Durst F. Fluid Mechanics: An Introduction to the Theory of Fluid Flows
Подождите немного. Документ загружается.

166 6 Hydrostatics and Aerostatics
6.2.2 Pressure-Measuring Instruments
The insights into the pressure distribution in containers gained in Sect. 6.2.1
were obtained through pressure relationships that were described for commu-
nicating systems. From the derived relationships for the pressure distribution
in the containers, information could be obtain for the location of the free
liquid surfaces. In return, it is possible, in the case that the established
fluid levels are known, to employ the general pressure relationships, indi-
cated in Sect. 6.1, in order to obtain information on the pressures occurring
in containers.
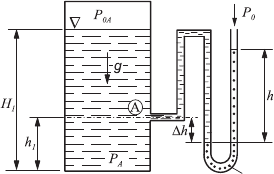
This is illustrated in Fig. 6.10, which shows a sketch for explaining the
basic principle according to which pressure measurements are carried out by
communicating systems.
To measure the pressure at point A in the container to which a “U-tube
manometer” is connected, the latter is filled with a measuring liquid (dotted
part of the U tube) and partly also with the liquid which enters into the U
tube from the container. The two liquids are assumed not to mix. For the
location of the separating plane between the two fluids, the following pressure
equilibrium holds:
p
A
+ ρ
A
g∆h = p
0
+ ρ
F
gh (6.47)
For the pressure to be measured at point A, it follows that:
p
A
= p
0
+ ρ
F
gh − ρ
A
g∆h (6.48)
Equation (6.48) makes it clear that it is possible to determine the pressure
at point A in the container by measurements of h and ∆h when the fluid
densities ρ
F
and ρ
A
are known. In Fig. 6.10, it was assumed that the pressure
in the container is high compared with the ambient pressure p
0
. When there
is a negative pressure in the container with respect to the outside pressure,
the conditions presented in Fig. 6.11 will exist for the fluid level in the U-tube
manometer.
Thus, for the pressure equilibrium at the parting surface of the two fluids,
the following relationship holds:
p
A
− ρ
A
g∆h = p
0
− ρ
F
gh (6.49)
Fig. 6.10 Diagram for explaining the ba-
sic principle of pressure measurements by
communicating systems
Measuring
Liquid
6.2 Connected Containers and Pressure-Measuring Instruments 167
Measuring
liquid
Fig. 6.11 Fluid columns in the U-tube manometer for “negative pressure” giving an
upward rise of the measuring liquid
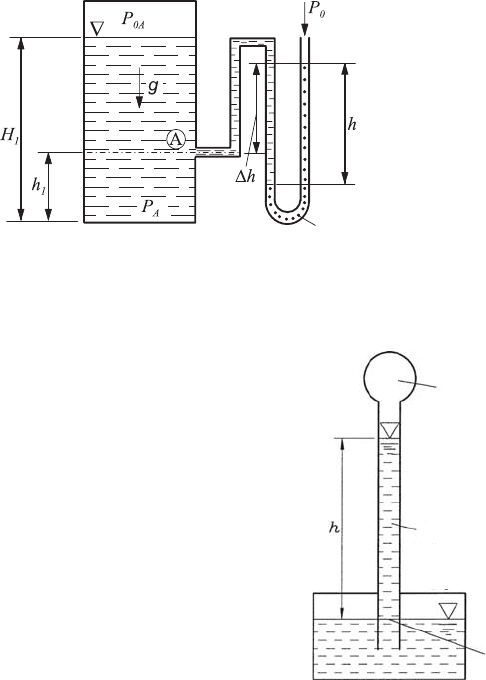
Fig. 6.12 Basic principle of barometric pressure
measurements
P
F
Vacuum
P
01
=0
Plain
A
P
0
For the pressure at point A one then obtains the following expression:
p
A
= p
0
− ρ
F
gh + ρ
A
g∆h (6.50)
On the basis of communicating containers, measuring devices can also be
designed and employed to measure the atmospheric pressure, i.e. to carry out
barometric measurements. To explain this, reference is made to the pressure
measuring equipment shown in Fig. 6.12.
