Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.


208 Part B Chemical and Microstructural Analysis
Table 5.1 (continued)
Methodology Subject under consideration Material type Atom
arrangement
Category
Method
Ref.
1
Structural order
Defects and impurities
Molecular architecture
2
Microstructure
3
/
Finite structures
4
Metal
Semiconductor
Insulator
Others
5
Crystalline
Amorphous
Requirements
Spectroscopy
Mößbauer
spectroscopy
5.3.1 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Limited
nuclear
species
Mechanical
Spectroscopy
24
5.1.2, 5.3.1,
5.3.2
◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Noise
Spectroscopy
5.3.1 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
DSC/DTA 4.1, 8 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Microanalysis
EDX
25
5.5.2 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Heavy
elements
TEM-EELS
26
5.5.2 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ HV,
thin sample
Micro-Auger 5.5.2, 6 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Intermediate
elements
EPMA
27
5.5.2 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ UHV
X-ray diffraction 5.5.1 ◦ ◦ ◦ ◦ ◦ ◦
SEM-electron
channeling pattern
5.5.1 ◦ ◦ ◦ ◦ ◦ Δ ◦ HV
Scanning tunneling
spectroscopy
5.3.1 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ UHV
SPM-
nanospectroscopy
5.5.2 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Nano-indenter 5.5.2 ◦ ◦ ◦ ◦ ◦ ◦ ◦
Fluorescence
microscopy
28
11 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Micro-Raman 5.5.2 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Stereology
Stereogram 5.5.3 ◦ ◦ ◦ ◦ ◦ ◦ ◦
LSCM 5.5.3 ◦ ◦ ◦ ◦ ◦ Transparent
sample
Light scattering
tomography
Ogawa
29
◦ ◦ ◦ ◦ ◦ ◦ ◦
Nonlinear
microscopy
30
5.5.3 ◦ ◦ ◦ ◦ ◦ ◦
3D-SEM 5.5.3 ◦ ◦ ◦ ◦ ◦ ◦ ◦
3D-TEM 5.5.3 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ HV
3D atom probe 5.5.3 ◦ ◦ ◦ ◦ ◦ ◦ UHV
Micro-XCT 5.5.3 ◦ ◦ ◦ ◦ ◦ ◦ ◦ Light
elements
Part B 5
Nanoscopic Architecture and Microstructure Introduction 209
Table 5.1 (continued)
Methodology Subject under consideration Material type Atom
arrangement
Category
Method
Ref.
1
Structural order
Defects and impurities
Molecular architecture
2
Microstructure
3
/
Finite structures
4
Metal
Semiconductor
Insulator
Others
5
Crystalline
Amorphous
Requirements
Miscellaneous
Density
measurements
Gupta
31
◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Quartz crystal
microbalance
Gupta
31
◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Radioactivation
analysis
Alfassi
32
◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Limitted
nuclear
species
SIMS Profile 6 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ UHV
Q-Mass 4 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ UHV
LC-MS
33
5.4.3, 4.1 ◦ ◦ ◦ ◦ ◦ ◦ ◦
GC-MS
34
5.4.3, 4.1 ◦ ◦ ◦ ◦ ◦ ◦ ◦
GPC/GFC
35
5.4.3, 4.1 ◦ ◦ ◦ ◦ ◦ ◦ ◦
ICP
36
4.2 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
AAS/AES
37
6 ◦ ◦ ◦ ◦ ◦ ◦ ◦ UHV
Electrophoresis 5.4.3 ◦ ◦ ◦ ◦ ◦ ◦
Electron
Holography
Tonomura
38
◦ ◦ ◦ ◦ ◦ ◦
FRET
39
5.4.3 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦
Pump & Probe Shah
40
, 11 ◦ ◦ ◦ ◦ ◦ ◦ ◦ ◦ Ultrafast
pulse laser
1
Chapter(s) and section(s) describing the detail or literature to which the interested readers should refer
2
Including macromolecules
3
Including phase distribution and texture
4
e.g., dendrites, cells, bones, fibres, nanoparticles, device structures etc.
5
Isolated molecules and molecular solids
6
High vacuum
7
Ultrahigh vacuum
8
Including anomalous
9
Including HRTEM
10
Including HAADF-STEM
11
Including back scattering EM, SEM-EBIC, SEM-CL, scanning reflection electron microscopy
12
Including etch pits observation
13
Laser scanning confocal microscopy
14
Total internal reflection fluorescence microscopy
15
[5.1]
16
Including photoacoustic spectroscopy, resonance-enhanced multiphoton ionization
17
Including pure rotation spectroscopy
Part B 5

210 Part B Chemical and Microstructural Analysis
Table 5.1 (continued)
18
Including DLTS, photocapacitance
19
Including optically-detected magnetic resonance
20
Including optical rotary dispersion and magnetic circular dichroism
21
Electron nuclear double resonance
22
Extended x-ray absorption fine structure
23
Including XPS,UPS
24
Including internal friction, anelasticity
25
Including SEM-based and (S)TEM-based
26
Including EXELFS (extended energy loss fine structure)
27
Electron probe micro analyzer
28
Flurometry
29
[5.2]
30
Including nonlinear fluorescence (two-photon) microscopy and second-harmonic generation microscopy
31
[5.3]
32
[5.4]
33
Liquid chromatography-mass spectroscopy
34
Gas chromatography-mass spectroscopy
35
Gel permeation (filtration) chromatography
36
Including flame photometry (FAAS/FAES/FAFS (flame atomic absorption (emission, fluorescence) spectrometry))
37
Atomic absorption (emission) spectrometry
38
[5.5]
39
Fluorescence resonance energy transfer
40
[5.6]
Table 5.2 Sample requirements in some common methods in their conventional form
Method Minimum center density Minimum sample amount Maximum molecular size
a
Element analysis
GC-MS 10 ppb ≈1ml Boiling temperature < 400
◦
C
Radioactivation analysis 0.1 ppb 0.01 ng −
X-ray fluorescence 10ppm 100 mg
b
−
Structure analysis
Single crystal XRD − 0.1mm
c
< 100 atoms
d
,
> 600 atoms
or 10
6
in molecular weight
e
Powder XRD − 0.2–0.5mm Several 10
3
atoms
NMR ≈ 1% 1mM,severalmg 3×10
4
in molecular weight
Defect analysis
ESR
f
10
12
–10
13
cm
−3g
< cavity size −
or 10
14
–10
16
cm
−3h
Positron annihilation 5×10
15
–5×10
18
cm
−3i
No serious limitation ≈ 10 vacancies
j
Raman scattering 0.01–1% (several ppb
k
) Several μl (liquid) −
FT-IR 0.01% sub μg
l
−
PL 0.1 ppm > mg −
Capacitance spectroscopy 10
14
cm
−3
1mm −
a
In crystal structure analysis, the size of atom assembly (molecule) occupying a lattice point
b
Reduced by orders of magnitude when a synchrotron radiation source is used
c
0.02 mm when a synchrotron radiation source is used
Part B 5
Nanoscopic Architecture and Microstructure 5.1 Fundamentals 211
Table 5.2 Sample requirements in some common methods in their conventional form, cont.
