Egerton R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope
Подождите немного. Документ загружается.


128 3 Physics of Electron Scattering
(Howie, 1995) because the energy losses involved are comparatively large. For
heavy elements, inner atomic shells make major contributions to both σ
i
and S.
3.2.2 Bethe Theory
In order to describe in more detail the inelastic scattering of electrons by an atom
(including the dependence of scattered intensity on energy loss), the behavior of
each atomic electron is specified in terms of transition from an initial state of wave-
function ψ
0
to a final state of wavefunction ψ
n
. Using the first Born approximation,
the differential cross section for the transition is (Inokuti, 1971)
dσ
n
d
=
m
0
2π
2
2
k
1
k
0
V(r)ψ
0
ψ
∗
n
exp(iq ·r) dτ
2
(3.20)
In Eq. (3.20), k
0
and k
1
are wavevectors of the fast electron before and after scat-
tering, q = (k
0
− k
1
) is the momentum transferred to the atom, r represents
the coordinate of the fast electron, V(r) is the potential (energy) responsible for the
interaction, and the asterisk denotes complex conjugation of the wavefunction; the
integration is over all volume elements dτ within the atom.
Below an incident energy of about 300 keV (see Appendix A), the interaction
potential that represents electrostatic forces between an incident electron and an
atom can be written as
V(r) =
Ze
2
4πε
0
r
−
1
4πε
0
Z
j=1
e
2
r −r
j
(3.21)
Although generally referred to as a potential, V(r) is actually the negative of the
potential energy of an electron and is related to the electrostatic potential φ by
V = eφ.
The first term in Eq. (3.21) represents Coulomb attraction by the nucleus,
charge =Ze; the second term is a sum of the repulsive effects from each atomic elec-
tron, coordinate r
j
. Because the initial and final state wavefunctions are orthogonal,
the nuclear contribution integrates to zero in Eq. (3.20), so whereas the elastic cross
section reflects both nuclear and electronic contributions to the potential, inelastic
scattering involves only interaction with the atomic electrons. Because the latter are
comparable in mass to the incident electron, inelastic scattering involves apprecia-
ble energy transfer. Combining Eqs. (3.20) and (3.21), the differential cross section
can be written in the form
dσ
n
d
=
4γ
2
a
2
0
q
4
k
1
k
0
|
ε
n
(q)
|
2
(3.22)
3.2 Inelastic Scattering 129
where the first term in parentheses is the Rutherford cross section for scattering
from a single free electron, obtained by setting Z = 1inEq.(3.3). The second term
(k
1
/k
0
) is very close to unity when the energy loss is much less than the incident
energy. The final term in Eq. (3.22), known as an inelastic form factor or dynamical
structure factor, is the square of the absolute value of a transition matrix element
defined by
ε
n
=
ψ
∗
n
j
exp(iq ·r
j
)ψ
0
dτ =
ψ
n
|
j
exp(iq ·r
j
)|ψ
0
(3.23)
Like the elastic form factor of Eq. (3.2), |ε
n
(
q
)
|
2
is a dimensionless factor that
modifies the Rutherford scattering that would take place if the atomic electrons were
free; it is a property of the target atom and is independent of the incident electron
velocity.
A closely related quantity is the generalized oscillator strength (GOS), given by
(Inokuti, 1971)
f
n
(q) =
E
n
R
|
ε
n
(q)
|
2
(qa
0
)
2
(3.24)
where R = (m
0
e
4
/2)(4πε
0
)
−2
=
2
/(2m
0
a
2
) = 13.6 eV is the Rydberg energy
and E
n
is the energy change associated with the transition. The differential cross
section can therefore be written in the form
dσ
n
d
=
4γ
2
R
E
n
q
2
k
1
k
0
f
n
(q) (3.25)
where (k
1
/k
0
) ≈ 1 −2E
n
(m
0
v
2
)
−1
can usually be taken as unity. In the limit q → 0,
the generalized oscillator strength f
n
(q) reduces to the dipole oscillator strength f
n
that characterizes the response of an atom to incident photons (optical absorption).
