Egerton R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope
Подождите немного. Документ загружается.

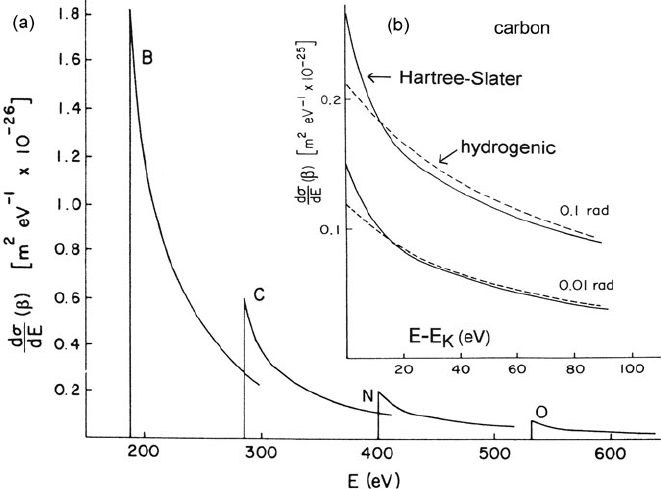
198 3 Physics of Electron Scattering
Fig. 3.42 (a) Energy-differential cross section for K-shell ionization in boron, carbon, nitrogen,
and oxygen, calculated for 80-keV incident electrons and 3-mrad collection semi-angle using the
Hartree–Slater method. (b) Comparison of Hartree–Slater and hydrogenic calculations for the car-
bon K-edge, taking E
0
= 80 keV and collection semi-angles of 10 and 100 mrad (Leapman et al.,
1980)
As seen in Fig. 3.43, measured K-shell edges conform to this same overall shape
but with the addition of some pronounced fine structure. The K-ionization edges
remain basically sawtooth shaped for third-period elements (Na to Cl). Their inten-
sities are lower, resulting in a relatively high noise content in the experimental
data.
Calculations of L
23
edges (excitation of 2p electrons) in third-period elements
(Na to Cl) show that they have a more rounded profile, as in Fig. 3.44. The intensity
exhibits a delayed maximum of 10–20 eV above the ionization threshold, resulting
from the l
’
(l + 1) t erm in the radial Schrödinger equation, Eq. (3.128), which causes
a maximum to appear in the effective atomic potential. At energies just above the
ionization threshold, this “centrifugal barrier” prevents overlap between the initial
(2p) and final-state wavefunctions, particularly for final states with a large angular
momentum quantum number l
. Measured L
23
edges display this delayed maximum
(Ahn, 2004), although excitonic effects can sharpen the edge, particularly in the case
of insulating materials (see page 212).
Fourth-period elements give rise to quite distinctive L-edges. Atomic calcula-
tions predict that the L
23
edges of K, Ca, and Sc will be sharply peaked at the
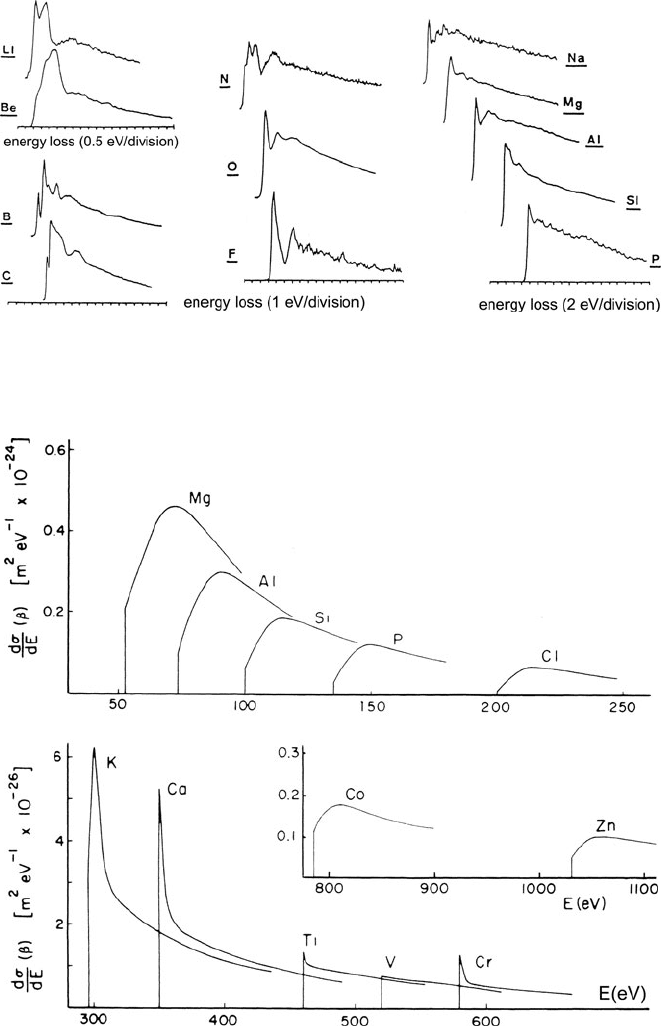
3.7 The Form of Inner-Shell Edges 199
Fig. 3.43 K-ionization edges (after background subtraction) measured by EELS with 120-keV
