Egerton R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope
Подождите немного. Документ загружается.

298 5 TEM Applications of EELS
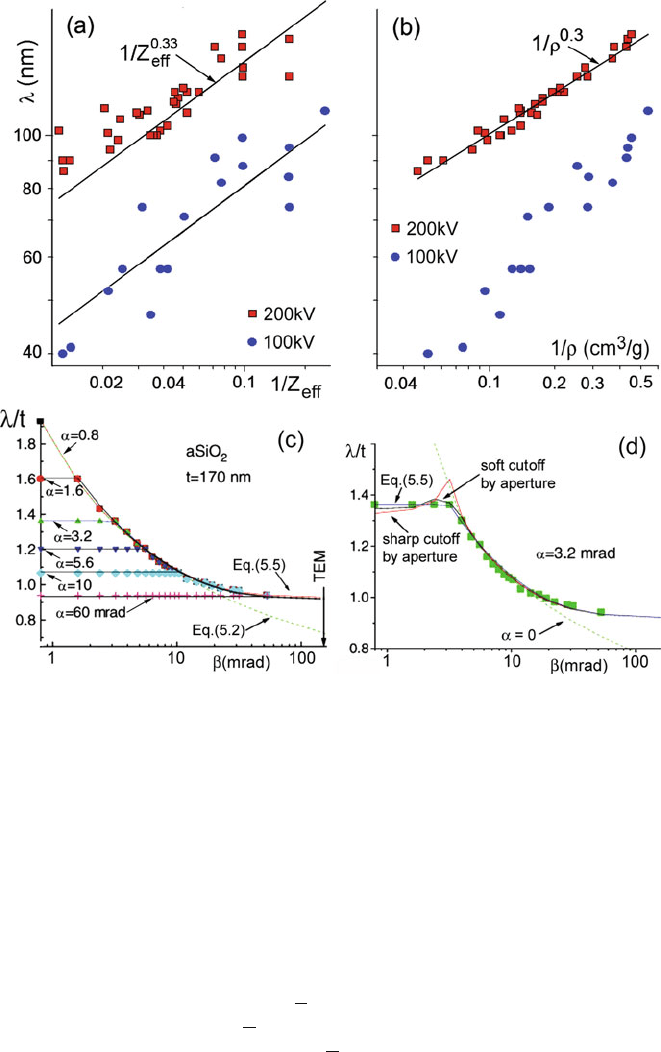
Fig. 5.2 Log–log plot of inelastic mean free path (for large β) as a function of (a) effective atomic
number Z
eff
and (b)densityρ of the specimen. Note the smaller amount of scatter in the latter
case. Squares represent values measured by Iakoubovskii et al. (2008b)atE
0
= 200 keV. Filled
circles are for E
0
= 100 keV and are derived from Eq. (5.2) by scaling to large collection angle by
the use of Eq. (3.16).(c) Measured inelastic mean free path λ for SiO
2
as a function of collection
semi-angle β and incident beam convergence angle α.Thevertical arrow shows the limit to β
imposed by the lens bore of a typical TEM. (d) Collection angle dependence of λ for α = 3.2
mrad. From Iakoubovskii et al. (2008a), copyright Wiley
and β are below the cutoff angle θ
c
, taken in Eq. (5.6) to be 20 mrad. The measured
α- and β-dependence of λ are shown for amorphous SiO
2
in Fig. 5.2c, d, together
with the parameterized formula, Eq. (5.5). Note that the value of λ is essentially
determined by the larger of α and β.
Equation (5.5a) implies θ
E
= E/(γ m
0
v
2
), where
¯
E represents some mean
energy loss, whereas θ
E
= E/(m
0
v
2
) relativistically; see Appendix A.Thisis
arguably a worse choice than θ
E
≈ E/(2E
0
) as assumed in Eq. (5.2), since

5.1 Measurement of Specimen Thickness 299
Fig. 5.3 Inelastic mean free
paths for elemental solids at
β = 10 mrad. Equation (5.5)
is represented by filled circles
at 200 keV and by open
circles at 100 keV. Equation
(5.2) is represented by a solid
line at 200 keV and a broken
line at 100 keV
(γ m
0
v
2
/2) = γ FE
0
= 172 keV at E
0
= 200 keV, whereas FE
0
= 124 keV.