A system can in principle be produced as follows:
(a) A glass tube with a length of more than 1 m is filled to the top with
mercury; at the lowest end of the tube a spherical extension of the tube
section has been made.
(b) The glass tube, filled with mercury, is turned upside down and its lower
end is displaced into a container also filled with mercury. This needs to be
achieved without mercury running out of the tube, as shown in Fig. 6.12.

168 6 Hydrostatics and Aerostatics
(c) The level of the mercury column in the glass tube over the surface of
the mercury in the external container is a measure of the barometric
pressure:
p
0
= ρ
F
gh (6.51)
A barometer, as shown in Fig. 6.12, can be employed to verify experimentally
the pressure distributions in the atmosphere stated in Sect. 6.4.1.
6.3 Free Fluid Surfaces
6.3.1 Surface Tension
In Chap. 1, it was emphasized that a special characteristic of fluids is that, in
contrast to solids, they have no form of their own, but always adopt the form
of the container in which they are stored. While doing this, a free surface
forms and it was shown in Sect. 6.1 that this surface adopts a position which
is perpendicular to the vector of the gravitational acceleration.
In this way, the fluid properties under gravitational influence were for-
mulated which are known from phenomena of every day life. It was always
assumed that the fluid at disposal possesses a total volume having the same
order of magnitude as the larger container in which it is stored. The fluid
properties hold only when these conditions are met. This is known from
observations of small quantities of liquids which form drops when put on
surfaces, as sketched in Fig. 6.13. It is seen that different shapes of drops
can form, depending on which surface and which fluid for forming drops are
used. More detailed considerations show, moreover, that the gas surrounding
the fluid and the solid surface all have an influence on the shape of a drop.
The latter is often neglected and one differentiates considerations of fluid–
solid combinations with reference to their “wetting possibility”, depending
on whether the angle of contact established between the edge of the fluid
surface and solid surface is smaller or larger than π/2.
The surface is classified as non-wetting by the fluid when
γ
gr
>π/2 (6.52)
(a)
(b)
γ
gr
γ
gr
Fig. 6.13 (a) Shape of a drop in the case of non-wetting fluid surfaces; (b)shapeof
a drop in the case of wetting fluid surfaces
6.3 Free Fluid Surfaces 169
It holds furthermore that for
γ
gr
<π/2 (6.53)
the surface is classified as wetting for the fluid.
Surfaces covered by a layer of fat are examples of surfaces that cannot be
wetted by water. Cleaned glass surfaces are to be classified as wetting for
many fluids.
The above phenomena can be explained by the fact that different “actions
of forces” are experienced by fluid elements. Equivalent physical consider-
ations can be made also by referring to the surface energy that can be
attributed to free fluid surfaces. The equivalence of forces and energy consid-
erations in mechanical systems is explained in Sect. 5.5. When a fluid element
is located in a layer that is far away from a free fluid surface, it is surrounded
from all sides by homogeneous fluid molecules and one can assume that the
cohesion forces occurring between the molecules cancel each other. This is,
however, no longer the case when one considers fluid elements in the prox-
imity of free surfaces. As the forces exerted by gas molecules on the water
particles are negligible in comparison with the interacting forces of the liq-
uid, a particle lying at the free surface experiences an action of forces in the
direction of the fluid. “Lateral forces” also act on the fluid element, which
thus finds itself, in the considered free interphase surface, in a state of ten-
sion that attributes special characteristics to the free surface. It is therefore
possible, for example, to deposit carefully applied flat metal components on
free surfaces without the metal piece penetrating the liquid. The floating of
razor blades on water surfaces is an experiment that is often presented in
basic courses of physics to demonstrate this. In nature, animals like “pond
skaters” make use of this particular property of the water surface in order to
cross pools and ponds skillfully and quickly.