In many cases (e.g., ionizing transitions to a “continuum” of states) the energy-
loss spectrum is a continuous rather than discrete function of the energy loss E,
making it more convenient to define a GOS per unit excitation energy (i.e., per unit
energy loss): df
(
q, E
)
/dE. The angular and energy dependence of scattering are
then specified by a double-differential cross section:
d
2
σ
ddE
=
4γ
2
R
Eq
2
k
1
k
0
df
dE
(q, E) (3.26)
To obtain explicitly the angular distribution of inelastic scattering, the scattering
vector q must be related to the scattering angle θ.Forθ 1 rad and E E
0
, where
E
0
is the incident beam energy, it is a good approximation to take the components
of q as k
0
θ and k
0
θ
E
(see p. 192, Fig. 3.39) and to write
q
2
≈ k
2
0
(θ
2
+θ
2
E
) (3.27)
130 3 Physics of Electron Scattering
where the characteristic angle θ
E
is defined as
θ
E
=
E
γ m
0
v
2
=
E
(E
0
+m
0
c
2
)(v/c)
2
(3.28)
and v is the speed of the incident electron. The factor γ = m/m
0
takes account of the
relativistic increase in mass of the incident electron. A nonrelativistic approximation
is θ
E
= E/(2E
0
), which gives characteristic angles 8, 14, and 18% too low at E
0
=
100, 200, and 300 keV, respectively. Equation (3.26) can now be written as
d
2
σ
ddE
≈
4γ
2
R
Ek
2
0
1
θ
2
+θ
2
E
df
dE
=
8a
2
0
R
2
Em
0
v
2
1
θ
2
+θ
2
E
df
dE
(3.29)
At low scattering angles, the main angular dependence in Eq. (3.29) comes from the
Lorentzian (θ
2
+θ
E
2
)
−1
factor. The importance of the characteristic angle θ
E
is that
it represents the half-width at half maximum (HWHM) of this Lorentzian function.
The regime of small scattering angle and relatively low energy loss, where df/dE is
almost constant (independent of q and θ), is known as the dipole region.
For typical TEM incident energies (e.g., E
0
= 100 keV), the width of the
inelastic angular distribution is quite small (FWHM = 2θ
E
∼ 0.1 for outer-shell
excitation, typically a few milliradians for inner-shell excitation) and considerably
less than the angular width of elastic scattering; see Fig. 3.5. Consequently, inelas-
tic scattering broadens only slightly the diffraction spots or rings in the diffraction
pattern recorded from a crystalline specimen. However, the Lorentzian function has
long tails and half of the outer-shell excitation corresponds to angles greater than
about 10 θ
E
(see Fig. 3.15), so inelastic scattering arising from electronic excitation
can contribute substantially to the background of an electron-diffraction pattern.
3.2.3 Dielectric Formulation
The equations given in Section 3.2.2 are most readily applied to single atoms or to
gaseous targets, in the sense that the required generalized oscillator s trength can be
calculated (as a function of q and E) using an atomic model. Even so, Bethe theory
is useful for describing the inelastic scattering that takes place in a solid, particu-
larly from inner atomic shells. Outer-shell scattering is complicated by the fact that
the valence-electron wavefunctions are modified by chemical bonding. In addition,
collective effects are important, involving many atoms. An alternative approach is
to describe the interaction of a transmitted electron with the entire solid in terms of
a dielectric response function ε(q, ω). Although the latter can be calculated from
first principles in only a few idealized cases, the same response function describes
the interaction of photons with a solid, so this formalism allows energy-loss data to
be compared with the results of optical measurements.