The E
0
scaling of Iakoubovskii’s Eq. (5.5) might therefore be improved by replac-
ing F in Eq. (5.5a)byF
g
= γ F. Note that Eq. (5.5a) implies
¯
E ≈ 11ρ
0.3
, whereas
Eq. (3.41a) gives E
p
∝ (zρ/A)
1/2
for the free-electron plasmon energy.
The main differences between Eqs. (5.2) and (5.5) are that the Iakoubovskii mean
free paths show pronounced oscillation with atomic number and are on average a
factor 1.4 (at 200 keV) or 1.3 (at 100 keV) larger than those given by the Malis et al.
formula; see Fig. 5.3.
Bonney (1990) reported that Eq. (5.2) gave thickness to within 10% when tested
on sub-micrometer vanadium spheres whose thickness was taken to be the same
as their diameter. The 100-keV measurements of Crozier ( 1990) are also within
±15% of the Malis et al. (1988) formula. The parameterization of Iakoubovskii
et al. (2008a) more accurately reflects the Z-dependence of Crozier’s measurements
but overestimates the absolute values by an average of 25%; see Fig. 5.4.
Fig. 5.4 Solid data points:
inelastic mean free path for
C, Al, Fe, Cu, Ag, and Au
(for E
0
= 100 keV, β = 5,
21, and 120 mrad) as
measured by Crozier (1990).
Hollow data points show
values predicted by Eq. (5.6),
the full and dashed lines are
the predictions of Eq. (5.2)
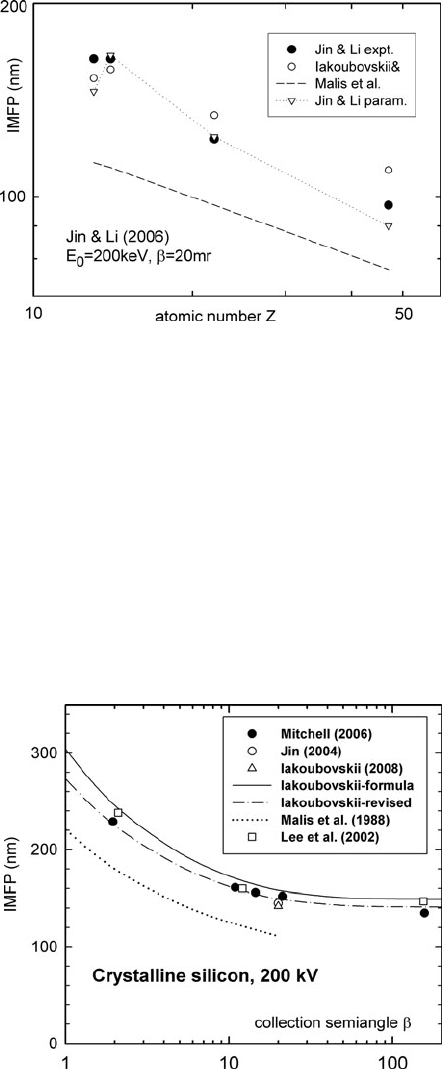
300 5 TEM Applications of EELS
Fig. 5.5 Solid circle:
inelastic mean free path of
Al, Si, Ti, and Ag (for
E
0
= 200 keV, α = 10 mrad,
and β = 20 mrad) measured
by Jin and Li (2006). Hollow
circles: values predicted from
Eq. (5.6). Dashed line:Eqs.
(5.2)and(5.3). Inverted
triangles:Eq.(5.2) with
E
m
= 43.5Z
0.47
ρ/A
Figure 5.5 shows 200-keV measurements of Jin and Li (2006). Here the
Iakoubovskii et al. (2008a) formula matches the experimental data within 10%. The
dipole approximation is not valid for this data, so the Malis et al. (1988)formula
underestimates λ. Jin and Li obtained a good fit to their data by replacing Eq. (5.2a)
by E
m
= 43.5 Z
0.47
ρ/A where A represents atomic weight.
Figure 5.6 shows 200-keV measurements on silicon, all within 10% of the
Iakoubovskii formula (solid curve) at medium values of β. The agreement is
improved further (dash-dot curve) by replacing F in Eq. (5.5a)byF
g
= γ F.