When a drop of liquid comes into contact with a firm support, attracting
forces also occur in addition to the internal interacting forces. When these
attracting forces are stronger than the internal forces that are typical for the
fluid, we have the case of a wetting surface and water drops form as shown in
Fig. 6.13b. If, however, the forces attributed to a thin layer of the free surface
are stronger, we have the case of a non-wetting surface and the shapes of the
drops correspond to those in Fig. 6.13a.
More detailed considerations of the processes in the proximity of the free
surface of a liquid show that we have to deal with a complicated layer (with
finite extension vertical to the fluid surface) from a liquid area to a gas area.
It suffices, for many considerations to be made in fluid mechanics however,
to introduce the surface as a layer with a thickness δ → 0. To the same
are attributed the properties that comprise the complex layer between fluid
and gas. The property that is of particular importance for the considerations
to be carried out here is the surface tension. This surface tension can be
demonstrated by immersing a strap, as shown in Fig. 6.14, in a fluid. When
pulling the strap through the free surface upwards, one observes that this

170 6 Hydrostatics and Aerostatics
Bend wire frame
Liquid film
Free liquid surface
Fig. 6.14 Wire frame experiment to prove the action of forces as a consequence of
surface tension
requires an action of forces which is proportional to the distance between the
strap arms. The proportionality constant describing this fact is defined as
the surface constant.
The surface tension thus represents a force of the free surface per unit linear
length. It can also be introduced as the energy that is required to build up
the tension in the liquid film in Fig. 6.14. The two definitions are identical. In
both ways of looking at the energy equation, the length of the liquid film in
the direction in which the wire frame is pulled can be understood as a process
to introduce energy to produce free surfaces. One can also look at the force
that is needed to pull the wire frame up to produce the free surface. This
makes it clear that both possibilities of introduction of the surface tension,
one as the action of forces per unit length and the other as the energy per
unit area, are identical.
In concluding these introductory considerations, the effect of the surface
tension on the areas above and below a free surface will be looked at more
closely. From observations of free surfaces in the middle of large contain-
ers one can infer that the surface tension there has no influence on the
fluid and the gas area lying above it, as the free surface forms vertically
to the field of gravity of the earth, as stated in Fig. 6.1. From this it follows
that considerations of liquids with free surfaces can be carried out far away
from solid boundaries (container walls), without consideration of the wall
effects.
When one considers a curved surface element, as shown in Fig. 6.15, one
understands that, as a consequence of the surface tensions that occur, actions
of forces are directed to the side of the surface on which the center points
of the curvatures are located. The forces acting on sides AD and BC of the
surface element are computed for each element ds
1
and the forces resulting