Ritchie (1957) derived an expression for the electron scattering power of an
infinite medium. The transmitted electron, having coordinate r and moving with a
3.2 Inelastic Scattering 131
velocity v in the z-direction, is represented as a point charge −eδ(r −vt) that gener-
ates within the medium a spatially dependent, time-dependent electrostatic potential
φ(r, t) satisfying Poisson’s equation:
ε
0
ε(q, ω)∇
2
φ(r, t) = eδ(r, t)
The stopping power (dE/dz) is equal to the backward force on the transmitted elec-
tron in the direction of motion and is also the electronic charge multiplied by the
potential gradient in the z-direction. Using Fourier transforms, Ritchie showed that
dE
dz
=
2
2
πa
0
m
0
v
2
q
y
ω Im[−1/ε(q, ω)]
q
2
y
+(ω/v)
2
dq
y
dω (3.30)
where the angular frequency ω is equivalent to E/ and q
y
is the component
of the scattering vector in a direction perpendicular to v. The imaginary part of
[−1/ε(q, ω)] is known as the energy-loss function and provides a complete descrip-
tion of the response of the medium through which the fast electron is traveling. The
stopping power can be related to the double-differential cross section (per atom) for
inelastic scattering by
dE
dz
=
n
a
E
d
2
σ
ddE
ddE (3.31)
where n
a
represents the number of atoms per unit volume of the medium. For small
scattering angles, dq
y
≈ k
0
θ and d ≈ 2πθdθ ,soEqs.(3.30) and (3.31)give
d
2
σ
ddE
≈
Im[−1/ε(q, E)]
π
2
a
0
m
0
v
2
n
a
1
θ
2
+θ
2
E
(3.32)
where θ
E
= E/(γ m
0
v
2
) is the characteristic angle, as before. Equation (3.32)
contains the same Lorentzian angular dependence and the same v
−2
factor as the cor-
responding Bethe equation, Eq. (3.29). Comparison of these two equations indicates
that the Bethe and dielectric expressions are equivalent if
df
dE
(q, E) =
2E
πE
2
a
Im
−1
ε(q, E)
(3.33)
where E
2
a
=
2
n
a
e
2
/(ε
0
m
0
), E
a
being a “plasmon energy” corresponding to one free
electron per atom (see Section 3.3.1).
In the small-angle dipole region, ε(q, E) varies little with q and can be replaced
by the optical value ε(0, E), which is the relative permittivity of the specimen at an
angular frequency ω = E/. An energy-loss spectrum that has been recorded using
a reasonably s mall collection angle can therefore be compared directly with opti-
cal data. Such a comparison involves a Kramers–Kronig transformation to obtain
Re[1/ε(0, E)], leading to the energy dependence of the real and imaginary parts

132 3 Physics of Electron Scattering
(ε
1
and ε
2
)ofε(0, E), as discussed in Section 4.2. At large energy loss, ε
2
is small
and ε
1
close to 1, so that Im(−1/ε) = ε
2
/(ε
2
1
+ε
2
2
)
1/2
becomes proportional to
ε
2
and (apart from a factor of E
−3
) the energy-loss spectrum is proportional to the
x-ray absorption spectrum.
The optical permittivity is a transverse property of the medium, in the sense
that the electric field of an electromagnetic wave displaces electrons in a direc-
tion perpendicular to the direction of propagation, the electron density remaining
unchanged. On the other hand, an incident electron produces a longitudinal dis-
placement and a local variation of electron density. The transverse and longitudinal
dielectric functions are precisely equal only in the random-phase approximation
(see Section 3.3.1) or at sufficiently small q (Nozieres and Pines, 1959); neverthe-
less, there is no evidence for a significant difference between them, as indicated
by the close similarity of Im( −1/ε) obtained from both optical and energy-loss
measurements on a variety of materials (Daniels et al., 1970).
3.2.4 Solid-State Effects
If Bethe theory is applied to inelastic scattering in a solid specimen, one might
expect the generalized oscillator strength (GOS) to differ from that calculated for
a single atom, due to the effect of chemical bonding on the wavefunctions and the
existence of collective excitations (Pines, 1963). These effects change mainly the
energy dependence of df/dE and of the scattered intensity; the angular dependence
of inelastic scattering remains Lorentzian (with half-width θ
E
), at least for small E
and small θ.