For most materials, the Iakoubovskii et al. (2008a) parameterization of λ appears
to represent an improvement over that of Malis et al. (1988) in terms of the accu-
racy, convenience of use (requiring density rather than an E
m
value), and the fact
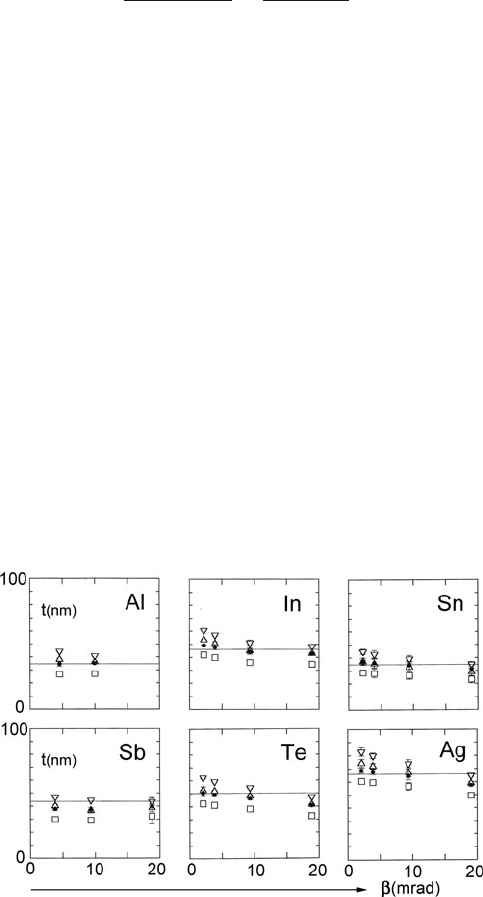
Fig. 5.6 Inelastic mean free
path for crystalline silicon as
a function of collection
semi-angle. Data points
represent TEM
measurements. The dash-dot
curve represents Eq. (5.5)
with F in Eq. (5.5a) replaced
by F
g
5.1 Measurement of Specimen Thickness 301
that the formula should apply to large β (e.g., 100 mrad, limited only by post-
specimen lenses). Advantages of large β are that its exact value need not be known
and convergence correction is unnecessary, so the incident probe convergence α is
not required. The programs IMFP and PMFP, described in Appendix B, calculate λ
for the different formulas described above.
Although Eq. (5.1) involves specimen thickness t, it is actually the total scat-
tering and mass thickness that is measured by EELS. If the physical density of a
material were reduced by a factor f, the scattering per atom would remain the same
(according to an atomic model) and the mean free path should increase by a factor f.
This prediction was confirmed by Jiang et al. (2010), using crystalline MgO and
nanoporous MgO whose density was about half the bulk value, resulting in a mea-
sured λ about a factor of 2 l arger. Such arguments ignore the presence of surface
plasmon losses at internal pores, and as pointed out by Batson (1993a) the presence
of surfaces increases the total scattering, despite the begrenzungs effect (Fig. 3.25).
In practice, this increase appears to be modest. Shindo et al. (2005) measured mean
free paths differing by a factor ≈1.7 for diamond-like carbon films prepared by
different methods, with physical densities between 1.4 and 2.1 g/cm
3
.
5.1.1.2 Organic Specimens
Biological specimens vary in porosity and are usually characterized in terms of mass
thickness ρt, which can be determined from a variant of Eq. ( 5.1), namely
ρt = ρλ ln(I
t
/I
0
) = (1/σ
)ln(I
t
/I
0
) (5.6)
where σ
is a cross section per unit mass. Calculations of Leapman et al.
(1984a, b) based on Thomas–Fermi, Hartree–Fock, and dielectric models (Ashley
and Williams, 1980) suggested that ρλ varies by no more than ±20% for biologi-
cal compounds, although these different models predicted values of ρλ differing by
almost a factor of 2 (e.g., 8.8 μg/cm
2
to 15 μg/cm
2
for protein at E
0
= 100 keV).
Other measurements and calculations based on the Bethe sum rule gave ρλ =
17.2 μg/cm
2
for protein at 100-keV beam energy (Sun et al., 1993).