from them, in the direction of the center points of the circles of curvature,
are:
dK
1
=2σ ds
1
sin β =2σ ds
1
ds
2
2R
1
dK
1
=
σ
R
2
ds
1
ds
2
=
σ
R
1
dO
(6.54)
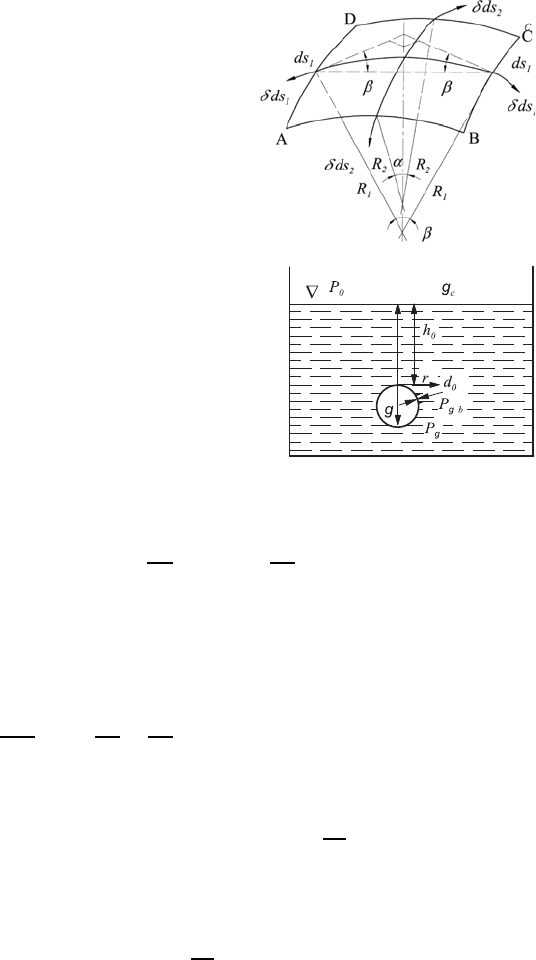
6.3 Free Fluid Surfaces 171
Fig. 6.15 Schematic representation of a
curved surface
Fig. 6.16 Diagram for the consideration of pres-
sure in bubbles
Similarly, the action of forces dK
2
is computed as
dK
2
=
σ
R
2
ds
2
ds
1
=
σ
R
2
dO (6.55)
This shows that as a consequence of the surface tension, pressure effects occur
that are directed towards the center points of the circles of curvature. This
pressure effect is computed as force per unit area. As the considerations show,
a differential pressure results, which is caused by the surface tension:
∆p
σ
=
dK
dO
= σ
1
R
1
+
1
R
2
, with dK =dK
1
+dK
2
(6.56)
If a spherical surface is considered, the following relationship holds:
R
1
= R
2
= R −→ ∆p
σ
=
2σ
R
(6.57)
This relation means that the gas pressure in a spherical bubble is higher
than the liquid pressure imposed from outside:
p
F
+
2σ
R
= P
g
(6.58)
For very small bubbles this pressure difference can be very large.
When one considers the equilibrium state of a surface element of a bubble
(see Fig. 6.16), the following relationship can be written for the pressure in
the upper apex:
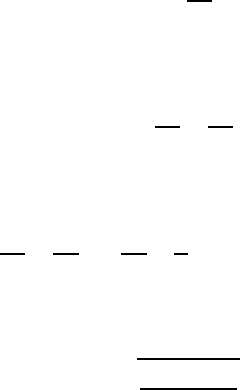
172 6 Hydrostatics and Aerostatics
p
0
+ ρ
F
gh
0
+ σ
2
R
0
= p
g,0
(6.59)
For a surface element of any height, the following pressure equilibrium
holds:
p
0
+ ρ
F
g (h
0
+ y)+σ
1
R
1
+
1
R
2
= p
g,0
+ ρ
g
gy (6.60)
When one now forms the difference of these pressure relationships, one
obtains:
1
R
1
+
1
R
2
−
2
R
0
+
1
σ
(ρ
F
− ρ
g
) gy = 0 (6.61)
Hence the characteristic quantity for the normalization of equation (6.61)
is to be introduced:
a =
$
2σ
g (ρ
F
− ρ
g
)
(6.62)
which is known as the Laplace constant or capillary constant. It has the
dimensions of length and indicates the order of magnitude when a perceptible
influence of the surface tension on the surface shape of a medium exists.
• When the Laplace constant of a free surface of a liquid is comparable to
the dimensions of the liquid body, an influence of the surface tension on
the free surface shape is to be expected.
• In the proximity of liquid rims (container walls), an influence of the surface
tension on the shape of the fluid surface is to be expected with linear
dimensions that are of the order of magnitude of the Laplace constant.
6.3.2 Water Columns in Tubes and Between Plates
From the final statements in Sect. 6.3.1, consequences result for considera-
tions of heights of liquid columns in pipes and channels of small dimensions.
Considerations, have been carried out already in Sect. 6.2, but influences of
the interactions between liquid, solid and gaseous phases remained uncon-
sidered there, i.e. the influence of the surface tension was not taken into
account. Considering the insights into the properties of connected containers
filled with liquid gained in Sect. 6.3.1, one sees that the considerations stated
for “communicating systems” in Sect. 6.2 hold only when the dimensions of
the systems are larger than the Laplace constant of the fluid interface. More-
over, the considerations only hold far away from fluid rims. In the immediate
proximity of the rim there exists an influence of the surface tension which
was neglected in the considerations in Sect. 6.2.
