Egerton R.F. Electron Energy-Loss Spectroscopy in the Electron Microscope
Подождите немного. Документ загружается.


108 2 Energy-Loss Instrumentation
solution is to increase the probe diameter as the magnification is lowered, although
this may still leave some of the specimen unirradiated because the probe is circular
and the pixels are square. An equally good solution, in the case of a digitally gener-
ated scan, is to use sub-pixel scanning: for an image having N×N pixels, waveforms
whose frequency is N times larger and amplitude N times smaller are added to the
line and frame scans. The very small probe is then scanned in a small square raster,
covering each pixel area, so that severe undersampling of the specimen is avoided.
2.6.6 Z-Contrast and Z-Ratio Imaging
The scanning-transmission electron microscope has the advantage of being able
to efficiently collect electrons that are scattered through large angles, by use of a
high-angle annular dark-field (HAADF) detector. This high-angle elastic scattering
approximates to Rutherford elastic scattering (Section 3.1.2), which ideally has a
Z
2
dependence on atomic number Z, although slightly lower exponents are com-
mon (Section 3.1.6). The STEM therefore provides Z-contrast images, useful for
imaging clusters of heavy atoms on a lower-Z substrate (e.g., catalyst s tudies) and
atomic columns in crystalline specimen (Pennycook and Jesson, 1991; Krivanek
et al., 2010). Channeling of the incident electrons limits the lateral spreading of
the beam in a crystalline specimen, allowing core-loss spectroscopy to identify the
atomic number of each column (Browning et al., 1993b, 1999; Pennycook et al.,
1995a; Varela et al., 2004). This spectroscopy can be precisely simultaneous with
the structural imaging because it makes use of electrons that pass through the central
hole of the HAADF detector, as in Fig. 2.35.
Z-ratio imaging refers to a technique first used by Crewe et al. (1975)todis-
play images of single atoms of Hg and U on a thin-carbon (<10 nm) support
film. The ADF signal I
d
was divided by the total-inelastic signal I
i
recorded
Fig. 2.35 Z-contrast imaging
in STEM mode. The inner
radius of the annular detector
subtends a semi-angle β at
the specimen, the outer radius
being considerably larger. An
electron spectrometer
separates the inelastic and
unscattered electrons
2.6 Energy-Selected Imaging (ESI) 109
through a spectrometer, using a wide energy-selecting slit that simply eliminated
the unscattered component I
u
; see Fig. 2.35. A single-channel detector, such as a
scintillator/PMT combination, is sufficient to measure I
i
. Because variations in mass
thickness of the support contribute almost equally to I
d
and I
i
, their unwanted effect
largely disappears in the image formed from the ratio R = I
d
/I
i
. Taking a ratio also
cancels any fluctuations in probe current but does involve some increase in noise,
particularly if the specimen is very thin.
Because of its relatively broad angular distribution (Section 3.2.1), elastic scatter-
ing provides the main contribution to I
d
, whereas the main contribution to I
i
comes
from electrons that suffer only inelastic scattering. If the specimen is amorphous
and very thin, the elastic and inelastic scattering are both proportional to specimen
thickness and to the appropriate atomic cross sections σ
e
and σ
i
. Assuming that all
elastic scattering is recorded by the dark-field detector and all inelastic scattering is
captured by the spectrometer, the ratio signal is
R = I
d
/I
i
= σ
e
/σ
i
= λ
i
/λ
e
(2.54)
where λ
e
and λ
i
are mean free paths for elastic and inelastic scattering. The inten-
sity in the ratio image is then proportional to the l ocal value of the elastic/inelastic
scattering ratio, which in theory and in practice (Section 3.2.1) is proportional to
atomic number Z.
In the case of specimens that are thicker than about λ
i
/2, the dark-field and
inelastic signals are given more accurately (Lamvik and Langmore, 1977; Egerton,
1982c)by
I
d
= I[1 −exp(−t/λ
e
)] (2.55)
I
i
= I exp(−t/λ
e
)[1 −exp(−t/λ
i
)] (2.56)
where I is the i ncident beam current and t is the local thickness of the specimen. The
exponential functions in Eqs. (2.55) and (2.56) occur because of plural scattering, as
a result of which neither I
i
nor I
d
is proportional to specimen thickness. For t/λ
i
< 1,
this nonlinearity can be removed by digital processing (Jeanguillaume et al., 1992).
For t/λ
i
>1,I
i
reaches a maximum and then decreases with increasing thickness,
as many of the inelastically scattered electrons also undergo elastic scattering and
therefore contribute to I
d
rather than to I
i
.