d
When direct method is used
e
When heavy atoms substitution method works
f
The sensitivity is quite dependent on what signal is detected. Here the signal is microwave absorption. Even a single spin can be detected if
a special technique is used [5.7]
g
In insulators
h
In semiconductors
i
For negatively charged vacancies at room temperature no serious limitation
j
Above which the vacancy clusters become indistinguishable from the surface
k
When resonant Raman scattering is employed
l
When combined with a chromatographic separation preprocess
5.1 Fundamentals
This section deals with the fundamental (minimum)
knowledge that the reader should have in advance. This
section is also intended to guide the reader to be able
to select properly the appropriate techniques for their
purpose by overviewing the range of energy, the length
scale, the spatial resolution and the time scale that are
covered by the entire range of these respective tech-
niques. Since it appears that diffraction methods are not
well covered in other parts of this handbook, the basic
physics of diffraction and the principles of microscopic
techniques (mainly TEM) based on beam diffraction
will be described in some depth. We leave most of the
basics of spectroscopic techniques to Chap. 11.
5.1.1 Diffraction and Scattering Methods
The scattering of particles by an interacting target
is a common phenomenon usually interpretable in
terms of classical mechanics with a minor correction.
Generally, the waves (electromagnetic, de Broglie, vi-
brational, etc.) are scattered with an efficiency that can
vary widely depending on the nature and the size of the
scatterers and the wavelength. The diffraction of waves
is a term used to describe the constructive interference
of waves coherently scattered by multiple scatterers.
Scattering and diffraction are, thus, inseparable con-
cepts that are sometimes treated together in textbooks.
Absorption is an apparently different aspect of waves
in which some wave energy is consumed by excitation
of a quasiparticle of different nature. In some cases,
however, absorption is an aspect of wave scattering in
which the excited state is derived from the interference
of the scattered waves. The wavelength dependence of
absorption is the central subject of various schemes of
spectroscopy, which are addressed mainly in Sect. 5.1.2.
The scatterers of electromagnetic waves from the
visible light to the x-ray regime are electrons, and those
in the infrared regime are phonons or molecular vi-
brations. Although the penetration of electromagnetic
waves through solids can vary significantly from ma-
terial to material due to the wavelength dependence of
the absorption coefficient, hard x-rays have a relatively
high penetrability through solids as long as the con-
stituent elements are light. In contrast, since electrons
have a negative electric charge, they interact so strongly
with matter that they practically cannot penetrate even
thin solids unless the energy is higher than 100 keV. On
the contrary, neutrons have no electric charge and so
can penetrate deeply into solids. Neutrons have a spin
magnetic moment and therefore interact with electrons,
which also have a magnetic moment. This fact pro-
vides unique experimental approaches for studies of
magnetism in solids. Furthermore, neutrons also inter-
act with nuclei with scattering cross sections that are
quite different from those of x-rays and electrons; for
example, the detection of protons that are difficult to
detect with x-rays and electrons but that are detectable
by neutrons due to the large scattering cross section.
The physical parameter of fundamental importance
in scattering and diffraction of waves or quantum beams
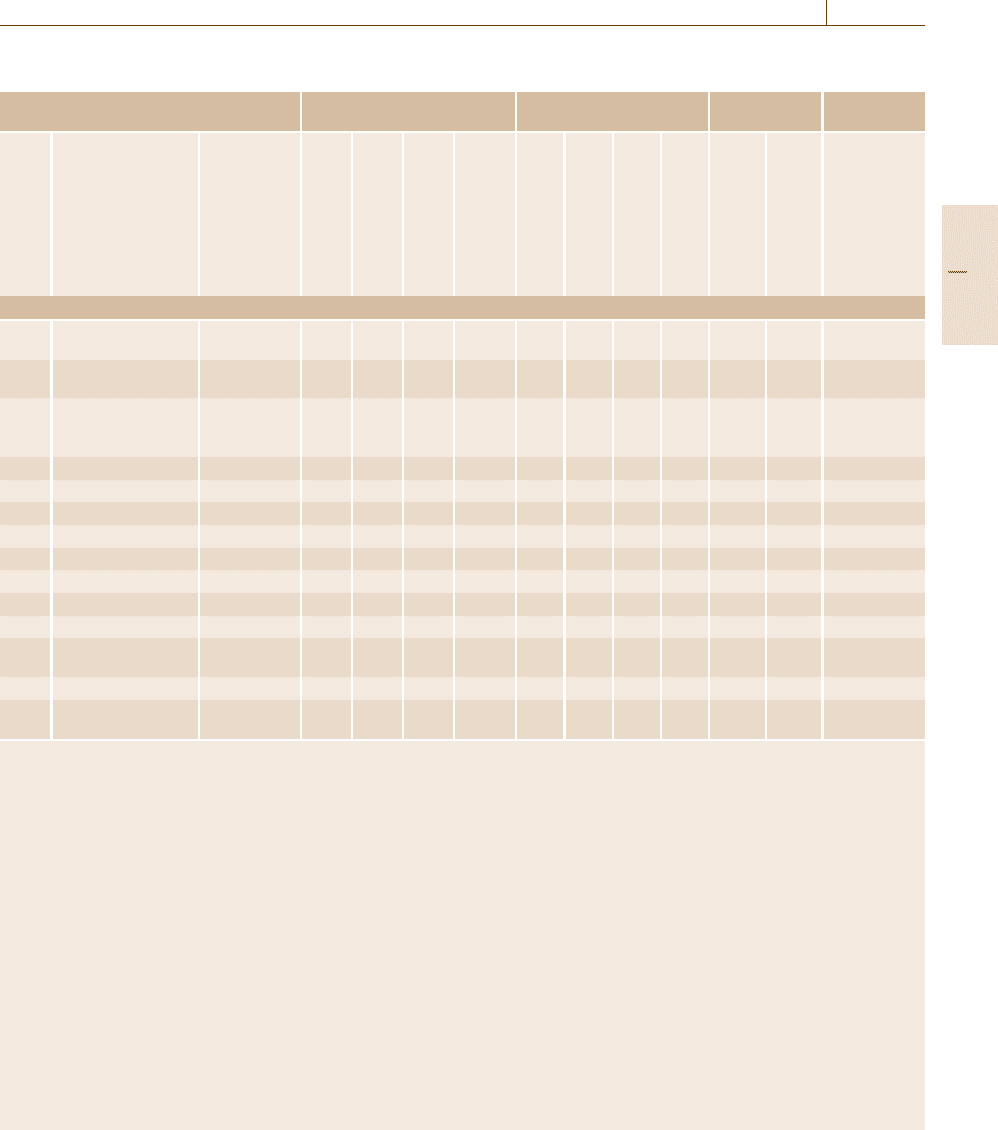
is the wavelength or wave number. Figure 5.1 shows
the wavelength of various beams plotted as a func-
tion of the quantum energy of the beam, the energy
quantum hν of a photon and the kinetic energy of an
electron and of a neutron. Since, as explained later,
diffraction occurs most significantly when the wave-
length is close to the separation of the scatterers, particle
beams with a wavelength of the order of 0.1 nm are
most suitable for diffraction studies of atomic arrange-
ment. Among electromagnetic waves, x-rays of 10 keV
Part B 5.1
212 Part B Chemical and Microstructural Analysis
10
4
10
2
10
0
10
–2
10
–4
10
–2
10
4
10
2
10
0
10
6
Wavelength (nm)
Energy (eV)
Photon
Electron
Neutron
Fig. 5.1 Wavelength versus energy relations in various
quantum beams
are in this range, whereas electrons of 100 eV also have
such a wavelength. The fast neutrons emitted from nu-
clear reactors or accelerator targets have energies of
the order of MeV but can be slowed down to energies
below 1 keV after passing through a moderator consist-
ing of materials such as water and graphite. Among
the slow neutrons, those whose energy is near room
temperature (0.025 eV) and those below room temper-
ature are called thermal neutrons and cold neutrons,
respectively. These are the neutrons used for diffrac-
tion studies. Neutron diffraction can be an alternative
to x-ray diffraction when x-rays do not detect low-Z
elements well, to distinguish near-Z elements or to pen-
etrate thick samples.