The only data processing involved in the log-ratio method is separation of the
spectrum into zero-loss and inelastic components, both of which are strong signals
and relatively noise free. Measurements can therefore be performed rapidly, with
an electron exposure of no more t han 10
−13
C. Even for organic materials, where
structural damage or mass loss can occur at a dose as low as 10
−3
C/cm
2
, thickness
can be measured with a lateral spatial resolution below 100 nm. In this respect, the
log-ratio technique is an attractive alternative to the x-ray continuum method (Hall,
1979), which requires electron exposures of 10
−6
C or more to obtain adequate
statistics (Leapman et al., 1984a). Rez et al. (1992) employed the log-ratio method to
measure the thickness of paraffin crystals, with a reported accuracy of 0.4 nm under
low-dose (0.003 C/cm
2
) and low-temperature (−170
◦
C) conditions. Leapman et al.
(1993a) used similar methods to measure 200-nm-diameter areas of protein (cro-
toxin) crystals and achieved good agreement with thicknesses determined using the

302 5 TEM Applications of EELS
STEM annular dark-field signal, after correcting the latter for nonlinearity arising
from plural elastic scattering.
Zhao et al. (1993) measured t/λ for a biological thin section by fitting its carbon
K-edge to a sum of components derived from K-loss and plasmon-loss single-
scattering distributions, both recorded from a pure carbon film, giving a “carbon
equivalent” thickness. This procedure is convenient to the extent that it does not
require recording of the low-loss region of the thin section, but it involves a radiation
dose about 100 times higher than that required by the log-ratio method.
5.1.2 Absolute Thickness from the K–K Sum Rule
As described in Section 4.2, Kramers–Kronig analysis of an energy-loss spectrum
gives a value for the absolute specimen thickness, along with energy-dependent
dielectric data, without requiring the chemical composition of the specimen. The
procedure involves extraction of the single-scattering distribution S(E) from a mea-
sured spectrum, use of the Kramers–Kronig sum rule to derive the energy-loss
function Im[−1/ε(E)], and removal of the surface scattering component of S(E)by
iterative computation; see Appendix B.
If specimen thickness is the only requirement, the procedure can be simplified.
Combining Eqs. (4.27) and (4.26)gives
t =
4a
0
FE
0
I
0
{1 −Re[1/ε(0)]}
∞
0
S(E)dE
E ln(1 + β
2
/θ
2
E
)
(5.7)
where a
0
= 0.0529 nm, F is the relativistic factor given by Eq. (5.2a), and
θ
E
is the characteristic angle defined by Eq. (3.28). In general, Re[1/ε(0)] =
ε
1
/(ε
1
2
+ε
2
2
)
2
, where ε
1
and ε
2
are the real and imaginary parts of the optical
permittivity. However, as discussed in Section 4.2,Re[1/ε(0)] can be taken as zero
for a metal or semimetal and as 1/n
2
for an insulator or semiconductor of refractive
index n.
The 1/E weighting factor makes the integral in Eq. (5.7) less sensitive to higher
orders of scattering. Provided the specimen is not too thick (t/λ < 1.2), the effect of
plural scattering can be approximated by dividing the integral by a correction factor
(Egerton and Cheng, 1987)
C ≈ 1 + 0.3(t/λ) = 1 + 0.3 ln(I
t
/I
0
) (5.8)
Kramers–Kronig analysis carried out on thin films of A1, Cr, Cu, Ni, and Au
(Egerton and Cheng, 1987) suggested that a more accurate value of thickness is
obtained by subtracting an amount t (≈8 nm) from the value given by Eq. (5.7)
to allow for surface plasmon scattering. With these approximations, and assuming
β
2
/θ
E
2
>> 1 over the energy range where the inelastic intensity is significant,
Eq. (5.7) can be simplified to
5.1 Measurement of Specimen Thickness 303
t =
2a
0
T
CI
0
{1 −n
−2
}
∞
0
J(E)dE
E ln(β/θ
E
)
−t (5.9)
Here J(E) represents the inelastic component of the energy-loss spectrum, including
plural scattering but excluding the zero-loss peak. A computer program (TKKs) that
evaluates Eq. (5.9) is described in Appendix B.