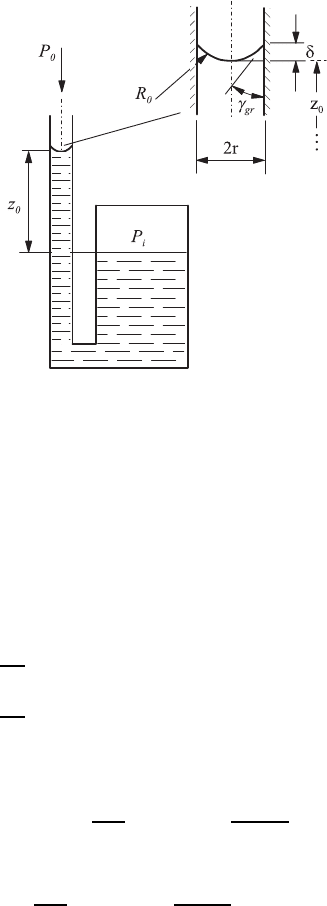
6.3 Free Fluid Surfaces 173
Fig. 6.17 Diagram for consideration of
heights of liquid column in tubes and
between plates
The processes taking place in fluid containers of small dimensions can be
treated easily if one carries out a division of the properties of container walls
into “wetting” ones and “non-wetting” ones. When making the considerations
first for wetting walls, experiments show that for such surfaces, in small tubes
and between plates with small distances forming small gaps, the fluid in the
tube or between the plates assumes a height which is above the height of the
surface of a connected larger container, as indicated in Fig. 6.17.
From pressure equilibrium considerations it follows that
Pressure between plates: p
0
−
σ
R
0
= p
F
= p
i
− ρ
F
gz
0
(6.63a)
Pressure in tubes: p
0
−
2σ
R
0
= p
F
= p
i
− ρ
F
gz
0
(6.63b)
or, written in another form,
Height of water surface between plates: z
0
=
1
ρ
F
g
(p
i
− p
0
)+
σ
ρ
F
gR
0
,
(6.64a)
Height of water surface in tubes: z
0
=
1
ρ
F
g
(p
i
− p
0
)+
2σ
ρ
F
gR
0
(6.64b)
In these relationships, the radius of curvature R
0
has to be considered as
an unknown before its determination, for which two possibilities exist. To
simplify the corresponding derivations one can assume, with a precision that
is sufficient in practice, that the surface in the rising pipe adopts the form
of a partial sphere for the tube and that of a partial cylinder for the gap
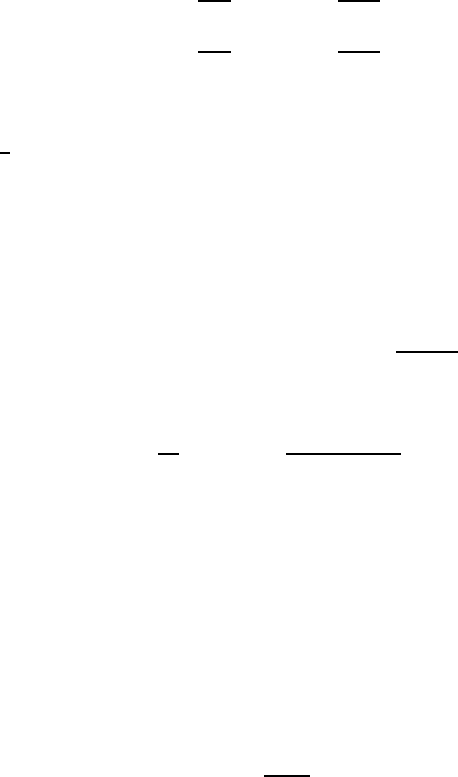
174 6 Hydrostatics and Aerostatics
of the plate. The angle of contact between the fluid surface and the tube
wall or plate wall has to be known from information regarding the wetting
properties of the wall materials. When one defines this angle as γ
gr
,one
obtains the following relation:
r = R
0
cos γ
gr
(6.65)
For the final relationships of the height z
0
of the water surface for plates
and tubes, the following expressions hold:
Plates: z
0
=
1
ρ
F
g
(p
i
− p
0
)+
σ
ρ
F
gr
cos γ
gr
(6.66a)
Tub e: z
0
=
1
ρ
F
g
(p
i
− p
0
)+
2σ
ρ
F
gr
cos γ
gr
(6.66b)
These final relationships now show that even in the case of pressure equal-
ity, i.e. p
i
= p
0
, the height z
0
of the free liquid surface assumes finite values
if γ
gr
<
π
2
exists. This has to be considered when employing communicating
systems for measurements of the height of the water level in containers and
when measuring pressures.
