding
energy. Because the total energy is conserved at each collision, the fast electron
loses an equal amount of energy and is scattered through an angle typically of
the order of 10 mrad for 100-keV incident energy. As a result of this inner-shell
scattering, the target atom is left in a highly excited (or ionized) state and will
quickly lose its excess energy. In the de-excitation process, an outer-shell elec-
tron (or an inner-shell electron of lower binding energy) undergoes a downward
transition to the vacant “core hole” and the excess energy is liberated as electro-
magnetic radiation (x-rays) or as kinetic energy of another atomic electron (Auger
emission).
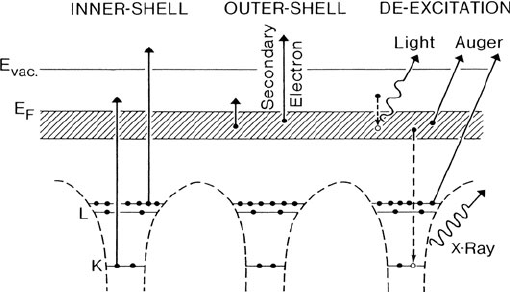
4 1 An Introduction to EELS
Fig. 1.2 Energy-level diagram of a solid, including K-andL-shell core levels and a valence band
of delocalized states (shaded); E
F
is the Fermi level and E
vac
the vacuum level. The primary pro-
cesses of inner- and outer-shell excitation are shown on the left, secondary processes of photon and
electron emission on the right
Outer-shell electrons can also undergo single-electron excitation. In an insula-
tor or semiconductor, a valence electron makes an interband transition across the
energy gap; in the case of a metal, a conduction electron makes a transition to a
higher state, possibly within the same energy band. If the final state of these transi-
tions lies above the vacuum level of the solid and if the excited atomic electron has
enough energy to reach the surface, it may be emitted as a secondary electron.As
before, the fast electron supplies the necessary energy (generally a few electron volts
or tens of electron volts) and is scattered through an angle of typically 1 or 2 mrad
(for E
0
≈ 100 keV). In the de-excitation process, electromagnetic radiation may be
emitted in the visible region (cathodoluminescence), although in many materials the
reverse transitions are radiationless and the energy originally deposited by the fast
electron appears as heat. Particularly in the case of organic compounds, not all of
the valence electrons return to their original configuration; the permanent disruption
of chemical bonds is described as ionization damage or radiolysis.
As an alternative to the single-electron mode of excitation, outer-shell inelastic
scattering may involve many atoms of the solid. This collective effect is known as
a plasma resonance (an oscillation of the valence electron density) and takes the
form of a longitudinal traveling wave, similar in some respects to a sound wave.
According to quantum theory, the excitation can also be described in terms of the
creation of a pseudoparticle, the plasmon, whose energy is E
p
= ω
p
, where is
Planck’s constant and ω
p
is the plasmon frequency in radians per second, which is
proportional to the square root of the valence electron density. For the majority of
solids, E
p
lies in the range 5–30 eV.
Plasmon excitation being a collective phenomenon, the excess energy is shared
among many atoms when viewed over an extended time period. At a given instant,
however, the energy is likely to be carried by only one electron (Ferrell, 1957),
1.2 The Electron Energy-Loss Spectrum 5
which makes plausible the fact that plasmons can be excited in insulators, E
p
being
generally higher than the excitation energy of the valence electrons (i.e., the band
gap). The essential requirement for plasmon excitation is that the participating elec-
trons can communicate with each other and share their energy, a condition that is
fulfilled for a band of delocalized states but not for the atomic-like core levels. The
lifetime of a plasmon i s very short; it decays by depositing its energy (via interband
transitions) i n the form of heat or by creating secondary electrons.
In addition to exciting volume or “bulk” plasmons within the specimen, a fast
electron can create surface plasmons at each exterior surface. However, these surface
excitations dominate only in very thin (<20 nm) samples or small particles.
Plasmon excitation and single-electron excitation represent alternative modes of
inelastic scattering. In materials in which the valence electrons behave somewhat
like free particles (e.g., the alkali metals), the collective form of response is predom-
inant. In other cases (e.g., rare gas solids), plasmon effects are weak or nonexistent.
Most materials fall between these two extremes.
1.2 The Electron Energy-Loss Spectrum
The secondary processes of electron and photon emission from a specimen can be
studied in detail by appropriate spectroscopies, as discussed in Section 1.4. In elec-
tron energy-loss spectroscopy, we deal directly with the primary process of electron
excitation, which results in the fast electron losing a characteristic amount of energy.
The transmitted electron beam is directed into a high-resolution electron spectrom-
eter that separates the electrons according to their kinetic energy and produces an
electron energy-loss spectrum showing the number of electrons (scattered intensity)
as a function of their decrease in kinetic energy.
A typical loss spectrum, recorded from a thin specimen over a range of about
1000 eV, is shown in Fig. 1.3. The first zero-loss or “elastic” peak represents
electrons that are transmitted without suffering measurable energy loss, including
electrons scattered elastically and those that excite phonon modes, for which the
energy loss is less than the experimental energy resolution. In addition, the zero-
loss peak includes electrons that can be regarded as unscattered, since they lose no
energy and remain undeflected after passing through the specimen. The correspond-
ing electron waves undergo a phase change but this is detectable only by holography
or high-resolution imaging.