Because of the 1/E weighting in Eq. (5.9), the value of t is particularly sensitive
to data at very low energy loss. The procedure used to separate the elastic scattering
peak from the inelastic intensity is therefore important. The simplest method is to
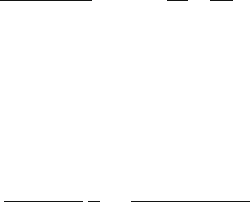
truncate the spectrum at the first minimum, E = δ in Fig. 5.1, but it results in
an underestimation of t (square data points in Fig. 5.7), since contributions below
E = δ are missing. Omitting the surface correction, by setting t to zero in Eq. (5.9),
compensates for this missing contribution and gives a more realistic thickness value
(solid circles in Fig. 5.7). Linear or parabolic extrapolation of the inelastic intensity
(at E = δ) to zero (at E = 0) also gives acceptable r esults (triangles in Fig. 5.7).
Another strategy is to model the tail of the zero-loss peak and subtract this from the
spectrum to give the inelastic intensity J(E).
The errors involved in these procedures can be minimized by optimizing the
energy resolution. Spectra read out from a CCD camera should be recorded with
a sufficiently high energy dispersion (low electron volt/channel) to minimize the
energy range over which tails of the zero-loss peak are significant, even though this
results in spectra with a restricted energy range. Because of the 1/E weighting in
Fig. 5.7 Thickness (in nm) of Al, In, Sn, Sb, Te, and Ag films measured using the Kramers–Kronig
sum rule with different treatments of the low-energy limit (Yang and Egerton, 1995). Inverted and
upright triangles denote linear and parabolic interpolation (respectively) between the origin and
the first intensity minimum. Eliminating data below the minimum gave values represented by the
squares and solid circles, the surface term t being set to zero in the latter case. Horizontal lines
represent the film thickness determined by weighing, assuming bulk densities
304 5 TEM Applications of EELS
Eq. (5.9), the upper limit of integration can be as low as 100 eV for reasonably
thin specimens. If t/λ > 1.2, the plural scattering correction in Eq. (5.9) becomes a
poor approximation. A better procedure is then to use Fourier log deconvolution to
remove the plural scattering component and employ Eq. (5.9) with C = 1.
Because the Kramers–Kronig procedure is based on the equivalence of energy
loss and optical data, the spectral intensity J(E) must be dominated by dipole scat-
tering, implying a small collection semi-angle β (which must be known). In practice,
values up to 18 mrad give acceptable results at E
0
= 100 keV (Fig. 5.7); at 200 keV,
this condition becomes β < 10 mrad.
Iakoubovskii et al. (2008a) found good agreement between thicknesses obtained
from the K–K sum rule (with E
0
= 200 keV, β
∗
≈ 12 mrad) and those deduced
from convergent-beam diffraction. These measurements were used to derive inelas-
tic mean free paths for many elements and oxides (Iakoubovskii et al., 2008b); see
Appendix C.
The advantage of the K–K sum rule method is that it gives thickness without
knowledge of the material properties of the specimen, if the latter is conducting
so that Re[1/ε(0)] ≈ 1 − n
−2
≈ 0. In the case of semiconducting and insulating
specimens, an optical permittivity or refractive index n is required, and in the case
of nanoporous materials, n will be reduced because of the lower physical density.
5.1.3 Mass Thickness from the Bethe Sum Rule
As in Section 3.2.2, the single-scattering intensity S(E) can be written in terms of a
differential oscillator strength df/dE rather than the energy-loss function Im(−1/ε).
Combining Eqs. (3.33) and (4.26)gives
S(E) =
4πI
0
Na
2
0
R
2
FE
0
E
ln
1 +
β
2
θ
2
E
df
dE
(5.10)
where N is the total number of atoms per unit area and df/dE is a dipole (small-q)
oscillator strength. Taking all the E-dependent terms to the right-hand side of this
equation, integrating over energy loss, and making use of t he Bethe sum rule of
Eq. (3.34),wehave
ρt = ANu =
uFE
0
4πa
2
0
R
2
I
0
A
Z
∞
0
ES(E)
ln
,
1 +β
2
/θ
2
E
-
dE (5.11)
where u is the atomic mass unit and ρt is the mass thickness of the specimen, A and
Z being its atomic weight and atomic number. For a compound, A is replaced by the
molecular weight and Z by the total number of electrons per molecule.