Since a comprehensive description of the funda-
mentals of crystal diffraction is found in many good
textbooks [5.8–12], only the essence is summarized
here so that the reader may understand the princi-
ples of the experimental techniques described in later
sections.
In cases in which the interaction of waves with mat-
ter is weak (typically in x-ray and neutron diffraction),
it is a good approximation to use the kinematical theory
which assumes that the wave can be scattered only once
in the matter. However, when the matter is crystalline
and the crystal is thick and nearly perfect, diffraction
may have to be treated by the dynamical theory that rig-
orously takes into account multiple diffractions in the
crystal. The same situation also arises in the diffraction
of charged particles such as electrons that interact very
strongly with solids. Nevertheless, although the quan-
titative interpretation of some phenomena requires the
dynamical theory, many of the phenomena encountered
in experiments can be understood in the framework of
the kinematical theory. For the dynamical theory, the
minimum knowledge is given in Sects. 5.1.2 and 5.3.2
where it is required.
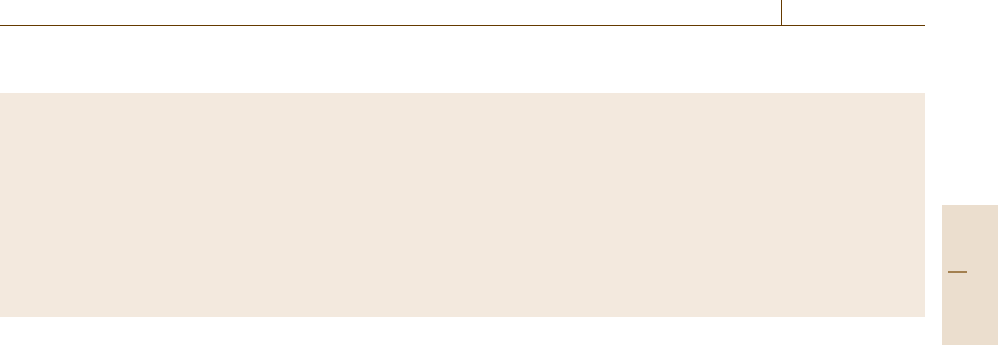
In real space, a strong diffraction of a wave of wave-
length λ is induced by a set of net planes (Note that
atoms on each net plane do not have to be regularly
arrayed.) regularly separated by a distance a (Fig. 5.2)
when the wave is incident on the net planes at an angle θ
satisfying the Bragg condition
2a sin θ =nλ (n : integer) . (5.1)
In the reciprocal space, the Ewald sphere is the most
commonly used graphical construction of wave diffrac-
tion, whether for x-ray or de Broglie waves. Precisely,
the sphere of radius 1/λ is three-dimensional but usu-
ally represented in two dimensions by a circle touching
the origin of the reciprocal lattice, as shown in Fig. 5.3.
The Bragg condition is satisfied when the sphere passes
through a reciprocal lattice point other than the origin.
The wave vector of the incident wave k
0
and that of
the diffracted wave k are indicated by the arrows drawn
a
a
a
k
υ
Bragg condition:
k
k
0
2asinυ =nμ
2υ
K = g ⏐g⏐=
n
a
k
0
Fig. 5.2 Diffraction by a set of net planes periodically
separated by a distance a. Constructive interference takes
place under the Bragg condition. Note that atoms on each
net plane are not necessarily periodic for specular reflec-
tion to occur
Part B 5.1
Nanoscopic Architecture and Microstructure 5.1 Fundamentals 213
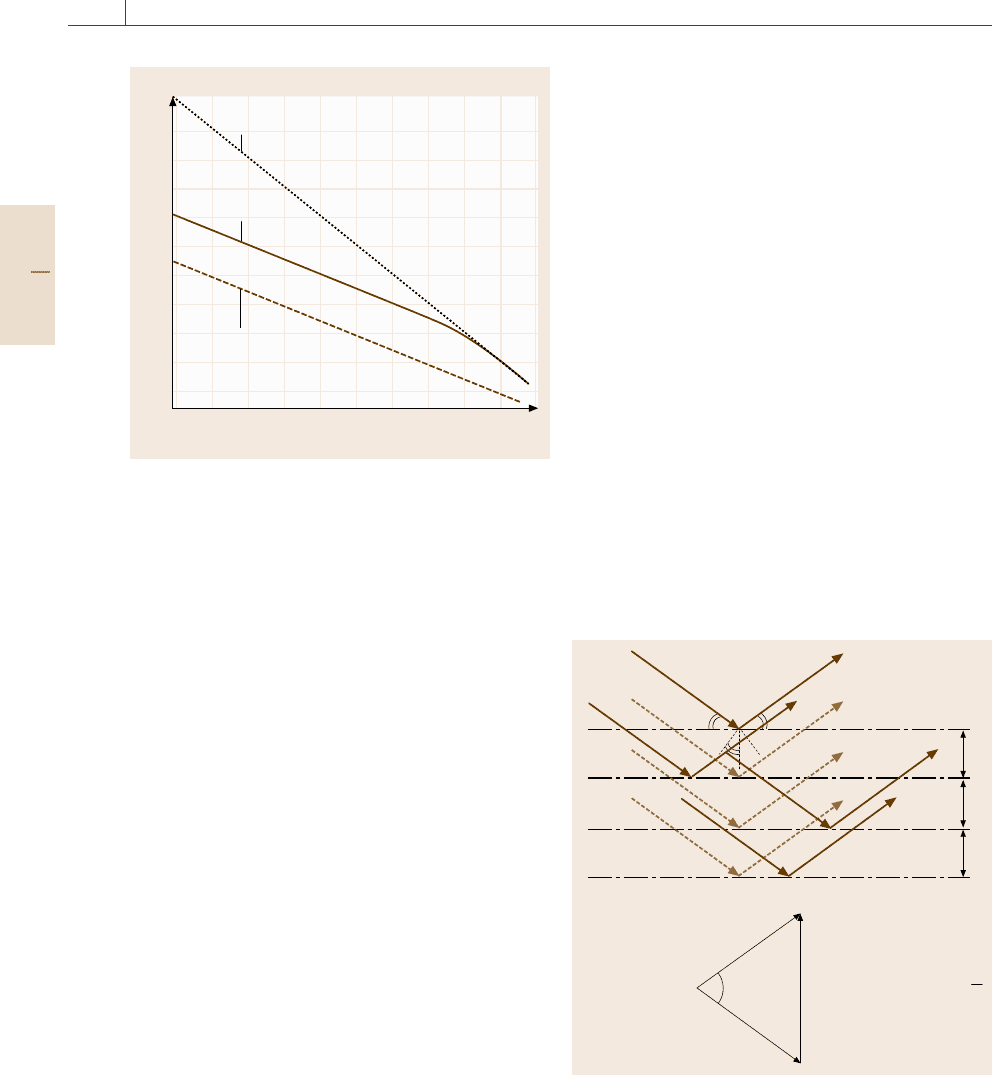
(0,0,0)
K = g
k
0
k
Fig. 5.3 Graphical reconstruction of wave diffraction by
Ewald sphere in the reciprocal lattice. K = g corresponds
