Czichos H., Saito T., Smith L.E. (Eds.) Handbook of Metrology and Testing
Подождите немного. Документ загружается.

1068 Part E Modeling and Simulation Methods
selected. The bcc-andfcc-based ordered phases are in-
cluded in the calculations. As can be seen in this table,
the calculated values and evaluated data are reasonably
consistent, and this close agreement encourages us to
proceed to the next step to evaluate the Gibbs free ener-
gies of solution phases.
First-principles Calculations
of the Gibbs Free Energy
The bcc phase in the Fe
−
Be binary system is illustrated
as an example to derive the Gibbs energy on the ba-
sis of ab initio method, since the phase is metastable
and located in the central part of the phase diagram, the
thermodynamic properties in this region are unknown.
An outline for deriving the Gibbs free energies of
the bcc-based structures incorporating the ab initio cal-
culations is as follows. First, a set of superstructures
{A-A2, A
3
B–D0
3
, AB-B2, AB-B32, AB
3
–D0
3
,B-A2}
are selected to be representative of a series of bcc-based
ordered phases, the total energies were calculated us-
ing the FLAPW method. With the known total energies,
the formation energy of the bcc-based superstructures,
ΔE
φ
form
, is defined by averaging the total energy of the
elements with chemical composition to the segregation
limit, as shown in the following equation
ΔE
φ
form
(V) = E
φ
tot
(V ) −
1−x
φ
Be
E
bccFe
tot
(V
Fe
)
−x
φ
Be
E
bccBe
tot
(V
Be
) , (20.25)
Table 20.3 The values of ξ
φ
i
for the bcc superstructures {A-A2, A
3
B–D0
3
, AB-B2, AB-B32, AB
3
–D0
3
,B-A2}
Ordered structures ξ
0
ξ
1
point ξ
2
n.n.pair ξ
3
n.n.n.pair ξ
4
triangle ξ
5
tetrahedron
A-bcc 1 1 1 1 1 1
A
3
B–D0
3
1 1/2 0 −1 −1 −1
AB-B2 1 0 −1 1 −1 1
AB-B32 1 0 0 −1 0 1
AB
3
–D0
3
1 −1/2 0 −1 1 −1
B-bcc 1 −1 1 1 −1 1
Table 20.4 The formation energies to the segregation limit for the Fe
−
Be binary system in the D0
3
, B2, B32, and A2
structures in the ground state
Basic lattice Composition of Be Structure Formation enthalpy (kJ/mol)
bcc 0 Fe A2 0.0
bcc 0.25 Fe
3
Be D0
3
−12.3
bcc 0.5 FeBe B2 −31.4
bcc 0.5 FeBe B32 −9.3
bcc 0.75 FeBe
3
D0
3
−18.6
bcc 1 Be A2 0.0
where φ denotes the type of superstructure and V is the
volume.
Then, the effective cluster interaction energies,
{ν
i
(V)}, can be extracted from these formation energies
using the cluster expansion method (CEM) developed
by Connolly and Williams [20.10]. This leads to a set of
composition-independent parameters, from which the
energy of the set of superstructures can be reproduced
in terms of a set of correlation functions {ξ
φ
i
},
ΔE
φ
form
(V) =
γ
i=0
ν
i
(V )×ξ
φ
i
, (20.26)
where ν
i
(V ) is the effective interaction energy of the
i-point cluster, and ξ
φ
i
is the correlation function for
cluster i in the phase φ; ξ
φ
i
is defined as the ensemble
average of the spin operator σ(p), which takes values
of ±1, depending on the atom occupancy of the lat-
tice site p. The values of ξ
φ
i
for the superstructures
considered in this study are summarized in Table 20.3.
The formation energies to the segregation limit for the
Fe
−
Be binary system in the D0
3
, B2, B32, and A2
structures in the ground state are summarized in Ta-
ble 20.4 [20.11].
The upper limit of the summation in (20.26) γ speci-
fies the largest cluster that participated in the expansion.