incident electrons and collection semi-angles in the range of 3–15 mrad. The background intensity
before each edge has been extrapolated and subtracted, as described in Chapter 4. From Zaluzec
(1982), copyright Elsevier
Fig. 3.44 Hartree–Slater calculations of L
23
edges, for 80-keV incident electrons and a collection
semi-angle of 10 mrad (Leapman et al., 1980)
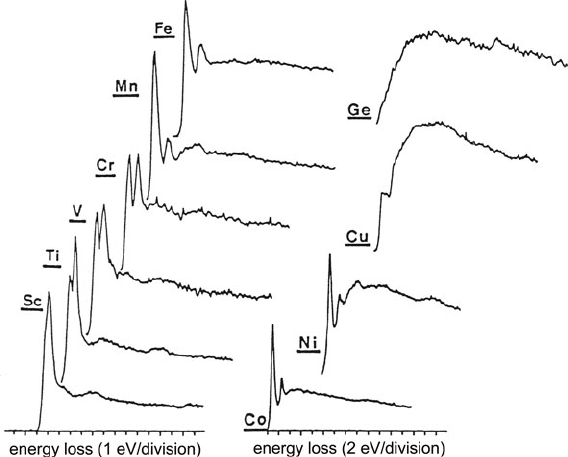
200 3 Physics of Electron Scattering
ionization threshold because of a “resonance” effect: the dipole selection rule favors
transitions to final states with d-character (l
= 2) and the continuum 3d wavefunc-
tion is sufficiently compact to fit mostly within the centrifugal barrier, resulting in
strong overlap with the core-level (2p) wavefunction and a large oscillator strength
at threshold (Leapman et al., 1980). These sharp threshold peaks are known as
white lines since they were first observed in x-ray absorption spectra where the
high absorption peak at the ionization threshold resulted in almost no blackening
on a photographic plate. The white lines are mainly absent in the calculated L
23
-
edge profiles of transition metal atoms (Fig. 3.44) because the calculations neglect
excitation to bound states (discrete unoccupied 3d levels). In a solid, however, these
atomic levels form a narrow energy band with a high density of vacant d-states,
leading to the strong threshold peaks observed experimentally; see Fig. 3.45.
Spin–orbit splitting causes the magnitude of the L
2
binding energy to be slightly
higher than that of the L
3
level. Consequently, two threshold peaks are observed,
whose separation increases with increasing atomic number (Fig. 3.45). The ratio
of intensities of the L
3
and L
2
white lines is found to deviate from the “statisti-
cal” value (2.0) based on the relative occupancy of the initial-state levels (Leapman
et al., 1982). This deviation is caused by spin coupling between the core hole and
the final state (Barth et al., 1983) and is useful for determining valence state; see
Section 5.6.4.