to the Bragg condition
from the center of the sphere to the origin and to the
reciprocal lattice point, respectively. Thus, the Bragg
condition (5.1) is equivalent to
K = g , (5.2)
where K ≡k−k
0
is called the scattering wave vector,
and g(
|
g
|
=n/a) is the reciprocal lattice vector normal
to the net planes. (Throughout this chapter, we fol-
low the convention that the factor 2π is not included
in the definition of reciprocal vectors g and wave vec-
tors k.)
In x-ray diffraction, the entities that scatter x-rays
in matter are electrons. Electrons forced to oscillate by
the alternating electric field of the electromagnetic wave
emit an electromagnetic wave of the same photon en-
ergy (elastic scattering or Thomson scattering) or of
a slightly reduced energy (inelastic scattering or Comp-
ton scattering), the latter constituting the background
for the coherent interference of the former, elastically
scattered, waves. Generally, waves incident with inten-
sity I
e
and wave vector k
0
are scattered by a collection
of electrons or an electron cloud of density ρ(r)toin-
terfere to yield a far-field electromagnetic wave with
a wave vector k and intensity
I(K) = I
e
ρ(r)exp
(
2πiK ·r
)
dv
2
. (5.3)
If the scatterers are electrons only in a single atom,
the scattering intensity is given by
I
a
(K) = I
e
|
f (K)
|
2
, (5.4)
with the atomic scattering factor defined by
f (K) ≡
ρ
a
(r)exp
(
2πiK ·r
)
dv, (5.5)
the magnitude of which is determined by the atomic
electron density ρ
a
(r) and hence specific for the atomic
element. The atomic scattering factor is a complex
quantity and may also vary depending on the beam en-
ergy, bringing about the anomalous dispersion effect
(Sects. 5.2.1 and 5.2.3).
In the Born approximation, or the kinematical the-
ory, in which a crystal is thin enough for x-ray scattering
to take place at most once in the solid, the intensity of
the x-ray scattered by the assembly of atoms is simply
given by
I(K) = I
e
n, j
f
j
(K)exp
2πiK ·
R
n
+r
j
2
≡ I
e
·G(K)·
|
F(K)
|
2
. (5.6)
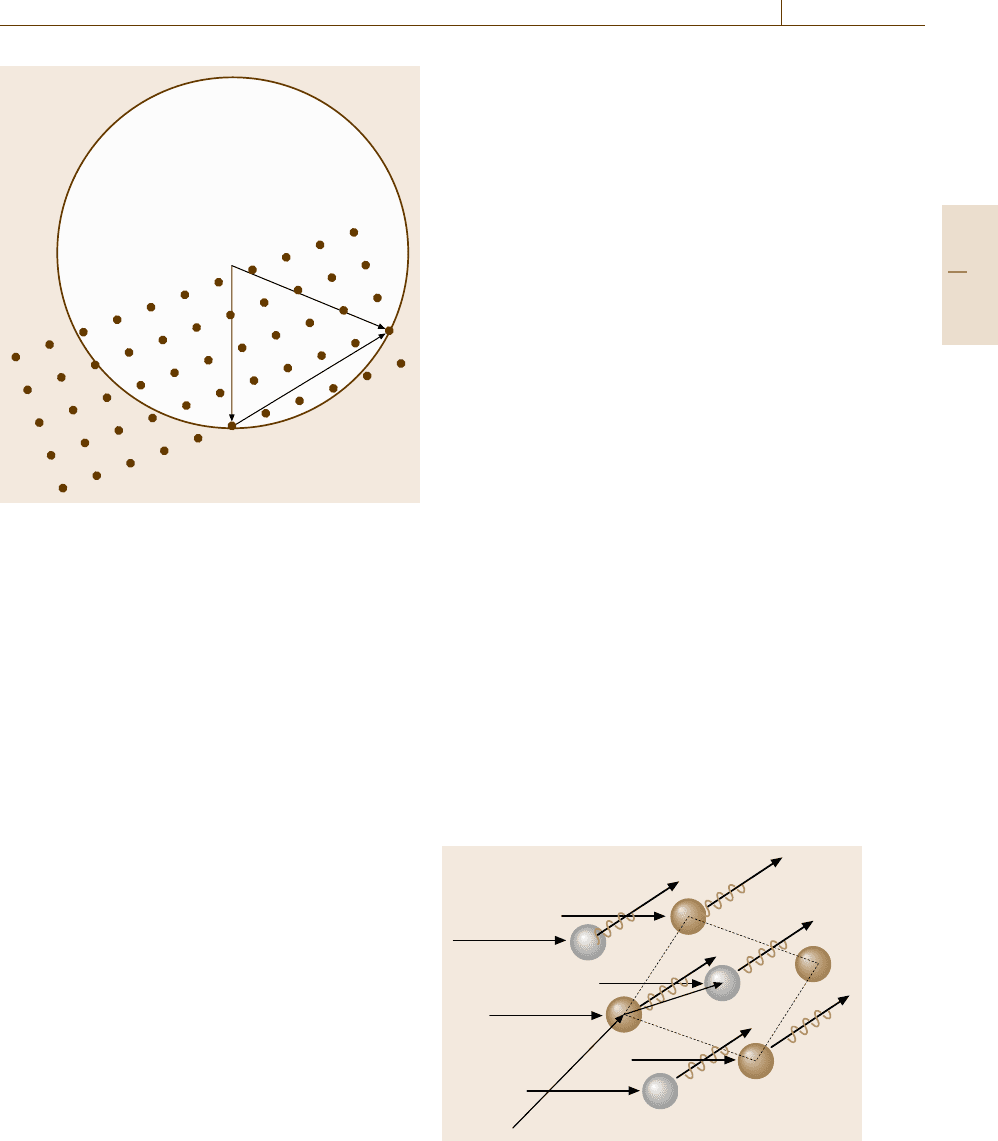
Here, as shown in Fig. 5.4, R
n
= n
1
a +n
2
b +n
3
c de-
notes the position of the nth crystalline unit cell (the
vectors a, b and c are the unit-cell translations while
n
1
, n
2
and n
3
are integers), r
j
is the relative position of
the jth atom within the unit cell, and f
j
is the atomic
scattering factor of the jth atom. The factor
F(K) ≡
unit cell
j
f
j
(K)exp
2πiK ·r
j
(5.7)
k
0
a
2
r
j
a
1
k
R
n
Fig. 5.4 X-ray diffraction by a crystal lattice the unit cell
of which is indicated with a paralellogram
Part B 5.1
214 Part B Chemical and Microstructural Analysis
is called the structure factor and characterizes the