Z-ratio imaging has been used to increase the contrast of thin (30-nm) sections
of stained and unstained tissues (Carlemalm and Kellenberger, 1982; Reichelt et al.,
1984). If the sample is crystalline and the ADF detector accepts medium-angle scat-
tering, both the elastic and inelastic images are strongly influenced by diffraction
contrast, which may increase rather than cancel when the ratio is taken (Donald and
Craven, 1979).
Chapter 3
Physics of Electron Scattering
It is convenient to divide the scattering of fast electrons into elastic and inelastic
components that can be distinguished on an empirical basis, the term elastic mean-
ing that any energy loss to the sample is not detectable experimentally. This criterion
results in electron scattering by phonon excitation being classified as elastic (or
quasielastic) when measurements are made using an electron microscope, where
the energy resolution is rarely better than 0.1 eV. The terms nuclear (for elastic scat-
tering) and electronic (for inelastic scattering) would be more logical but are not
widely used.
3.1 Elastic Scattering
Elastic scattering is caused by interaction of incident electrons with the electrostatic
field of atomic nuclei. The atomic electrons are involved only to the extent that
they terminate the nuclear field and therefore determine its range and magnitude.
Because a nucleus is some thousands of times more massive than an electron, the
energy transfer involved in elastic scattering is usually negligible. However, for the
small fraction of electrons that are scattered through large angles (up to 180
◦
), the
transfer can amount to some tens of electron volts, as evidenced by the occurrence
of displacement damage and electron-induced sputtering at high incident energies
(Jenkins and Kirk, 2001).
Although not studied directly by electron energy-loss spectroscopy, elastic
scattering is relevant for the following reasons:
1. Electrons can undergo both elastic and inelastic interactions within the sample,
so elastic scattering modifies the angular distribution of the inelastically scattered
electrons.
2. In a crystalline material, elastic scattering can redistribute the electron flux
(current density) within each unit cell and change the probability of localized
inelastic scattering (see Section 3.1.4).
3. The ratio of elastic and inelastic scattering can provide an estimate of the local
atomic number or chemical composition of a specimen (Sections 2.6.6 and
5.4.1).
111
R.F. Egerton, Electron Energy-Loss Spectroscopy in the Electron Microscope,
DOI 10.1007/978-1-4419-9583-4_3,
C
Springer Science+Business Media, LLC 2011

112 3 Physics of Electron Scattering
3.1.1 General Formulas
A quantity of basic importance in scattering theory is the differential cross section
dσ/d, representing the probability of an incident electron being scattered (per unit
solid angle ) by a given atom. For elastic scattering, one can write
dσ/d =
|
f
|
2
(3.1)
where f is a (complex) scattering amplitude or atomic scattering factor, which is
a function of the scattering angle θ or scattering vector q. The phase component
of f is important in high-resolution phase-contrast microscopy (Spence, 2009)but
for the calculation of scattered intensity only the amplitude is required, as implied
in Eq. (3.1). Within the first Born approximation (equivalent to assuming only sin-
gle scattering within each atom), f is proportional to the three-dimensional Fourier
transform of the atomic potential V(r).
Alternatively, the differential cross section can be expressed in terms of an elastic
form factor F(q):
dσ
d
=
4
a
2
0
q
4
|
F(q)
|
2
=
4
a
2
0
q
4
|
Z −f
x
(q)
|
2
(3.2)
Here a
0
= 4πε
0
2
/m
0
e
2
= 0.529 × 10
−10
m is the first Bohr radius and γ =
(1 −v
2
/c
2
)
−1/2
is the relativistic factor tabulated in Appendix E; f
x
(q) is the atomic
scattering factor (or form factor) for an incident x-ray photon and is equal to the
Fourier transform of the electron density within the atom. The atomic number Z in
Eq. (3.2) represents the nuclear charge and denotes the fact that incident electrons
are scattered by the entire electrostatic field of an atom, whereas x-rays interact
mainly with the atomic electrons.
3.1.2 Atomic Models
The earliest and simplest model for elastic scattering of charged particles is based
on the unscreened electrostatic field of a nucleus and was first used by Rutherford
to account for the scattering of α-particles (Geiger and Marsden, 1909). While an
α-particle is repelled by the nucleus, an incident electron is attracted, but in either
case, classical mechanics indicates that the trajectories are hyperbolic (Fig. 3.1).