Fig. 3.45 L-edges of fourth-period elements measured using 120-keV electrons and a collection
semi-angle of 5.7 mrad. From Zaluzec (1982), copyright Elsevier
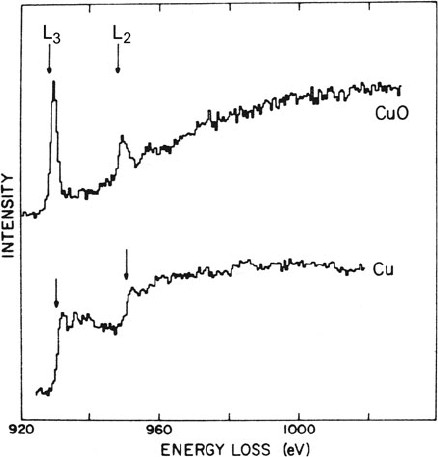
3.7 The Form of Inner-Shell Edges 201
Fig. 3.46 Cu L
23
edges in
metallic copper and in cupric
oxide, measured using
75-keV electrons scattered up
to β =2 mrad. From
Leapman et al. (1982),
copyright American Physical
Society. Available at http://
link.aps.org/abstract/PRB/
v26/p614
In the case of metallic copper, the d-band is full and threshold peaks are absent,
but in compounds such as CuO electrons are drawn away from the copper atom,
leading to empty d-levels and sharp L
2
and L
3
threshold peaks; see Fig. 3.46. Fourth-
period elements of higher atomic number (Zn to Br) have full d-shells and the L
23
edges display delayed maxima, as in the case of Ge (Fig. 3.45).
Hartree–Slater calculations of L
1
edges indicate that they have a sawtooth shape
(like K-edges) but relatively low intensity. They are usually observable as a small
step on the falling background of the preceding L
23
edge; see Fig. 3.45.
M
45
edges are prominent for fifth-period elements and appear with the intensity
maximum delayed by 50–100 eV beyond the threshold (Fig. 3.47) because the cen-
trifugal potential suppresses the optically preferred 3d → 4f transitions just above
the threshold (Manson and Cooper, 1968). Within the s ixth period, between Cs
(Z = 55) and Yb (Z = 70), white-line peaks occur at the threshold due to a high
density of unfilled f-states (Fig. 3.48a). The M
4
–M
5
splitting and the M
5
/M
4
inten-
sity ratio increase with the atomic number (Brown et al., 1984; Colliex et al., 1985).
Above Z = 71, the M
4
and M
5
edges occur as rounded steps (Ahn, 2004), making
them harder to recognize.
M
23
edges of elements near the beginning of the fourth period (K to Ti) occur
below 40 eV, superimposed on a rapidly falling valence-electron background that
makes them appear more like plasmon peaks than typical edges. M
23
edges of the
elements V to Zn are fairly sharp and resemble K-edges (Hofer and Wilhelm, 1993);
M
1
edges are weak and are rarely observed in energy-loss spectra.
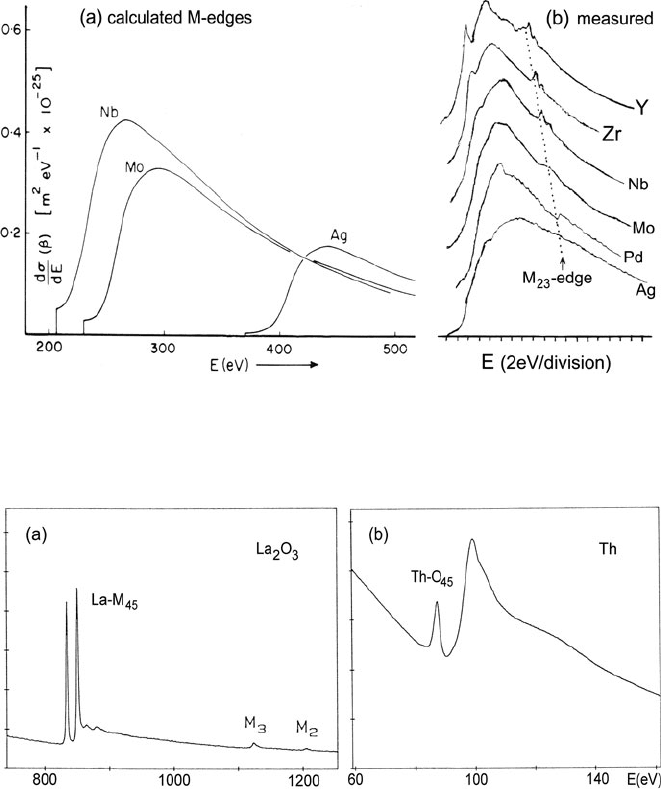
202 3 Physics of Electron Scattering
Fig. 3.47 (a) M
45
edges for E
0
= 80 keV and β = 10 mrad, according to Hartree–Slater calcula-
tions (Leapman et al., 1980). (b) M
45
edges of fifth-period elements measured with E
0
= 120 keV
and β = 5.7 mrad (Zaluzec, 1982)
Fig. 3.48 (a) Energy-loss spectrum of a lanthanum oxide thin film, recorded with 200-keV elec-
trons and collection semi-angle of 100 mrad, showing M
5
and M
4
white lines followed by M
3
and
M
2
edges. (b)O
45
edge of thorium recorded using 120-keV electrons and 100-mrad acceptance
angle. Reproduced from Ahn and Krivanek (1983)
N
67
,O
23
, and O
45
edges have been recorded for some of the heavier elements
(Ahn and Krivanek, 1983). In thorium and uranium, the O
45
edges are prominent
as a double-peak structure (spin–orbit splitting 10 eV) between 80- and 120-eV
energy loss; see Fig. 3.48b.