atomic arrangement in the unit cell. Another factor
G(K) ≡
n
exp
(
2πiK ·R
n
)
2
=
n
1
exp (2πik
1
n
1
a)
n
2
exp (2πik
2
n
2
b)
×
n
3
exp (2πik
3
n
3
c)
2
(5.8)
is called the interference function, which is written
in the second formula in terms of the components of
the vector K = (k
1
, k
2
, k
3
) projected onto the unit-cell
translation vectors. In diffraction of a monochromatic
wave from a sufficiently large single crystal (n
1
, n
2
and n
3
are all large), G(K) is nonzero (n
1
·n
2
·n
3
) only
when
k
1
a =h, k
2
b =k and k
2
c =, (5.9)
for integers h, k and . This is again equivalent to the
Bragg condition (5.2), the condition for constructive
interference. In such cases, the diffraction intensity is
a)
b)
sin υ
sin υ
sin υ
400
300
200
100
0
100
80
60
40
20
0
25
20
15
10
5
0
–1.0 –0.5 0.0 0.5 1.0
–1.0 –0.5 0.0 0.5 1.0
–1.0 –0.5 0.0 0.5 1.0
N =20
N =10
N =5
Fig. 5.5a,b As the sharpness of diffraction decreases with the decreasing size of a grating in a monochromator (a),
diffraction peaks in small crystals show broadening, which is represented by the diffuseness of reciprocal lattice points
(b)
simply given by |F(g)|
2
times the number of unit cells
in the sample. In small crystals, however, diffraction
also occurs in directions other than those satisfying (5.2)
with intensities that depend on the interference function,
G(K), which reflects the size and external shape of the
crystal.
Thus, we have to consider two cases: the case of
large crystals for which the effects of G(K) can be ne-
glected, and the case of small crystals for which the
morphological information contained in G(K) is our
main concern.
Combining (5.5)and(5.7)wefindthatF(K) is re-
lated to the electronic density ρ(r) through a Fourier
transformation. This means that for large crystals we
could deduce the electronic density ρ(r) or the atomic
arrangement in the crystalline unit cell by inverse
Fourier transformation of the structure factors that can
be found from the experimental scattering intensity.
This forms the basis of structural determination by
x-ray diffraction from crystalline samples (Sects. 5.2.1
and 5.4.1).
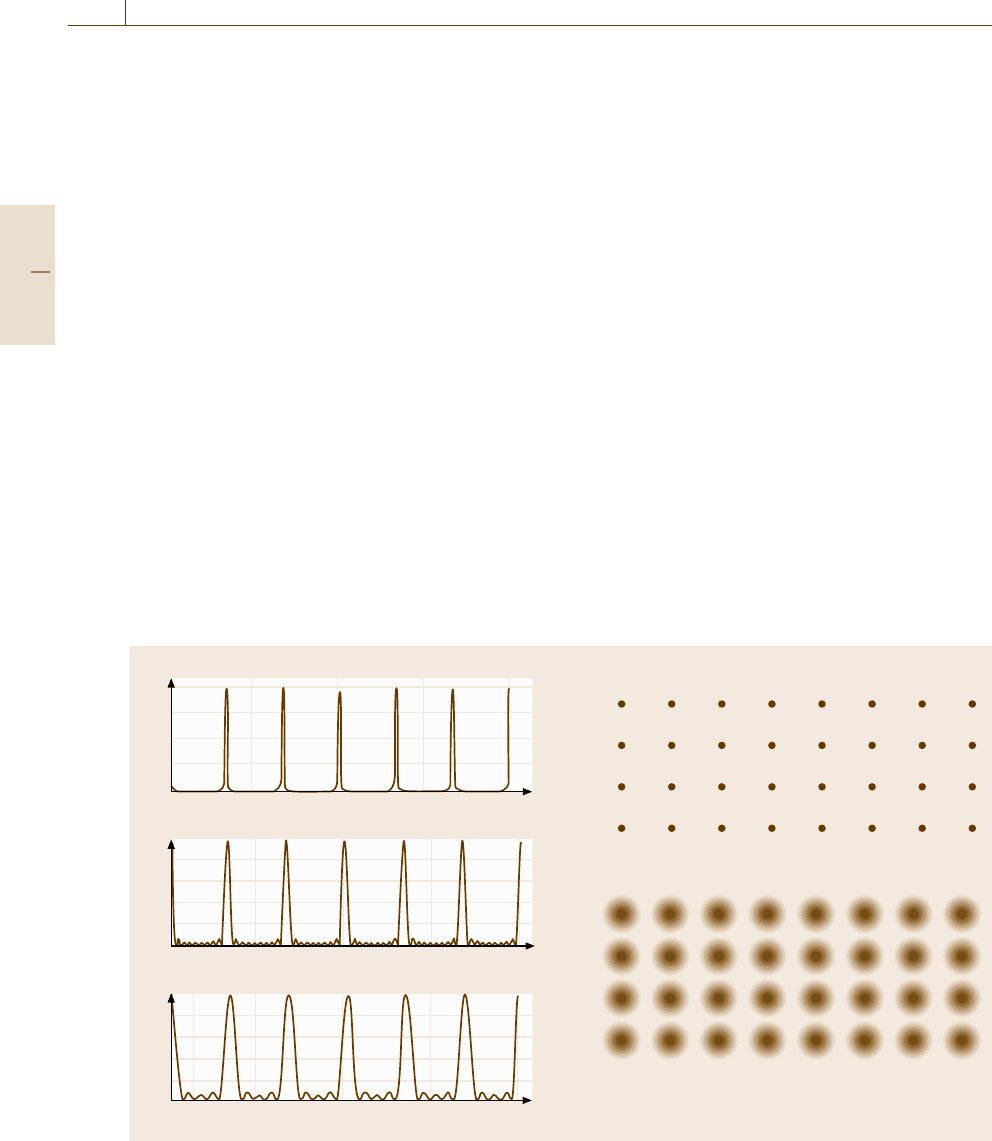
For small crystals, we have broadening of diffrac-
tion spots as happens in the diffraction of light by an
optical grating of finite size (Fig. 5.5a). This diffraction
broadening could be regarded as due to the aug-
Part B 5.1
Nanoscopic Architecture and Microstructure 5.1 Fundamentals 215
Real space Reciprocal space
a) b)
c) d)
Fig. 5.6a–d Broadening and sharpening of reciprocal lat-
tice points (b),(d) due to the external shape of the crystals
(a),(c)
mentation of the reciprocal lattice points (Fig. 5.5b).