The second possibility for computing the pressure difference below and
above interphases is given by the fact that it is experimentally possible, al-
though with greater inaccuracy, to determine the quantity δ in Fig. 6.17 by
means of the following considerations:
r
2
+(R
0
− δ)
2
= R
2
0
R
0
=
r
2
+ δ
2
2δ
(6.67)
The height z
0
of the liquid surface is calculated from this as follows:
z
0
=
1
ρg
(p
i
− p
0
)+
4σδ
ρ
F
g (r
2
+ δ
2
)
(6.68)
This proves that for σ = 0 no height difference exists and a liquid level
increase due to surface effects is not to be expected in tubes or between
plates. Under such conditions, for considerations of wetting of a surface, the
relations derived in Sect. 6.2 hold also for small tube diameters and small
gaps between plates.
In the case of non-wetting surfaces, it is observed that the fluid in the
interior of a rising tube or the gap between plates does not reach the height
which the fluid outside the tube (or the gap of the plate) assumes, as indicated
in Fig. 6.18. Analogous to the preceding considerations for wetting fluids, it
can be stated that the following relationship holds:
z
0
= −
2σ
R
0
ρg
(6.69)
where R
0
can be introduced again as indicated above.
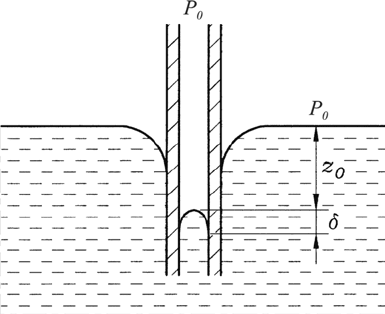
6.3 Free Fluid Surfaces 175
Fig. 6.18 Considerations of the height of the meniscus in tubes and between plates
for non-wetting surfaces
The equation thus obtained indicates that the final relationships derived
for the wetting surfaces can often be applied also to non-wetting media,
if one considers the sign of γ
gr
and δ,Thusδ needs, for example, to be
introduced positively in the above relationships for wetting fluids, whereas
for non-wetting surfaces δ has to be inserted negatively.
6.3.3 Bubble Formation on Nozzles
The injection of gases into fluids for chemical reactions or for an exchange of
gases represents a process which is employed in many fields of process engi-
neering. Thus bubble formation on nozzles as an introduction process for the
injected gases is of interest for these applications. Moreover, the simulation
of boiling processes, where steam bubbles are replaced by gas bubbles, repre-
sents another field where precise knowledge of bubble formation is required.
When gas bubbles form on nozzles during the gassing of liquids, the pres-
sure in the interior of bubbles changes. For the theoretically conceivable static
bubble formation, this is attributed to different curvatures of the bubble in-
terface during the formation of bubbles and thus to changes of the pressure
difference caused by capillary effects . Superimposed upon these are changes
in pressure which have their origin in the upward movement of the bubble
vertex taking place during the formation. With the dynamic formation of
bubbles, additional changing pressure effects are to be expected which are
essentially caused by accelerative and frictional forces.
By the term “static bubble formation”, one understands the formation
of bubbles under pressure conditions, which allow one to neglect the pres-
sure effects on an element of the interface due to accelerative and frictional