In the case of the Fe
−
Be system, a tetrahedron cluster is
considered, as schematically illustrated in Fig. 20.4,as
being the largest cluster, since in the bcc structure, the
tetrahedron forms an irregular shape containing both the
Part E 20.2
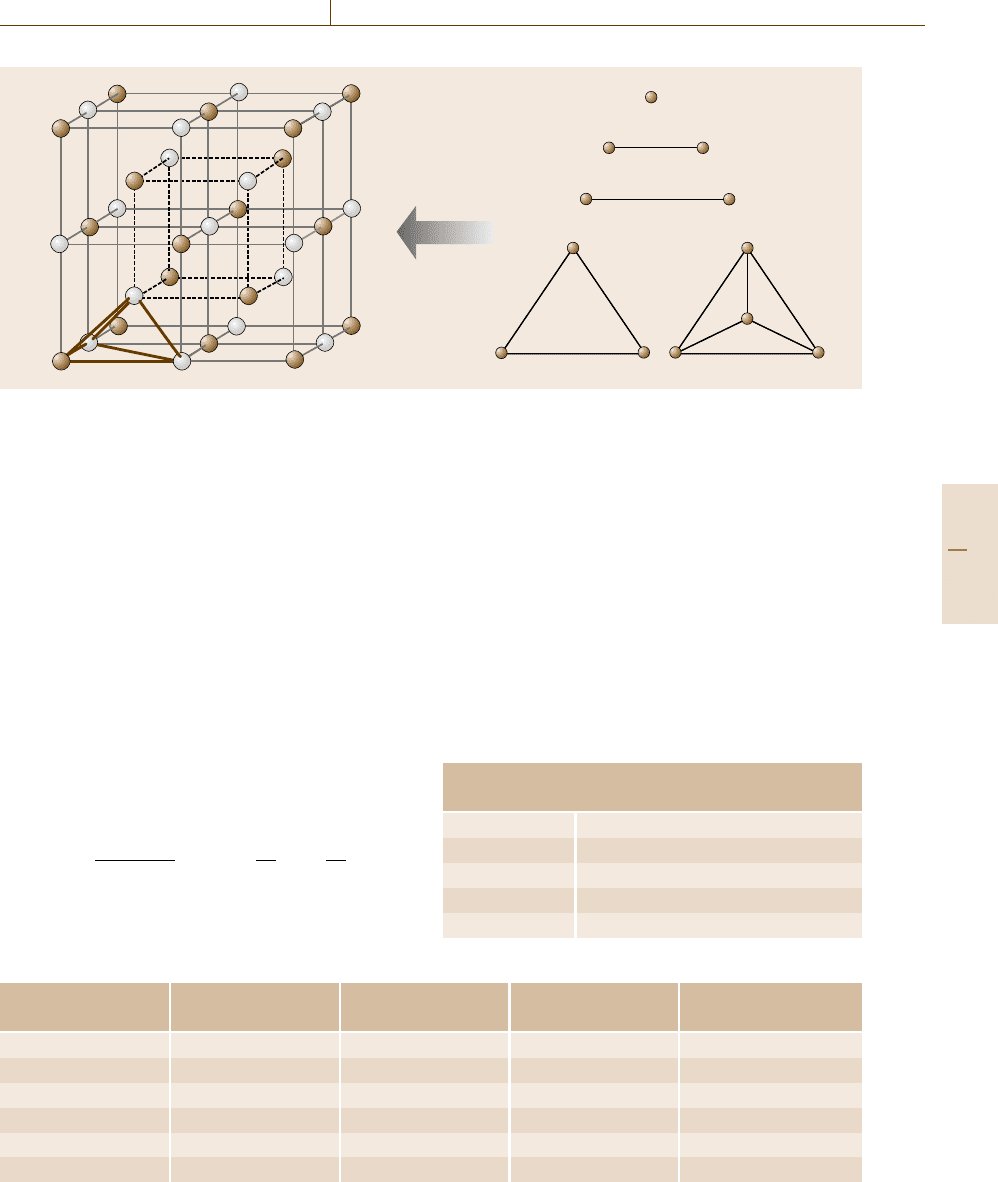
The CALPHAD Method 20.2 Incorporation of the First-principles Calculations into the CALPHAD Approach 1069
Fig. 20.4 Various clusters consisting of ordered structures
first- and second-nearest neighbor distances. The sec-
ond pair interaction was also taken into account, which
leads to the consequence that mathematically, five γ
should represent six types of cluster. From Table 20.3,
we can see that {ξ
φ
i
} is a 6× 6 matrix and that it is a reg-
ular matrix. The matrix inversion of (20.26) yields the
effective interaction energies as
ν
i
(V) =
5
i=0
ξ
φ
i
−1
ΔE
φ
form
(V) . (20.27)
Thus the interatomic interactions can be estimated by
expanding the total energies of the ordered structures
obtained from the band-energy calculations.