Mathematically, G(K) is again the Fourier transform
of the external shape of the crystal, so thin crystals
elongated in one direction, as shown in Fig. 5.6a, yield
sharpening of the reciprocal lattice points along that
direction but broadening perpendicular to it, as shown
in Fig. 5.6b. Similarly, plate-like flat crystals (Fig. 5.6c)
broaden the diffraction peaks along the surface normal,
as shown in Fig. 5.6d. This fact makes it possi-
ble to analyze the medium-range order (Sect. 5.2.2),
to determine experimentally the small particle size
(Sect. 5.5.3), and to conduct high-resolution TEM ob-
servations (Sect. 5.1.2).
5.1.2 Microscopy and Topography
Microscopy allows one to study material structures with
spatial resolution. The resolving power of microscopes
depends on the beam that is used to construct images.
Most microscopic methods may be grouped into one
of two types: ordinary microscopy, which uses certain
waves for sample illumination and a set of lenses for
construction of an image; and scanning microscopy,
which uses a tiny probe that is scanned over the sam-
ple to construct a microscopic image synchronously
displayed on a two-dimensional screen. In the former
type of microscopes, the dominant factor governing the
spatial resolution is the wavelength of the wave or the
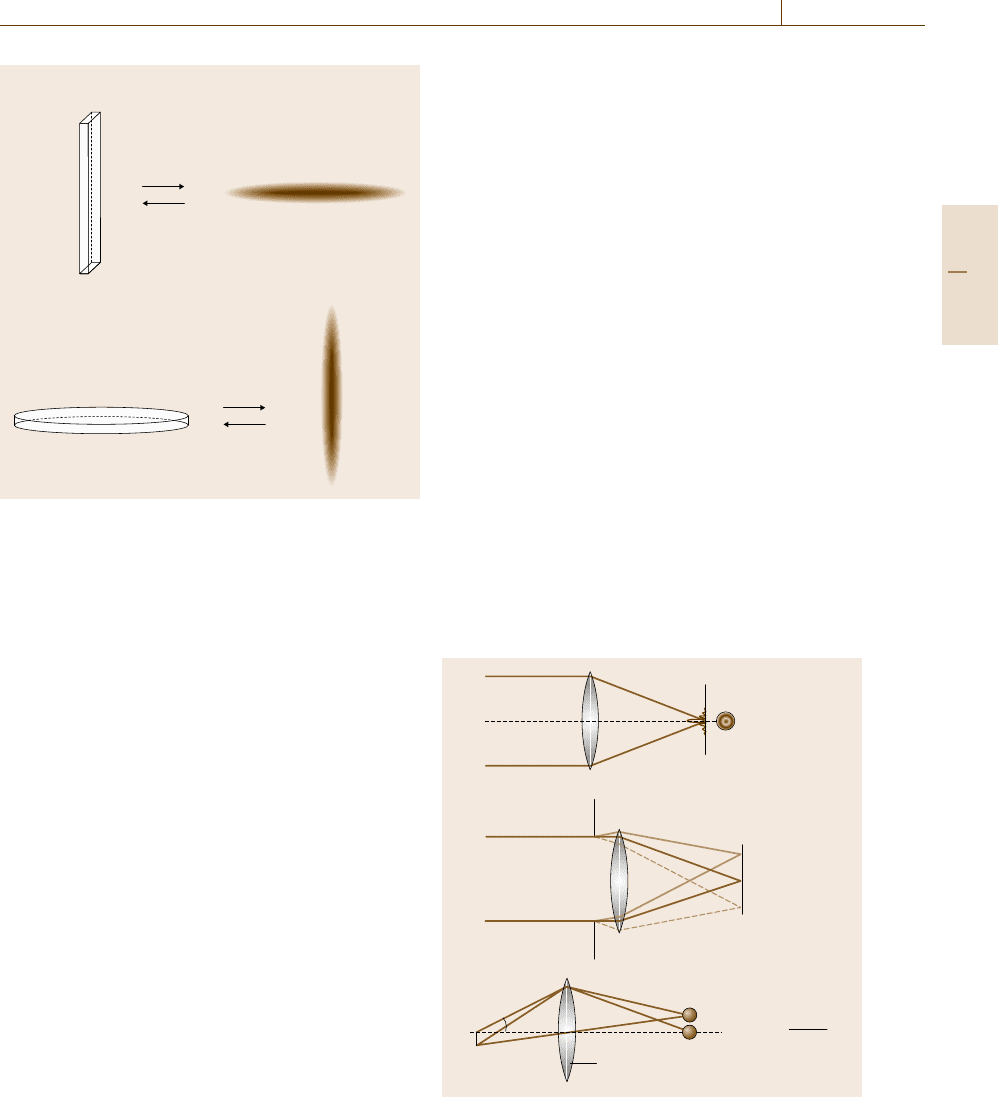
aberration of the objective lens (the convex lens near-
est to the object being observed). Even if the lens is
free of aberration, its focus is not a perfect point but is
blurred by the effect of wave diffraction due to the finite
lens diameter (Fig. 5.7). Owing to the parallel imaging
capability in the former type of microscopes, one may
observe in situ the dynamic change or motion of objects,
which could be recorded by a video camera, although
the same may also be possible in the latter type if the
scanning rate is high enough to follow the events. Fig-
ure 5.8 shows the range of scales that is covered by var-
ious microscopes. The lower limit and the upper limit
of the lateral scale indicate the lateral resolution and the
maximum size of the specimen that can be surveyed,
respectively. The lower and upper limits of the vertical
scale indicate the sample thinness required for micro-
scopic observations and the sample thickness allowed
for experiments, respectively. Other features of micro-
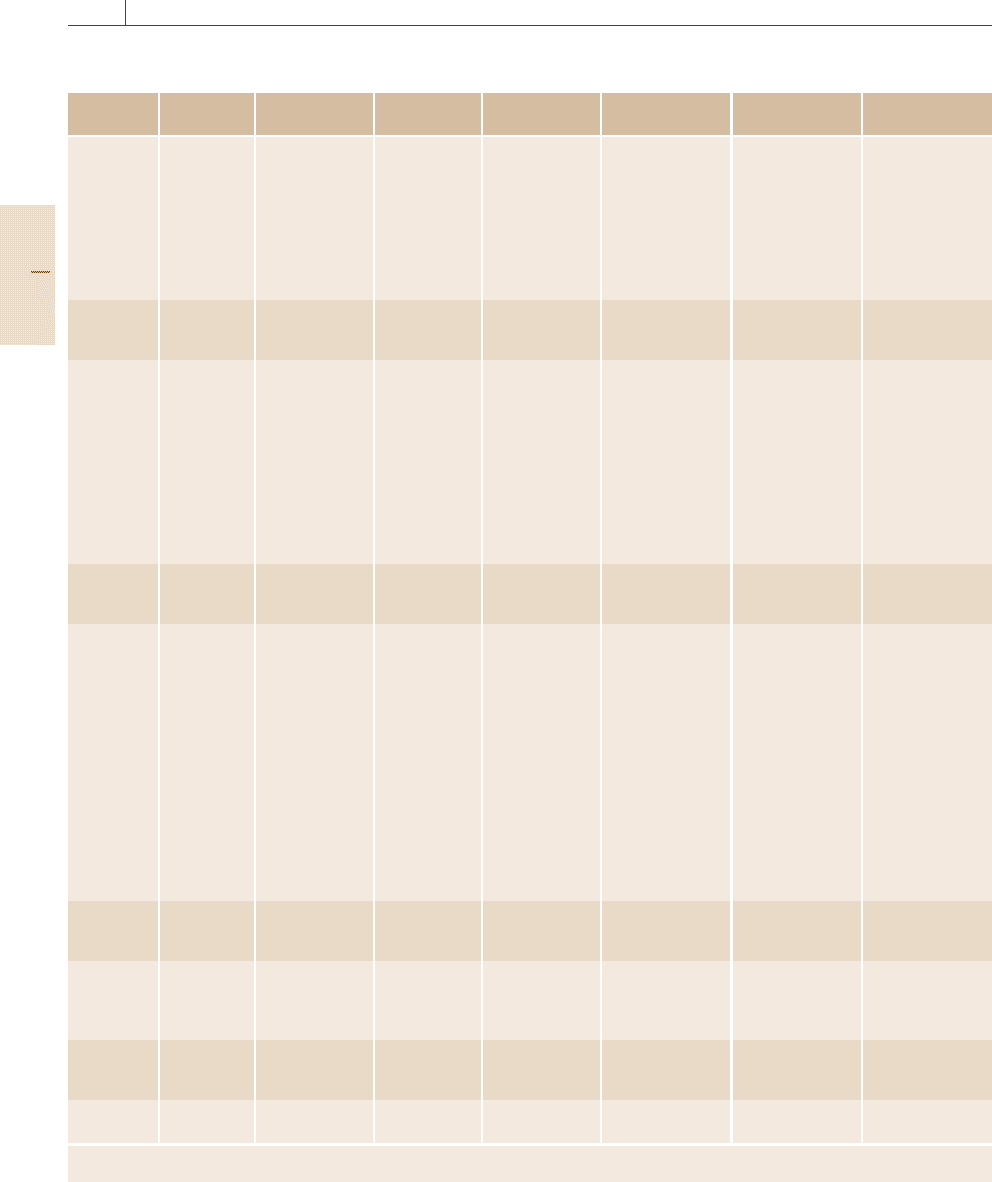
scopic techniques are also summarized in Table 5.3.
Optical Microscopy (OM)
We mention here only briefly three types of optical
microscopy (OM): interference microscopy, which is
mature but plays important roles in the assessment
of material microstructures; laser scanning confocal
α
ε
n
Point resolution:
ε
= 0.67
λ
n sinα:
numerical aperture
n sinα:
a)
b)
c)
Fig. 5.7a–c Point resolution in microscopes limited by
wave diffraction. In lenses of finite size, even if aberration-