Inelastic scattering from outer-shell electrons is visible as a peak (or a series of
peaks, in thicker specimens) in the 4–40 eV region of the spectrum. At higher energy
loss, the electron intensity decreases rapidly, making it convenient to use a logarith-
mic scale for the recorded intensity, as in Fig. 1.3. Superimposed on this smoothly
decreasing intensity are features that represent inner-shell excitation; they take the
form of edges rather than peaks, the inner-shell intensity rising rapidly and then
falling more slowly with increasing energy loss. The sharp rise occurs at the ioniza-
tion threshold, whose energy-loss coordinate is approximately the binding energy
of the corresponding atomic shell. Since inner-shell binding energies depend on the
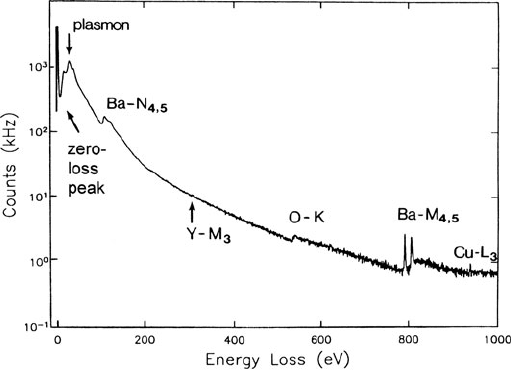
6 1 An Introduction to EELS
Fig. 1.3 Electron energy-loss spectrum of a high-temperature superconductor (YBa
2
Cu
3
O
7
) with
the electron intensity on a logarithmic scale, showing zero-loss and plasmon peaks and ionization
edges arising from each element. Courtesy of D.H. Shin, Cornell University
atomic number of the scattering atom, the ionization edges present in an energy-loss
spectrum reveal which elements are present within the specimen. Quantitative ele-
mental analysis is possible by measuring an area under the appropriate ionization
edge, making allowance for the underlying background.
When viewed in greater detail, both the valence electron (low-loss) peaks and the
ionization edges possess a fine structure that reflects the crystallographic or energy
band structure of the specimen. Even with an energy resolution of 2 eV, it is possible
to distinguish between different forms of an element such as carbon, as illustrated
in Fig. 1.4.
If the energy-loss spectrum is recorded from a sufficiently thin region of the spec-
imen, each spectral feature corresponds to a different excitation process. In thicker
samples, there is a substantial probability that a transmitted electron will be inelas-
tically scattered more than once, giving a total energy loss equal to the sum of the
individual losses. In the case of plasmon scattering, the result is a series of peaks at
multiples of the plasmon energy (Fig. 1.5). The plural (or multiple) scattering peaks
have appreciable intensity if the specimen thickness approaches or exceeds the mean
free path of the inelastic scattering process, which is typically 50–150 nm for outer-
shell scattering at 100-keV incident energy. Electron microscope specimens are
typically of this thickness, so plural scattering is usually significant and generally
unwanted, since it distorts the shape of the energy-loss spectrum. Fortunately it be
removed by various deconvolution procedures.
On a classical (particle) model of scattering, the mean free path (MFP) is an
average distance between scattering events. More generally, the MFP is inversely
proportional to a scattering cross section, which is a direct (rather than inverse)
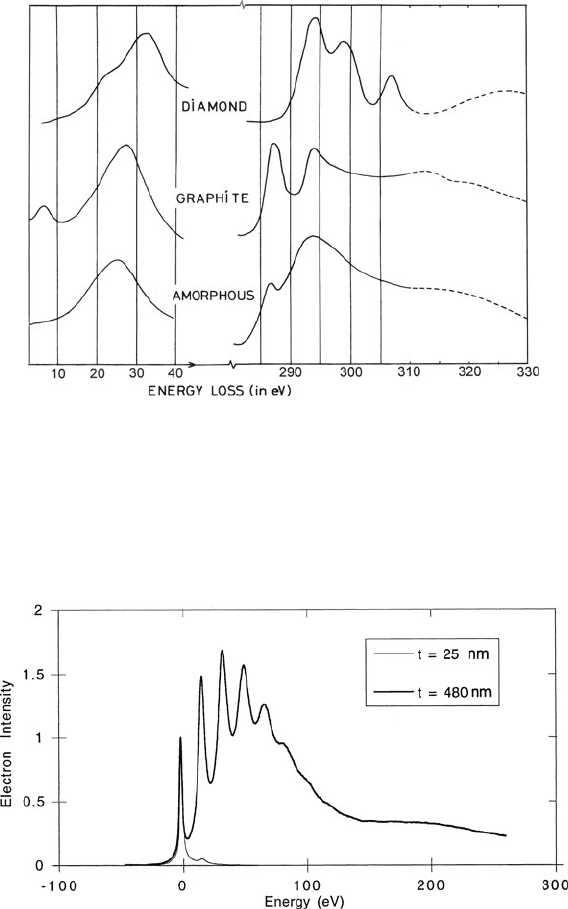
1.2 The Electron Energy-Loss Spectrum 7
Fig. 1.4 Low-loss and K-ionization regions of the energy-loss spectra of three allotropes of car-
bon, recorded on photographic plates and then digitized (Egerton and Whelan, 1974a, b). The
plasmon peaks occur at different energies (33 eV in diamond, 27 eV in graphite, and 25 eV in
amorphous carbon) because of the different valence electron densities. The K-edge threshold is
shifted upward by about 5 eV in diamond due to the formation of an energy gap. The broad peaks
indicated by dashed lines are caused by electrons that undergo both K-shell and plasmon scattering
Fig. 1.5 Energy-loss spectra recorded from silicon specimens of two different thicknesses. The
thin sample gives a strong zero-loss peak and a weak first-plasmon peak; the thicker sample
provides plural scattering peaks at multiples of the plasmon energy