The integral in Eq. (5.11) is relatively insensitive to the instrumental energy res-
olution, allowing S(E) to be taken as the intensity J
1
(E) obtained from Fourier log
deconvolution of experimental data. The combined effect of the other terms within
5.1 Measurement of Specimen Thickness 305
the integral is to weight S(E) by a factor typically between E and E
2
, implying
that the spectrum must be measured up to rather high energy loss to ensure conver-
gence of the integral. Convergence is further delayed because inner atomic shells
contribute to S(E) at energy losses above their binding energy. This means that
Eq. (5.11) is useful only for specimens composed of light elements, with K-shell
binding energies below 1000 eV. However, this category includes most organic and
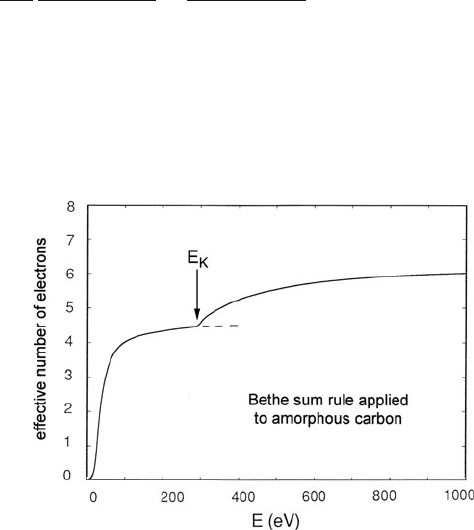
biological materials, containing mainly carbon, oxygen, and hydrogen. Figure 5.8
shows the E-dependence of Eq. (5.11) for pure carbon, where the integral reaches
its saturation value at about 1000 eV.
The need for such an extended energy range means that the spectrum must be
recorded in segments with different integration times and spliced together. Because
the intensity is integrated in Eq. (5.11), good statistics and energy resolution are
not important and the high-E data can be quite noisy. However, any detector back-
ground (dark current) must be carefully removed, together with any spectrometer
background that arises from stray scattering, as discussed in Chapter 2. Unless
the specimen is extremely thin, plural scattering should be removed by Fourier log
deconvolution.
For light elements, the ratio A/Z in Eq. (5.11) is close to 2, but a better approx-
imation for most biological materials is to take A/Z = 1.9 (Crozier and Egerton,
1989), in which case Eq. (5.11) can be rewritten as
ρt =
BE
0
I
0
(1 +E
0
/1022)
(1 +E
0
/511)
2
∞
0
EJ
1
(E)
ln
,
1 +β
2
/θ
2
E
-
dE (5.12)
where B = 4.88 × 10
−11
g/cm
2
E is in eV, and E
0
in keV. This equation was
tested on thin films of copper phthalocyanine (ρt up to 30 μg/cm
2
, corresponding
to t/λ ≈ 1.5) and yielded mass thickness values within 10% of those determined
Fig. 5.8 Value of the integral
in Eq. (5.11), expressed as an
effective number of
contributing electrons defined
by Eq. (4.33). Note that n
eff
almost saturates as E
approaches the K-shell
binding energy (284 eV) but
approaches its full value (6)
only for energy losses around
1000 eV. From Sun et al.
(1993), copyright Elsevier
306 5 TEM Applications of EELS
by weighing (Crozier and Egerton, 1989). The Bethe sum r ule has also been used to
determine the cross section per unit mass of protein and water (Sun et al., 1993).
The Bethe sum rule method involves an electron exposure (typically 10
−10
Cfor
parallel recording) higher than that needed to apply the log-ratio method (≈10
−13
C) but its potential accuracy is higher. The bremsstrahlung continuum method (Hall,
1979) is useful for thicker specimens (t >0.5μm) but involves an electron exposure
of the order of 10
−6
C, sufficient to cause significant mass loss in most organic
materials if the diameter of the incident beam is less than 1 μm (Leapman et al.,
1984a).
With suitable calibration, local mass thickness can also be obtained by integrat-
ing the inelastic scattering over chosen ranges of scattering angle and energy loss,
using an electron spectrometer to reject the elastic and unscattered components (Feja
et al., 1997). This may be a lower dose alternative to measuring high-angle elastic
scattering with a STEM and ADF detector (Wall and Hainfeld, 1986; Feja and Aebi,
1999).