free, the focal point is blurred due to the diffraction effect.
(a) is equivalent to (b); (c) defines the point resolution
Part B 5.1
216 Part B Chemical and Microstructural Analysis
Table 5.3 Comparison of various microscopic techniques
Method Operation
mode
Object Resolution Sample
requirement
Test
environment
Merit Demerit
Optical
microscopy
Interference
microscopy
Steps < 1μm Transparent or
reflective
Ambient, liquid Easy operation,
wide survey
range,
dynamic
observation
Limited
magnification
LSCM Light scatterers,
fluorochromes
TIRFM fluorochromes
X-ray
topography
Internal
architecture
< afewμm
a
High quality
single crystal
Ambient,
gaseous, vacuum
Applicable to
bulky samples
Low
magnification
TEM Two-wave
diffraction
Dislocations,
planar defects
Several tens
nm
Thickness
< 1 μm
a
Vacuum Crystallographic
analysis,
dynamic
observation
Narrow survey
range
Weak beam Dislocations 0.5nm Thickness
< 1 μm
a
Vacuum
HRTEM Extended
defects, layer
stacking
0.2nm Thickness
< 100 nm
a
Vacuum Atomic-level
resolution,
dynamic
observation
Critical sample
preparation
STEM HAADF Extended
defects,
impurities
< 0.2nm Thickness
< several tens
nm
a
Vacuum Insensitivity to
focusing and
sample thickness
Slow image
acquisition
SEM SEM-SE Surface
morphology
< 1nm High SE
efficiency,
electrically
conductive
Vacuum, gas Easy operation,
wide survey
range, dynamic
observation
Little
information on
material
substance
SEM-EBIC Recombination
centers in
semiconductors
≈1 μm
a
Semiconductors
with electrodes
High Vacuum
(HV)
High sensitivity Needs electrical
contacts
SEM-CL Cathodo-
luminescent/
nonradiative
centers
≈1 μm
a
Luminescent
semiconductors
HV Nondestructive
spectroscopic
Limited to
luminescent
materials
EPMA Element
analysis
≈1 μm X-ray
fluorescence
HV, Ultra-High
Vacuum (UHV)
Established Vulnerable to
contamination
FIM Surface atoms < 0.1nm Electrically
conductive thin
needles
UHV Atomic
resolution
Difficult sample
preparation
STM Constant
current
Surface LDOS,
subsurface
defects
< 0.1nm Electrically
conductive
clean surface
Ambient, UHV,
liquid
Affinity with
various micro-
spectroscopic
techniques
Limited imaging
speed, difficulty
in reproducible
tip preparation
AFM Noncontact Surface atoms,
surface
morphology
≈0.2nm
(depends)
Roughness
< 1 μm
a
Ambient, UHV,
liquid
Applicable to
nonconductive
samples
Limited imaging
speed
SNOM Va ri ab le Var ia bl e Several tens
nm
Optically
active
Ambient,
UHV, liquid
Many potential
applications
Limited imaging
speed
a
Variable depending on the material and experimental conditions
Part B 5.1
Nanoscopic Architecture and Microstructure 5.1 Fundamentals 217
microscopy (LSCM) of the scanning type; and total
internal reflection fluorescence microscopy (TIRFM),
a relatively new technique with expanding applications
particularly to biological substances. For the principles
of conventional OM, the interested reader should refer
to [5.13].