3.7 The Form of Inner-Shell Edges 203
3.7.2 Dipole Selection Rule
Particularly when a small collection aperture is employed, the transitions that
appear prominently in energy-loss spectra are mainly those for which the dipole
selection rule applies, as in the case of x-ray absorption spectra. From Eq. (3.145),
the momentum exchange is approximately q
min
≈ k
0
θ
E
= E/v for θ<
θ
E
, whereas the momentum exchange upon absorption of a photon of energy E
is q(photon) = E/c. The ratio of momentum exchange in the two cases is
therefore
q
min
/q(photon) ≈ c/v (3.161)
and is less than 2 for incident energies above 80 keV. Therefore the optical selection
rule l =±1 applies approximately to the energy-loss spectrum. This dipole rule
accounts for the prominence of L
23
edges (2p → 3d transitions) in transition metals
and their compounds (high density of d-states just above the Fermi level) and of M
45
edges in the lanthanides (high density of unfilled 4f states).
In the case of a large collection aperture, the momentum transfer can be several
times q
min
(the median scattering angle for inner-shell excitation is typically 5θ
E
;
see Fig. 3.41) and dipole-forbidden transitions are sometimes observed (Section
3.8.2). For example, sharp M
2
and M
3
peaks are seen in the spectrum of lanthanum
oxide (Fig. 3.48a), representing l = 2 transitions from the 3p core level to a high
density of unfilled 4f states. These peaks almost disappear when a small (1.6 mrad)
collection angle is used (Ahn and Krivanek, 1983).
3.7.3 Effect of Plural Scattering
In discussing edge shapes, we have so far assumed that the specimen is very thin
(t/λ ≤ 0.3, where λ is the mean free path for all inelastic scattering) so that we
can ignore the possibility of a transmitted electron being inelastically scattered by
valence electrons, in addition to exciting an inner shell. In thicker samples, this sit-
uation no longer applies and a broad double-scattering peak appears at an energy
loss of approximately E
k
+ E
p
, where E
p
is the energy of the main “plasmon”
peak observed in the low-loss region. In even thicker specimens, higher order satel-
lite peaks merge with the double-scattering peak to produce a broad hump beyond
the edge, completely transforming its shape and obliterating any fine structure; see
Figs. 3.33 and 3.49. This behavior points to the advantage of using very thin spec-
imens for the identification of ionization edges and the analysis of fine structure.
Within limits, however, such plural or “mixed” scattering can be removed from the
spectrum by deconvolution (Section 4.3).
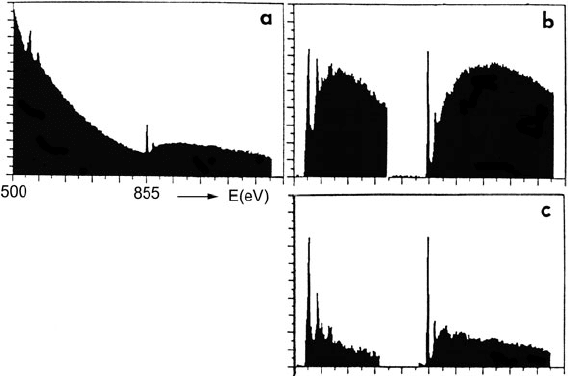
204 3 Physics of Electron Scattering
Fig. 3.49 (a) Energy-loss spectrum recorded from a thick (t/λ ≈ 1.5) specimen of nickel
oxide. (b) Oxygen-K and nickel-L edges, after background removal. (c) Edge profiles after plural
scattering was removed by Fourier ratio deconvolution (Zaluzec, 1983)
3.7.4 Chemical Shifts in Threshold Energy
When atoms combine to form a molecule or a solid, the outer-shell wavefunctions
change to become molecular orbitals or Bloch functions, their energy levels then
reflecting the overall chemical or crystallographic structure. Although core-level
wavefunctions are altered to a much smaller extent, the energy of the core level may
change by several electron volts, depending on the chemical and crystallographic
environment of the atom involved.