Likewise, changes in the total inelastic cross section σ
i
are limited to a mod-
est factor (generally ≤3) because the GOS is constrained by the Bethe f-sum rule
(Bethe, 1930):
df
dE
dE = z (3.34)
The integral in Eq. (3.34) is over all energy loss E (at constant q) and should be
taken to include a sum over excitations to “discrete” final states, which in many
atoms make a substantial contribution to the total cross section. If the sum rule is
applied to a whole atom, z is equal to the total number Z of atomic electrons and
Eq. (3.34) is exact. If applied to a single atomic shell, z can be taken as the number
of electrons in that shell, but the rule is only approximate, since the summation
should include a (usually small) negative contribution from “downward” transitions
to shells of higher binding energy, which are in practice forbidden by the Pauli
exclusion principle (Pines, 1963; Schnatterly, 1979).
Using Eq. (3.33), the Bethe sum rule can also be expressed in terms of the energy-
loss function:
3.2 Inelastic Scattering 133
Im
−1
ε(E)
EdE=
π
2
zn
a
e
2
2ε
0
m
0
=
π
2
E
2
p
(3.35)
where E
p
= (ne
2
/ε
0
m
0
)
1/2
is a “plasmon energy” corresponding to the number of
electrons, n per unit volume, that contribute to inelastic scattering within the range
of integration.
According to Eq. (3.29), the differential cross section (for θ θ
E
) is propor-
tional to E
−1
df /dE, and df/dE is constrained by Eq. (3.34); therefore, the cross
section σ
i
(integrated over all energy loss and scattering angle) must decrease if
contributions to the oscillator strength shift toward higher energy loss. This upward
shift applies to most solids because the collective excitation of valence electrons
generally involves energy losses that are higher than the average energy of atomic
valence-shell transitions. As a rough estimate of the latter, one might consider the
first ionization energy (Inokuti, personal communication); most often, the mea-
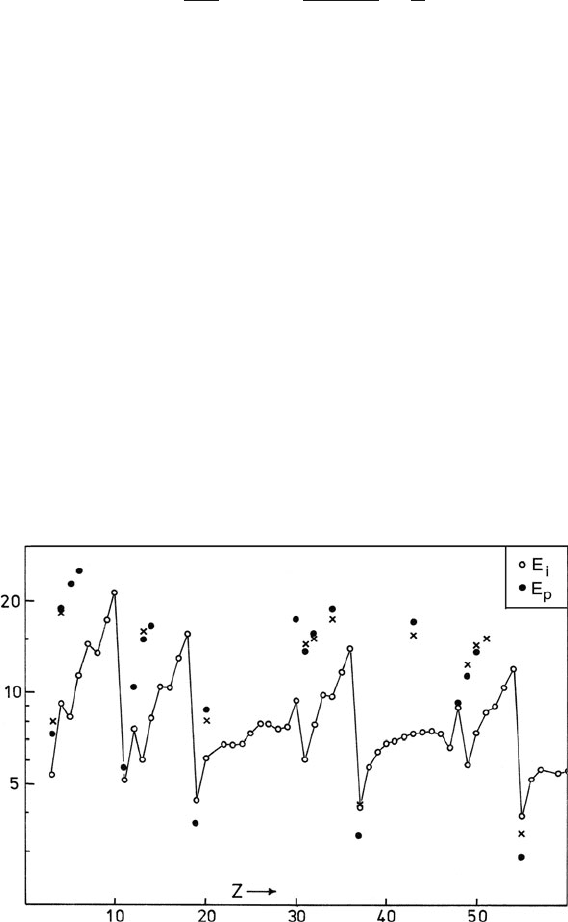
sured valence-peak energy loss is above this value, as shown in Fig. 3.10,sothe
valence-electron contribution to σ
i
is reduced when atoms form a solid. The result-
ing decrease in σ
i
should be more marked for light elements where the valence shell
accounts for a larger fraction of the atomic electrons and therefore makes a larger
percentage contribution to the cross section (Inokuti et al., 1981). A fairly extreme
example is aluminum, where the inelastic cross section per atom is a factor of about
3 lower in the solid, in rough agreement with the ratio of the plasmon energy (15 eV)
and the atomic ionization threshold (6 eV).