5.2 Low-Loss Spectroscopy
The 1–50 eV region of the energy-loss spectrum contains peaks that arise from
inelastic scattering by outer shell electrons. In most materials, the major peak can be
called a plasmon peak; its energy is related to valence electron density and its width
reflects the damping effect of single-electron transitions (Section 3.3.2). In some
cases, these transitions appear directly in the low-loss spectrum as peaks or fine
structure oscillations superimposed on the plasmon peak. The low-loss spectrum
is then characteristic of the material present within the electron beam and can be
used to identify that material, if suitable comparison standards are available and the
spectral data have a sufficiently low noise level.
5.2.1 Identification from Low-Loss Fine Structure
If a specimen contains regions that give rise to sharp plasmon peaks, the materials
involved are easily identified, as demonstrated for metallic sodium, aluminum, or
magnesium by Sparrow et al. (1983) and Jones et al. (1984). Other materials are
harder to characterize because their plasmon peaks are broad and occur within a
limited range, typically 15–25 eV, yet by careful comparison with low-loss spectra
recorded from candidate materials, it is sometimes possible to identify an unknown
phase. This fingerprinting method was used to identify 25–250 nm precipitates
in internally oxidized Si/Ni alloy as amorphous SiO
2
(Cundy and Grundy, 1966)
and 10–100 nm precipitates in silicon as SiC (Ditchfield and Cullis, 1976). More
recently, Evans et al. (1991) quantified the depth profile of aluminum in spinel
implanted with 2-MeV Al
+
ions, the depth-dependent low-loss spectrum being fitted
to reference spectra of aluminum and spinel; see Fig. 5.9.
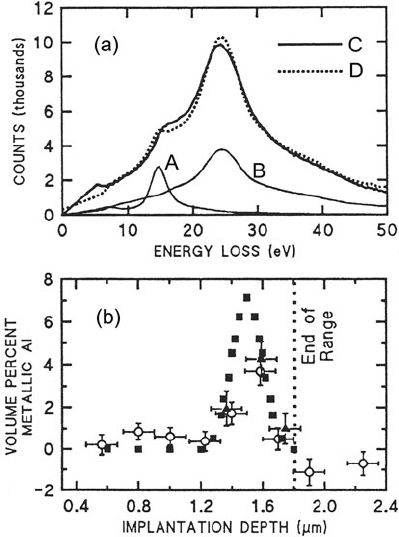
5.2 Low-Loss Spectroscopy 307
Fig. 5.9 (a) Low-loss spectra
with plural scattering
removed: A = metallic Al, B
= undamaged spinel, C =
material at implant depth of
1.6 μm, D = best fit from
multiple regression analysis,
indicating 3.7 ±0.6 vol.% Al.
(b) Profile showing aluminum
concentration as a function of
depth. From Evans et al.
(1991), with permission from
San Francisco Press, Inc
Because of plural scattering, the overall shape of the low-loss spectrum depends
on specimen thickness, so to avoid errors due to thickness differences between
unknown and reference materials, plural scattering should be removed, for example,
by Fourier log deconvolution. A published library allows comparison of low-loss
spectra with those of some common materials and is now available in digital form
(Ahn, 2004).
Plasmon peak width can sometimes be indicative. Chen et al. (1986)showed
that the plasmon peak measured from quasi-crystalline Al
6
Mn was wider (3.1 eV)
than that of the amorphous (2.4 eV) or crystalline phases (2.2 eV), suggesting that
the icosahedral material had a distinct electronic band structure, favoring plasmon
decay via interband transitions. On the other hand, Levine et al. (1989) found the
plasmon widths of icosahedral Pd
59
U
21
/Si
20
and Al
75
Cu
15
V
10
to be the s ame as
those of amorphous materials of the same composition.
Many organic compounds provide a distinctive fine structure at energies below
the main plasmon peak (Hainfeld and Isaacson, 1978); see Fig 5.10.Evenifthe
structure is not prominent, a careful comparison using multiple least-squares fit-
ting can allow the composition to be measured. Sun et al. (1993) used this method
to determine the water content of cryosectioned red blood cells as 70 ± 2%,
with water and frozen solutions of bovine serum albumin (BSA) as standards.
Acceptable statistical errors in the MLS fitting were obtained with an electron dose