Core-level binding energies can be measured directly by x-ray photoelectron
spectroscopy (XPS). In this technique, a bulk specimen is illuminated with
monochromatic x-rays and an electron spectrometer is used to measure the kinetic
energies of photoelectrons that escape into the surrounding vacuum. The final state
of the electron transition therefore lies in a continuum far above the vacuum level
and is practically independent of the specimen. In the case of a compound, any
increase in binding energy of a core level, relative to its value in the pure (solid)
element, is called a chemical shift. For metallic core levels in oxides and most other
compounds, the XPS chemical shift is positive because oxidation removes valence-
electron charge from the metal atom, reducing the screening of its nuclear field and
deepening the potential well around the nucleus.
The ionization-edge threshold energies observed in EELS or in x-ray absorption
spectroscopy (XAS) represent a difference in energy between the core-level initial
state and the lowest energy final state of the excited electron. The corresponding
chemical shifts in threshold energy are more complicated than in XPS because the
lowest energy final state lies below the vacuum level and its energy depends on the
3.7 The Form of Inner-Shell Edges 205
valence-electron configuration. For example, going from a conducting phase (such
as graphite) to an insulator (diamond) introduces an energy bandgap, raising the
first-available empty state by several electron volts and increasing the ionization
threshold energy (Fig. 1.4). On the other hand, the edge threshold in many ionic
insulators corresponds to excitation to bound exciton states within the energy gap,
reducing the chemical shift by an amount equal to the exciton binding energy.
The situation is further complicated by a many-body effect known as relaxation.
When a positively charged core hole is created by inner-shell excitation, nearby
electron orbitals are pulled inward, reducing the magnitude of the measured binding
energy by an amount equal to the relaxation energy. In XPS, where the excited
electron leaves the solid, measured relaxation energies are s ome tens of electron
volts. In EELS or XAS, however, a core electron that receives energy just slightly
in excess of the threshold value remains in the vicinity of the core hole and the
screening effect of its negative charge reduces the relaxation energy. In a metal,
conduction electrons provide additional screening that is absent in an insulating
compound, so while relaxation effects may be less in EELS than in XPS, differences
in relaxation energy between a metal and its compounds can have an appreciable
influence on the chemical shift (Leapman et al., 1982).
Muller (1999) has pointed out that core-level shifts can be opposite in sign to
those expected from electronegativity arguments, and in binary alloys the shift can
be of the same sign for both elements. Measured core-level shifts in Ni–Al and
Ni–Si alloys were found to be proportional to valence band shifts deduced from
linear muffin-tin orbital calculations. In metals, therefore, the core-loss shift appears
to be largely determined by changes in the valence band, rather than by charge
transfer. The width of the valence band varies with changes in the type, number,
and separation of neighboring atoms, the core level tracking these valence band
shifts to within 0.1 eV. As a result, the EELS chemical shift is capable of providing
information about the occupied electronic states of a metal.
Measured EELS chemical shifts of metal-atom L
3
edges in transition metal
oxides are typically 1 or 2 eV and either positive or negative (Leapman et al., 1982).
The shifts of K-absorption edges in the same compounds are all positive and in the
range of 0.7–10.8 eV (Grunes, 1983). XAS chemical shifts, largely equivalent to
those registered by EELS, have been studied extensively. The absorption edge of
the metal atom in a compound is usually shifted to higher photon energy (compared
to the metallic element) and these positive chemical shifts range up to 20 eV (for
KMnO
4
) i n the case of K-edges of transition metals.
Because t ransition series elements can take more than one valency, there exist
mixed-valency compounds (e.g., Fe
3
O
4
and Mn
3
O
4
) containing differently charged
ions of the same species. Since the chemical shift increases with increasing oxida-
tion state, a double edge or multiple edges may be observed. In chromite spinel, for
example, the L
3
and the L
2
white lines are each split by about 2 eV because of the
presence of both Cr
2+
and Cr
3+
ions. Since these two ions occupy different sites
(tetrahedral and octahedral) within the unit cell, the observed splitting will include
a contribution (estimated as 0.7 eV) arising from the different site symmetry (Taftø
and Krivanek, 1982b).