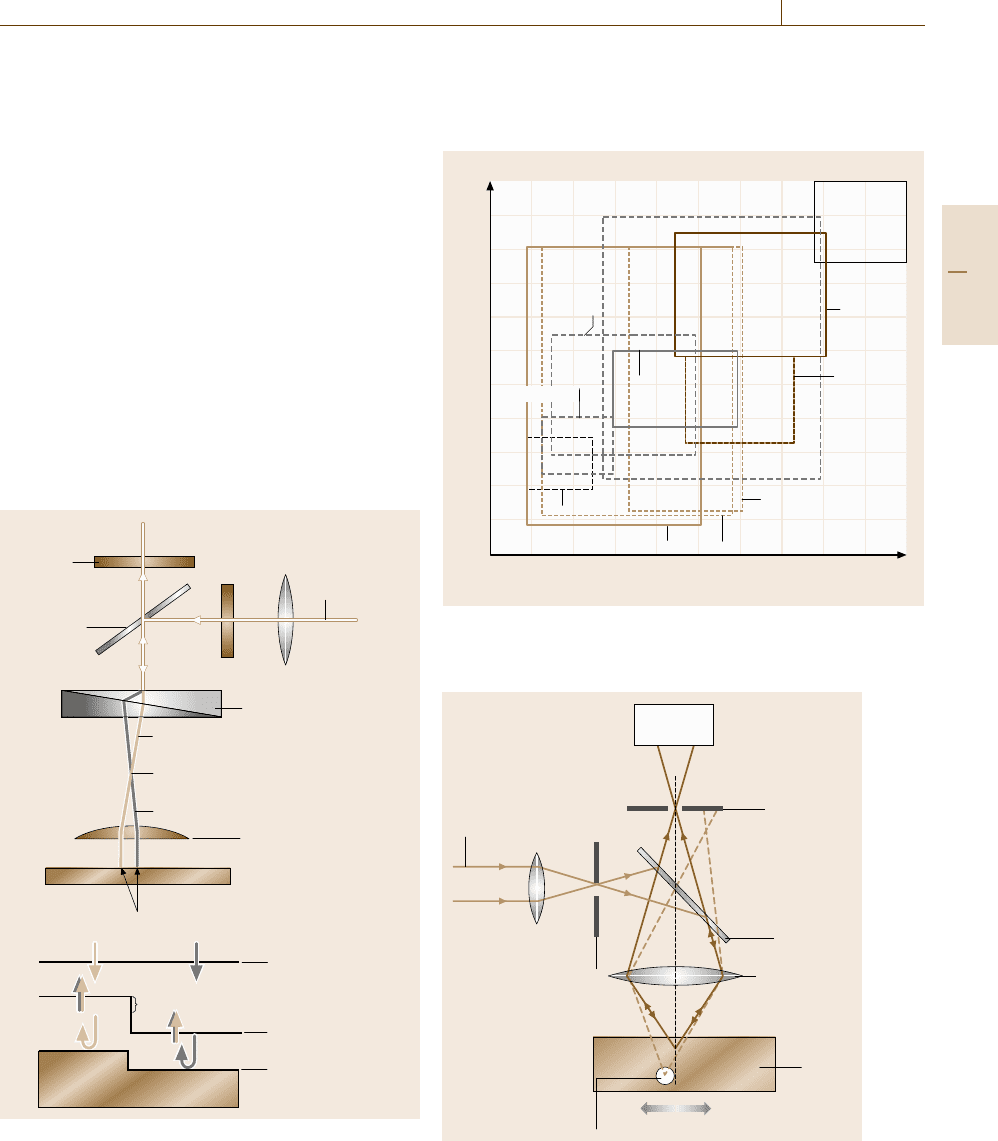
Interference microscopy is based on the phase shifts
of the illuminating light that take place when the light
passes through a transparent sample with different re-
fraction indices or when it is reflected at a surface
with steps of different heights. The interference of this
light with a reference light forms interference fringes
or, in differential interference Nomarski microscopes
(Fig. 5.9), the interference of two slightly displaced
beams is converted to different colors with high spa-
tial resolution, which allows the measurement of step
heights with a precision of 20 nm.
LSCM is classified as a scanning microscope, us-
ing a laser beam as a probe. The principle is shown in
Analyzer
Interference color
Half mirror
White light
Nomarski prism
Extraordinary ray
Backfocal point of the objective lens
Ordinary ray
Objective lens
Sample
Slightly displaced
a)
b)
Incident wave front
Reflected wave front
Sample surface
Phase shift
Polarizer
Fig. 5.9 (a) The principle of differential interference opti-
cal (Nomarski) microscopy. (b) Phase shift that occurs in
reflecting waves at a step edge
Fig. 5.10, where a parallel laser beam focused at an en-
trance aperture is further focused by an objective lens
to a small spot in the sample and the scattered light is
10
–2
10
–3
10
–4
10
–5
10
–6
10
–7
10
–8
10
–9
10
–10
10
–11
10
–12
10
–11
10
–10
10
–9
10
–8
10
–7
10
–6
10
–5
10
–4
10
–3
10
–2
10
–1
Vertical scale (m)
Lateral scale (m)
Human
eye
SEM
OM
PCM
STEM
TEM
HRTEM
FIM
STM AFM
SNOM
Fig. 5.8 Scale range covered by various types of microscopic tech-
niques. For the meaning of the horizontal and vertical axes, see the
text
Photo-
detector
Exit aperture
Half mirror
Objective lens
Sample
ScanRegion to be omitted
Laser beam
Entrance
aperture
Fig. 5.10 The principle of laser scanning confocal micro-
scopy
Part B 5.1