Using the Murnaghan equation of states [20.12], as
shownin(20.28), the total energies of the A2, B2, B32,
and the D0
3
structures were expressed as a function of
the volume
E(V ) =
BV
B
(B
−1)
B
1 −
V
0
V
+
V
0
V
B
−1
+E
V
0
, (20.28)
Table 20.5 The coefficient of the Murnaghan equation of states for the bcc superstructures
Structures B B
E(V
0
) V
0
(GPa) (Ry/a.u.
3
) (Ry) (a.u.
3
)
Fe (bcc) 334.2450 5.5525 −5091.1796 155.5298
Fe
3
Be (D0
3
) 75.3890 0.7851 −3833.1698 144.3524
FeBe (B2) 180.8774 3.6243 −2575.1689 124.0438
FeBe (B32) 87.6360 5.9438 −2575.1367 122.6823
FeBe
3
(D0
3
) 81.5496 3.6914 −1317.1225 112.6662
Be (bcc) 244.0350 3.0866 −59.0657 105.6658
where B, B
,andV
0
are the bulk modulus, its pressure
derivative, and the equilibrium volume, respectively,
at normal pressures. Table 20.5 shows the coefficients
in (20.28) for each ordered structure. In this table,
E(V ) is the total energy of each ordered structure
in the equilibrium volume of the B2 structure; i. e.,
V
0
= 124.0438 a.u.
3
. The formation energy of each
structure is represented as ΔE
φ
form
, by defining the
average concentration of the total energy of the bcc
Fe and the bcc Be phases at the segregation limit.
By applying (20.28) to these formation energies, the
Table 20.6 The effective interaction {ν
i
(V )} correspond-
ing to the clusters
Effective cluster interactions (kJ/mol)
Tetrahedron CVM
ν
1
−4.0
ν
2
28.8
ν
3
−4.8
ν
4
4.0
ν
5
6.2
Part E 20.2
1070 Part E Modeling and Simulation Methods
effective interaction, {v
i
(V )}, corresponding to each
cluster is calculated, as shown in Table 20.6, where
the positive numbers define the attractive force work-
ing between unlike atoms. The value of ν
2
represents
the interaction energy between the nearest neighbor
atoms, while ν
3
denotes the next-nearest neighbor
interaction.