Fig. 3.10 First ionization energy E
i
(open circles) and measured plasmon energy E
p
(filled circles)
as a function of atomic number. Where E
p
> E
i
, an atomic model is expected to overestimate the
total amount of inelastic scattering. Crosses represent the plasmon energy calculated on a free-
electron model with m = m
0

134 3 Physics of Electron Scattering
Some solid compounds show less pronounced collective effects and the inelastic
scattering from valence electrons retains much of its atomic character. As a rough
approximation, each atom then makes an independent contribution to the scattering
cross section. The effect of chemical bonding is to remove valence electrons from
electropositive atoms (e.g., Na and Ca) and reduce their scattering power, whereas
electronegative atoms (O, Cl, etc.) have their electron complement and scattering
power increased. On this basis, the periodic component of the Z-dependence of σ
i
(Fig. 3.9), which is related to the occupancy of the outermost atomic shell, should be
less in the case of solids. In any event, what is measured experimentally is the sum of
the scattering from all atoms (anions and cations); if the reductions and increases in
scattering power are equal in magnitude, the total scattering power is simply the sum
of the scattering powers calculated on an atomic model. This additivity principle
(when applied to the stopping power) is known as Bragg’s rule and is believed to
hold t o within ≈5% accuracy (Zeiss et al., 1977) except for any contribution from
hydrogen, which is usually small anyway. It provides some justification for the use
of atomic cross sections to calculate the stopping power and range of electrons in
solids ( Berger and Seltzer, 1982).
Fano (1960) suggested that the extent of collective effects depends on the value
of the dimensionless parameter:
u
F
(E) =
2
ne
2
ε
0
m
0
df
d(E
2
)
=
2
ne
2
2ε
0
m
0
1
E
df
dE
(3.36)
where n is the number of electrons per unit volume (with binding energies less
than E) that can contribute to the scattering at an energy E. Collective effects can
be neglected if u
F
1, but are of importance where u
F
approaches or exceeds 1
within a particular region of the loss spectrum (Inokuti, 1979). Comparison with Eq.
(3.33) shows that u
F
= Im[−1/ε(E)]/π , so the criterion for neglecting collective
excitations becomes
Im[−1/ε(E )] π (3.37)
Equation (3.37) provides a convenient criterion for assessing the importance of
collective effects, since any energy-loss spectrum that has been measured up to a
sufficiently high energy loss can be normalized, using Eq. (3.35)or(4.27), to give
the energy-loss function Im[−1/ε], as described in Section 4.2. A survey of experi-
mental data indicates that Im[−1/ε] rises to about 30 in Al, 3 to 4 for InSb, GaAs,
and GaSb (materials that support well-defined plasma oscillations), reaches 2.2 in
diamond, and does not rise much above 1 in the case of Cu, Ag, Pd, and Au, where
plasma oscillations are strongly damped (Daniels et al., 1970).
Organic solids are similar in the sense that their energy-loss function generally
reaches values close to 1 for energy losses around 20 eV (Isaacson, 1972a), imply-
ing that both atomic transitions and collective effects contribute to their low-loss
spectra. Aromatic compounds and those containing C = C double bonds also show
a sharp peak around 6–7 eV, sometimes interpreted as a plasmon resonance of the π
3.3 Excitation of Outer-Shell Electrons 135
electrons. However, vapor-phase aromatic hydrocarbons give a similar peak, which
must therefore be interpreted in terms of π − π
∗
single-electron transitions (Koch
and Otto, 1969).