Both classical and wave-mechanical theory leads to the same expression for the
differential cross section, which is obtained by setting the electronic term f
x
(q)to
zero in Eq. (3.2), giving
dσ/d = 4γ
2
Z
2
/a
2
0
q
4
(3.3)
Here q is the magnitude of the scattering vector and is given by q = 2k
0
sin(θ/2),
as illustrated in Fig. 3.2; k
0
= γ m
0
v is the momentum of the incident electron;
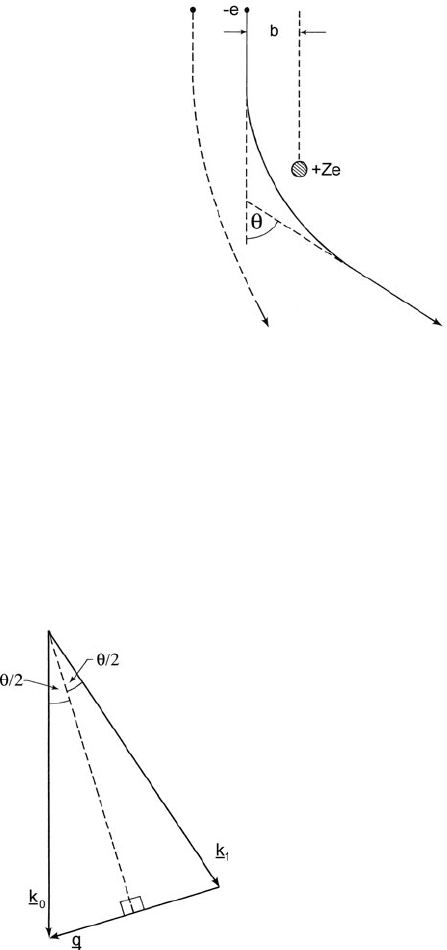
3.1 Elastic Scattering 113
Fig. 3.1 Rutherford
scattering of an electron by
the electrostatic field of an
atomic nucleus, viewed from
a classical (particle)
perspective. Each value of
impact parameter b gives rise
to a different scattering angle
θ,andasb increases, θ
decreases because the
electron experiences a weaker
electrostatic attraction. For
small θ, dσ /dΩ is
proportional to θ
–4
and q is the momentum transferred to the nucleus. For lighter elements, Eq. (3.3)
is a reasonable approximation at large scattering angles (see Fig. 4.24) and is use-
ful for estimating rates of backscattering (θ>π/2) in solids (Reimer, 1989). But
since no allowance has been made for screening of the nuclear field by the atomic
electrons, the Rutherford model greatly overestimates the elastic scattering at small
θ (corresponding to larger impact parameter b) and gives an infinite cross section if
integrated over all angles.
Fig. 3.2 Vector diagram for elastic scattering, where k
0
and k
1
are the wavevectors of the fast
electron before and after scattering. From geometry of the right-angled triangles, the magnitude of
the scattering vector is q = 2k
0
sin(θ/2). The direction of q has been chosen to represent momen-
tum transfer to the specimen (opposite to the wavevector change of the fast electron), as normally
required in equations that deal with the effects of elastic and inelastic scattering
114 3 Physics of Electron Scattering
The simplest way of incorporating screening is through a Wentzel (or Yukawa)
formula, in which the nuclear potential is attenuated exponentially as a function of
distance r from the nucleus:
φ(r) = [Ze/(4πε
0
r)] exp(−r/r
0
) (3.4)
where r
0
is a screening radius. Equation (3.4) leads to the angular distribution:
dσ
d
=
4γ
2
a
2
0
Z
q
2
+r
−2
0
2
≈
4γ
2
Z
2
a
2
0
k
4
0
1
(θ
2
+θ
2
0
)
2
(3.5)
where θ
0
= (k
0
r
0
)
−1
is a characteristic angle of elastic scattering. The angular
dependence for a mercury atom is shown in Fig. 3.3, and for a carbon atom in
Fig. 3.5. The fraction of elastic scattering that lies within the angular range 0 < θ < β
is (for β 1 rad)
σ
e
(β)
σ
e
=
1
1 +[2k
0
r
0
sin(β/2)]
−2
≈
1 +
θ
2
0
β
2
−1
(3.6)
Following Wentzel (1927) and Lenz (1954), an estimate of r
0
can be obtained
from the Thomas–Fermi statistical model, treating the atom as a free-electron gas,
namely
r
0
= a
0
Z
−1/3
(3.7)