The Gibbs free energies of the metastable bcc-based
phase in the Fe
−
Be binary system are evaluated us-
ing the effective cluster interaction energies up to the
tetrahedron cluster, including a second pair interaction
as
ΔE =
5
i=0
ν
i
ξ
i
. (20.29)
At a finite temperature T the free energy of a phase
of interest ΔG can be obtained by adding a config-
urational entropy term ΔS to the internal energy as
follows [20.13,14]:
ΔG = ΔE −TΔS . (20.30)
The cluster variation method (CVM) with the tetrahe-
dron approximation are used to calculate the config-
urational entropy. For the bcc structure, the entropy
0
–5
–10
–15
–20
–25
–30
–35
0.2 0.4 0.6 0.8Fe Be
0
–5
–10
–15
–20
–25
–30
–35
0.2 0.6 0.8Fe Be0.4
Gibbs free energy ΔG (kJ mol) Gibbs free energy ΔG (kJ mol)
227°C
ab initio calculation
727°C
bcc–A2
bcc–B2
bcc–A2
bcc–B2
Molar fraction x
B
Molar fraction x
B
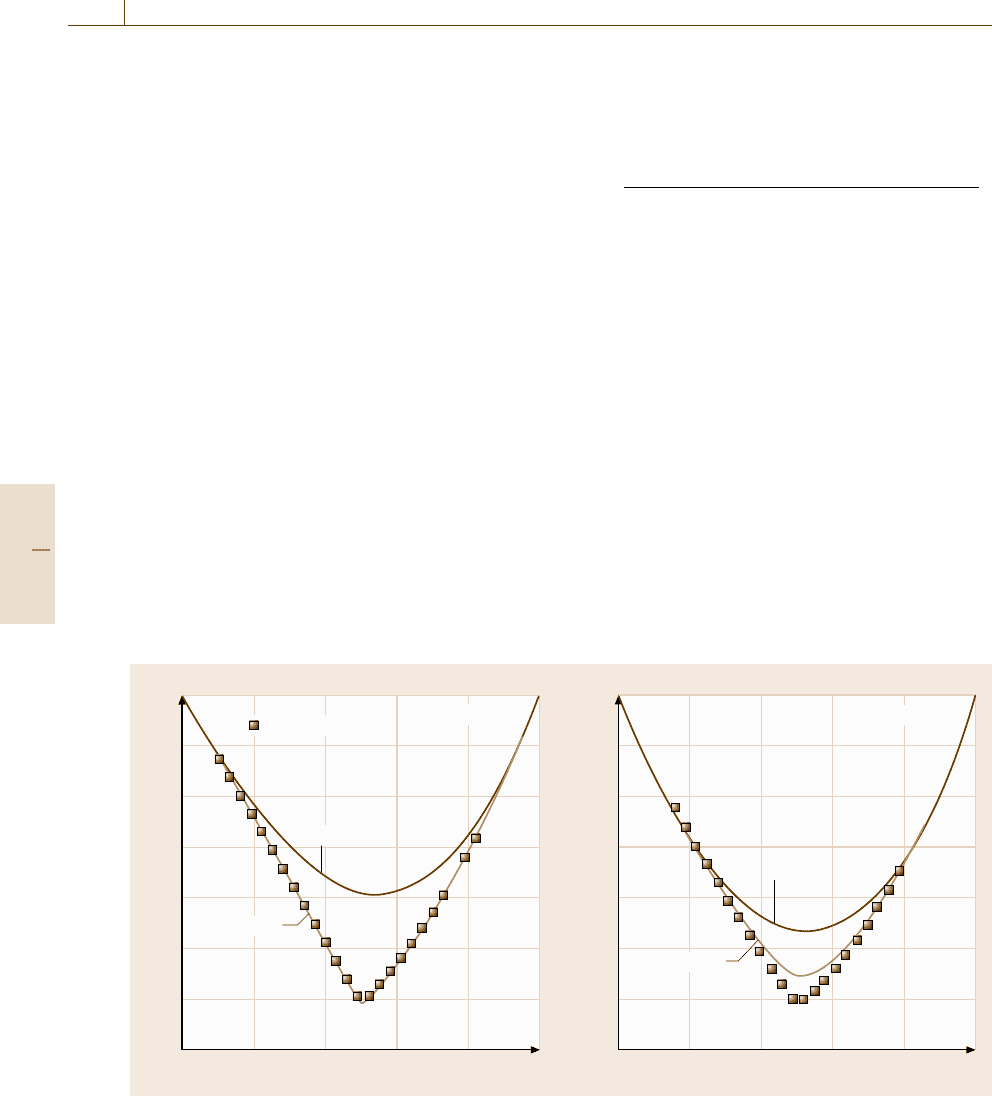
Fig. 20.5 The Gibbs energies of mixing in the α bcc solid solution at 227
◦
C and 727
◦
C based on ab initio energetic
calculations
formula is
ΔS = k
B
×
ln
i, j,k
Nz
ijk
!