Ehrenreich and Philipp (1962) proposed more definitive criteria for the occur-
rence of collective effects (plasma resonance), based on the energy dependence of
the real and imaginary parts ( ε
1
and ε
2
) of the permittivity. According to these cri-
teria, liquids such as glycerol and water, as well as solids such as aluminum, silver,
silicon, and diamond, all respond in a way that is at least partly collective (Ritchie
et al., 1989).
3.3 Excitation of Outer-Shell Electrons
Most of the inelastic collisions of a fast electron arise from interaction with elec-
trons in outer atomic shells and result in an energy loss of less than 100 eV. In a
solid, the major contribution comes from valence electrons (referred to as conduc-
tion electrons in a metal), although in some materials (e.g., transition metals and
their compounds) underlying shells of low binding energy contribute appreciable
intensity in the 0–100 eV range. We begin this section by considering plasmon exci-
tation, an important process in most solids and one that exhibits several features not
predicted by atomic models.
3.3.1 Volume Plasmons
The valence electrons in a solid can be thought of as a set of coupled oscilla-
tors that interact with each other and with a transmitted electron via electrostatic
forces. In the simplest situation, the valence electrons behave almost as free parti-
cles (although constrained by Fermi–Dirac statistics) and constitute a “free-electron
gas,” also known as a “Fermi sea” or “jellium.” Interaction with the ion-core lattice
is assumed to be a minor perturbation that can be incorporated phenomenologically
by using an effective mass m for the electrons, rather than their rest mass m
0
, and by
introducing a damping constant or its reciprocal τ, as in the Drude theory of elec-
trical conduction in metals. The behavior of the electron gas is described in terms of
a dielectric function, just as in Drude theory. In response to an applied electric field,
such as that produced by a transmitted charged particle, a collective oscillation of
the electron density occurs at a characteristic angular frequency ω
p
and this resonant
motion would be self-sustaining if there were no damping from the atomic lattice.
3.3.1.1 Drude Model
The displacement x of a “quasi-free” electron (mass m) due to a local electric field
E must satisfy the equation of motion:

136 3 Physics of Electron Scattering
m
¨
x +m
˙
x =−eE (3.38)
For an oscillatory field: E = E exp(−iωt), Eq. (3.38) has a solution:
x = (eE/m)(ω
2
+iω)
−1
(3.39)
The displacement x gives rise to a polarization P =−enx = ε
0
χE, where n is
the number of electrons per unit volume and χ is the electronic susceptibility, and
Eq. (3.39) leads to
χ =
−enx
ε
0
m
=
−ne
2
ε
0
m
1
ω(ω +i)
=−ω
2
p
1
ω
2
+
2
−
i/ω
ω
2
+
2
(3.39a)
The relative permittivity or dielectric function ε(ω) is then
ε(ω) = ε
1
+iε
2
= 1 +χ = 1 −
ω
2
p
ω
2
+
2
+
i ω
2
p
ω (ω
2
+
2
)
(3.40)
Here ω is the angular frequency (rad/s) of forced oscillation and ω
p
is the natural or
resonance frequency for plasma oscillation, given by
ω
p
= [ne
2
/(ε
0
m)]
1/2
(3.41)
A transmitted electron represents a sudden impulse of applied electric field, contain-
ing all angular frequencies (Fourier components). Setting up a plasma oscillation
of the loosely bound outer-shell electrons in a solid is equivalent to creating a
pseudoparticle of energy E
p
= ω
p
, known as a plasmon (Pines, 1963).
Taking m = m
0
and writing electron density as n = zρ/(uA) where z is the
number of free (valence) electrons per atom, u is the atomic mass unit, A represents
atomic weight, and ρ is the specific gravity (density in g/cm
3
)ofthesolid,the
free-electron plasmon energy is conveniently evaluated as
E
p
= (28.82 eV) (zρ/A)
1/2
(3.41a)
For a compound, A becomes the molecular weight and z the number of valence
electrons per molecule.