206 3 Physics of Electron Scattering
Site-dependent chemical shifts can also occur in organic compounds in which
carbon atoms are present at chemically dissimilar sites within a molecule. For exam-
ple, the carbon K-edge recorded from a nucleic acid base shows a series of peaks,
interpreted as being the result of several edges chemically shifted relative to one
another due to the different effective charges on the carbon atoms (Isaacson, 1972b).
3.8 Near-Edge Fine Structure (ELNES)
Core-loss spectra recorded from solid specimens often s how a pronounced fine
structure, taking the form of peaks or oscillations in intensity within 50 eV of the
ionization threshold. Most of this structure reflects the influence of atoms surround-
ing the excited atom and requires a solid-state explanation. The basic principles are
described in several review articles (Brydson, 1991; Sawatzky, 1991; Rez, 1992;Rez
et al., 1995; M izuguchi, 2010). In the sections below, we outline several approaches
that have been used to interpret the fine structure in different types of material,
namely
1. the band structure approach, in which the final states of the excited core electron
are Bloch states in an infinite solid;
2. the multiple scattering concept, which considers backscattering of the excited
electron within a cluster of typically a few hundred atoms;
3. the molecular orbital picture, in which the final state involves just a few atoms;
4. multiplet processes, in which several electrons are involved, but all within the
same atom.
3.8.1 Densities-of-States Interpretation
Modulations of the single-scattering intensity J
k
1
(E) can be related to the band
structure of the solid in which scattering occurs. The theory is greatly simplified
by making a one-electron approximation: excitation of an inner-shell electron is
assumed to have no effect on the other atomic electrons. According to the Fermi
golden rule of quantum mechanics (Manson, 1978), the transition rate is then pro-
portional to a product of the density of final states N(E) and an atomic transition
matrix M(E):
J
1
k
(E) ∝ dσ/dE ∝|M(E)|
2
N(E) (3.162)
M(E) represents the overall shape of the edge, discussed in Section 3.7.1, and is
determined by atomic physics, whereas N(E) depends on the chemical and crystal-
lographic environment of the excited atom. To a first approximation, M(E) can be
assumed to be a slowly varying function of energy loss E, so that variations in J
1
k
(E)
represent the energy dependence of the densities of states (DOS) above the Fermi
level. However, the following qualifications apply.

3.8 Near-Edge Fine Structure (ELNES) 207
(1) Transitions occur only to a final state that is empty; like x-ray absorption spec-
troscopy, EELS yields information on the density of unoccupied states above
the Fermi level.
(2) Because the core-level states are highly localized, N(E)isalocal density of
states (LDOS) at the site of the excited atom (Heine, 1980). As a result, there
can be appreciable differences in fine structure between edges representing dif-
ferent elements in the same compound; see Fig. 3.50. Even in the case of a
single element, the fine structure may be different at sites of different symmetry,
as demonstrated by Tafto (1984) for Al in sillimanite.
(3) The strength of the matrix element term is governed by the dipole selection rule:
l =±1, with l = 1 transitions predominating (see Sections 3.7.2 and 3.8.2).
As a result, the observed DOS is a symmetry-projected density of states. Thus,
modulations in K-edge intensity (1s initial state) reflect mainly the density of 2p
final states. Similarly, modulations in the L
2
and L
3
intensities (2p initial states)
are dominated by 3d final states, except where p → d transitions are hindered
by a centrifugal barrier (for example, p → s transitions are observed close to
silicon L
23
threshold). As a result of this selection rule, a dissimilar structure can
be expected in the K- and L-edges of the same element in the same specimen.
(4) N(E) is in principle a joint density of states, the energy dependence of the final-
state density being convolved with that of the core level. The core-level width
i
is given approximately by the uncertainty relation
i
τ
h
≈ , where the lifetime
τ
h
of the core hole is determined by the speed of the de-excitation mechanism
Fig. 3.50 Dashed curves: near-edge fine structure calculated for cubic boron nitride using pseudo-
atomic orbital band theory. Solid curves: core-loss intensity measured by P. Fallon. From Weng
et al. (1989), copyright American Physical Society. Available at http://link.aps.org/abstract/PRB/
v40/p5694