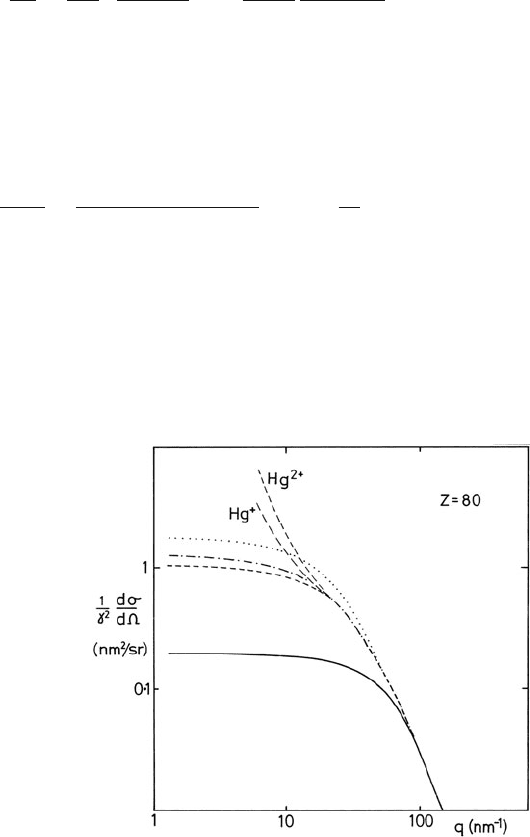
Fig. 3.3 Angular
dependence of the differential
cross section for elastic
scattering of 30-keV electrons
from a mercury atom,
calculated using the Lenz
model with a Wentzel
potential (solid curve) and on
the basis of Hartree–Fock
(dotted curve), Hartree–Slater
(chained curve), and
Dirac–Slater (dashed curve)
wavefunctions. Dirac–Slater
results are also shown for a
single- and double-ionized
atom. From Langmore et al.
(1973), copyright Springer

3.1 Elastic Scattering 115
Integrating Eq. (3.5) over all scattering angles gives
σ
e
=
π
0
dσ
d
2π sin θ dθ =
4πγ
2
k
2
0
Z
4/3
= (1.87 ×10
−24
m
2
)Z
4/3
(v/c)
−2
(3.8)
where v is the velocity of the incident electron and c is the speed of light in vacuum.
For an element of low atomic number, Eq. (3.8) gives cross sections that are accurate
to about 10%, as confirmed by measurement on gases (Geiger, 1964). For a heavy
element such as mercury, the Lenz model underestimates small-angle scattering by
an order of magnitude (see Fig. 3.3), due largely to t he neglect of electron exchange;
for 100-keV electrons Eq. (3.8) gives only about 60% of the value obtained from
more sophisticated calculations (Langmore et al., 1973). Some authors use a coef-
ficient of 0.885 in Eq. (3.7)ortaker
0
= 0.9a
0
Z
−1/4
. However, the main virtue of
the Lenz model is that it provides a rapid estimate of the angular dependence of
scattering, as in the L
ENZPLUS program described in Appendix B.
More accurate cross sections are achieved by calculating the atomic potential
from an iterative solution of the Schrödinger equation, as in the Hartree–Fock
and Hartree–Slater methods (Ibers and Vainstein, 1962; Hanson et al., 1964).
Alternatively, electron spin and relativistic effects within the atom can be included
by using the Dirac equation (Cromer and Waber, 1965), leading to the so-called
Mott cross sections. Partial wave methods can be used to avoid the Born approxi-
mation (Rez, 1984), which fails if Z approaches or exceeds 137 (v/c), in other words
for heavy elements or low incident energies.
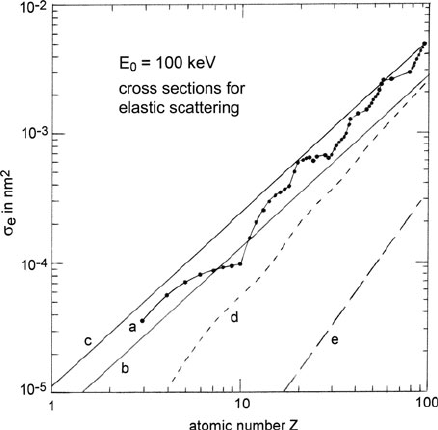
Langmore et al. (1973) proposed the following equation for estimating the total
elastic cross section of an atom of atomic number Z:
σ
e
=
(1.5 ×10
−24
m
2
)Z
3/2
(v/c)
2
1 −
Z
596(v/c)
(3.9)
The coefficient and Z-exponent are based on Hartree–Slater calculations; the term
in brackets represents a correction to the Born approximation. The accuracy of
Eq. (3.9) is limited to about 30% because the graph of σ
e
against Z is in reality
not a smooth curve but displays irregularities that reflect the outer-shell structure of
each atom; see Fig. 3.4. A compilation of elastic cross sections (dσ/d and σ
e
)is
given by Riley et al. (1975), based on relativistic Hartree–Fock wavefunctions.