In this free-electron approximation, the energy-loss function is given by
Im
−1
ε(ω)
=
ε
2
ε
2
1
+ε
2
2
=
ωω
2
p
(ω
2
−ω
2
p
)
2
+(ω)
2
(3.42)
AsshownbyEq.(3.32), Im(−1/ε) represents the energy dependence of the inelastic
intensity at an energy loss E = ω (the energy-loss spectrum), so Eq. (3.42) can be
written as
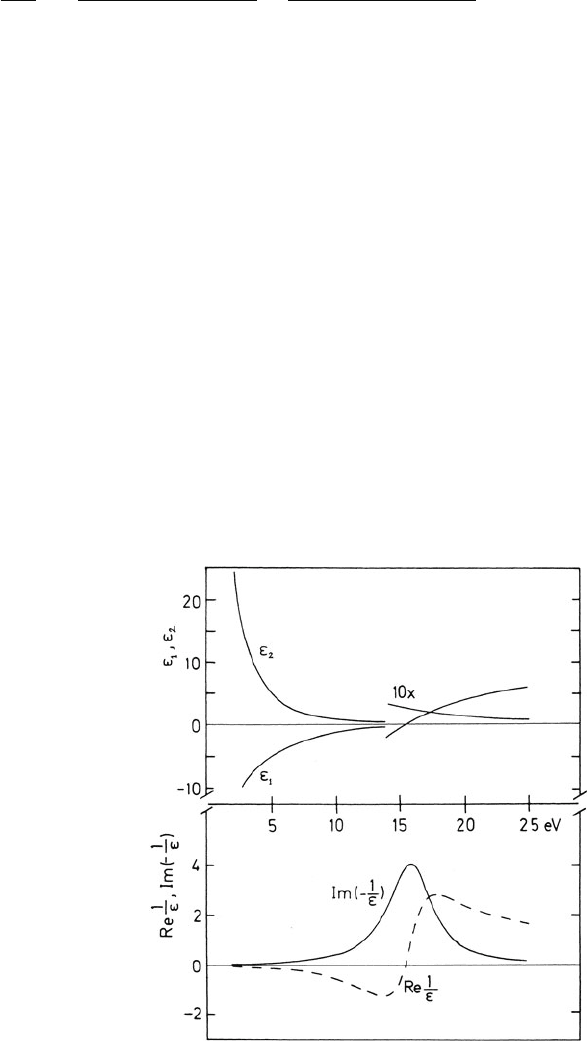
3.3 Excitation of Outer-Shell Electrons 137
Im
−1
ε(E)
=
E
2
p
(E/τ )
(E
2
−E
2
p
)
2
+(E/τ )
2
=
E(E
p
)E
2
p
(E
2
−E
2
p
)
2
+(E E
p
)
2
(3.43)
where E
p
is the plasmon energy and τ = 1/ is a relaxation time. The energy-loss
function Im(−1/ε) has a full width at half-maximum (FWHM) given by E
p
=
= /τ and reaches a maximum value of ω
p
τ at an energy loss given by
E
max
= [E
2
p
−(E
2
p
/2)]
1/2
(3.43a)
For a material such as aluminum, where the plasmon resonance is sharp (E
p
=
0.5 eV), the maximum is within 0.002 eV of E
p
, but for the broad resonance found
in carbon, Eq. (3.43a)impliesthatE
max
is shifted to lower energy by about 2.1 eV.
From Eq. (3.40), the energy at which ε
1
(E) passes through zero with positive slope
(see Fig. 3.11)is
E(ε
1
= 0) = [E
2
p
−(E
p
)
2
]
1/2
(3.43b)
This zero crossing is sometimes taken as evidence of a well-defined collective
response in the solid under investigation.
Although based on a simplified model, Eq. (3.43) corresponds well with the
observed line shape of the valence-loss peak in materials with sharp plasmon peaks,
Fig. 3.11 Real and
imaginary parts of the relative
permittivity and the
energy-loss function
Im(−1/ε), calculated using a
free-electron (jellium) model
with E
p
= 15 eV and
E
p
= 4 eV (Raether, 1980),
copyright Springer-Ve rlag