For an ionized atom, the atomic potential remains partially unscreened at large r,
so dσ/d continues to increase with increasing impact parameter (decreasing θ );
see Fig. 3.3. As a result, the amount of elastic scattering can appreciably exceed that
from a neutral atom, particularly in the range of low scattering angles (Anstis et al.,
1973; Fujiyoshi et al., 1982).
The scattering theory just described is based on the properties of a single isolated
atom. In a molecule, the cross section per atom is reduced at low scattering angles,
116 3 Physics of Electron Scattering
Fig. 3.4 Cross section σ
e
for elastic scattering of 100-keV electrons, calculated by Humphreys
et al. (1974). Curve a shows individual data points derived from Doyle–Turner scattering factors,
based on Hartree–Fock wavefunctions. Lines b and c represent the Lenz model, with and without
a multiplying factor of 1.8 applied to Eq. (3.8). Curves d and e give cross sections [σ
e
− σ
e
(β)]
for elastic scattering above an angle β of 24 and 150 mrad, respectively. Note that the atomic-shell
periodicity in the Z-dependence disappears as β becomes large and the scattering approximates
to Rutherford collision with small impact parameter, where screening by outer-shell electrons is
unimportant
typically by 10–20%, as a result of chemical bonding (Fink and Kessler, 1967). In
a crystalline solid, the angular dependence of elastic scattering is changed dramat-
ically by diffraction effects but in an amorphous solid, diffraction i s weak and an
atomic model can be used as a guide to the magnitude and angular distribution of
elastic scattering. As an alternative to describing the amount of scattering in terms of
a cross section (σ
e
per atom), one can use an inverse measure: λ
e
= (σ
e
n
a
)
−1
, where
n
a
is the number of atoms per unit volume of the specimen. In an amorphous mate-
rial at least, the mean free path λ
e
can be thought of as the mean distance between
elastic collisions.
3.1.3 Diffraction Effects
In a crystalline material, the regularity of the atomic arrangement requires that the
phase difference between waves scattered from each atom be taken into account by
introducing a structure factor F(θ) defined by
3.1 Elastic Scattering 117
F(θ) =
j
f
j
(θ)exp(−iq ·r
j
) (3.10)
Here, r
j
and f
j
are the coordinate and scattering amplitude of atom j, with q·r
j
the
associated phase factor; the sum is carried out over all atoms (j = 1, 2, etc.) in the
unit cell. Equation (3.10) can also be expressed in the form
F(θ) ∝
V(r)exp(− q ·r) dτ (3.11)
where V(r) is the scattering potential and the integration is carried out over all vol-
ume elements within the unit cell. Equation (3.11) indicates that the structure factor
is related to the Fourier transform of the lattice potential.
The intensity scattered in a direction θ relative to the incident beam is |F(θ)|
2
and peaks at values of θ for which the scattered waves are in phase with one
another. Each diffraction maximum (Bragg beam) can also be regarded as repre-
senting “reflection” from atomic planes, whose spacing d depends on the Miller
indices and unit-cell dimensions. Bragg reflection occurs when the angle between
the incident beam and the diffracting planes coincides with a Bragg angle θ
B
defined by
λ = 2d sin θ
B
(3.12)
where λ = 2π/k
0
is the incident electron wavelength. The scattering angle θ is
twice that of θ
B
,soEq.(3.12) is equivalent (for small θ
B
, large v)toλ = θd.For
100-keV incident electrons, λ = 3.7 pm and the scattering angles corresponding to
Bragg reflection exceed 10 mrad in simple materials. Larger values of θ
B
correspond
to reflection from planes of smaller separation or to higher order reflections whose
phase difference is a multiple of 2π.
The Bragg-reflected beams can be recorded by a two-dimensional detector such
as a CCD array. For a single-crystal specimen, the diffraction pattern consists of an
array of sharp spots whose symmetry and spacing are closely related to the crys-
tal symmetry and lattice constants. In the case of a polycrystalline sample whose
crystallite size is much less than the incident beam diameter, random rotational aver-
aging produces a diffraction pattern consisting of a series of concentric rings, rather
than a spot pattern.
The relative intensities of the lowest order Bragg beams (for the case of a thin
diamond specimen) are shown in Fig. 3.5 and are seen to follow the overall trend
predicted by a single-atom model. Distributions of inelastic scattering from the inner
(K-shell) and outer-shell (valence) electrons are also shown and are seen to be much
narrower in angular range. In fact, each Bragg beam generates inelastic scattering
within the specimen, so each Bragg spot in a diffraction pattern is surrounded by
a halo of inelastic scattering. This inelastic scattering can be removed by zero-loss
filtering of the diffraction pattern, which sharpens the Bragg spots and reduces the
background between them.