12
i
(
Nx
i
)
!
i, j,k,l
Nw
ijkl
!
6
i, j
Ny
ij
!
4
i, j
Ny
ij
!
3
,
(20.31)
where x
i
, y
ij
, y
ij
, z
ijk
,andw
ijkl
are the cluster probabil-
ities of finding the atomic configurations specified by
the subscript(s) at a point, the nearest neighbour pair,
the second-nearest neighbour pair, the triangle, and the
tetrahedron clusters, respectively, and N is the number
of lattice points. Minimizing the grand potential with
respect to all the correlation functions allows for the
Gibbs energy of mixing to be obtained as a function of
composition at a constant temperature, T.
The compositional variation of the formation en-
thalpy derived by the cluster expansion and the cluster
variation methods is shown in Fig. 20.5 [20.11]. The
solid lines show the results of thermodynamic analyses
described in the following section. The open squares de-
note the corresponding Gibbs free energy derived by the
method shown in this section.
Part E 20.2
The CALPHAD Method 20.2 Incorporation of the First-principles Calculations into the CALPHAD Approach 1071
20.2.3 Thermodynamic Analysis
of the Gibbs Energies Based
on the First-principles Calculations
The Gibbs free energy of the bcc phase derived using
the first-principles calculations is analyzed according to
the two-sublattice model. According to Table 20.4,the
most stable ordered structure in the Fe
−
Be bcc phase
is recognized as a B2 structure, and hence the Gibbs
free energy for this simple structure is described in this
section. The Gibbs energy for one mole of φ phase,
denoted as (Fe, Be)
m
(Fe, Be)
n
, is represented by the
two-sublattice model as described in Sect. 20.1.1 as the
following equation:
G
φ
= y
1
Fe
y
2
Fe
0
G
φ
Fe:Fe
+y
1
Fe
y
2
Be
0
G
φ
Fe:Be
+y
1
Be
y
2
Fe
0
G
φ
Be:Fe
+y
1
Be
y
2
Be
0
G
φ
Be:Be
+RT
m
m +n
y
1
Fe
ln y
1
Fe
+y
1
Be
ln y
1
Be
+
n
m +n
y
2
Fe
ln y
2
Fe
+y
2
Be
ln y
2
Be
+
ex
G
φ
+G
mag
. (20.32)
The term y
s
i
denotes the site fraction of element i in
the sublattice s. The terms m and n are variables denot-
ing the size of the sublattice s, and straightforwardly,
the relationships of m = 0.5andn = 0.5 hold for the
B2 structure.
0
G
i:j
denotes the Gibbs energy of a hy-
pothetical compound i
0.5
j
0.5
, and terms relative to the
same stoichiometry are identical, whatever the occupa-
tion of the sublattice. The excess Gibbs energy term,
ex
G
φ
, contains the interaction energy between unlike
atoms, and is expressed using the following polynomial
ex
G
φ
= y
1
Fe
y
1
Be
y
2
Fe
L
Fe,Be:Fe
+y
1
Fe
y
1
Be
y
2
Be
L
Fe,Be:Be
+y
2
Fe
y
2
Be
y
1
Fe
L
Fe:Fe,Be
+y
2
Fe
y
2
Be
y
1
Be
L
Be:Fe,Be
,
(20.33)
where L
i, j:k
(or L
i:j,k
) is the interaction parameter
between unlike atoms on the same sublattice. The mag-
netic contribution to the Gibbs free energy G
mag
was
given by (20.11).
The thermodynamic parameters obtained by fitting
the ab initio values to (20.32) are shown as follows:
G
β
Be:Fe
−0.5
0
G
bcc
Be
−0.5
0
G
bcc
Fe
=−37 100 +9T (J/mol) ,
G
β
Fe:Be
−0.5
0
G
bcc
Be
−0.5
0
G
bcc
Fe
=−37 100 +9T (J/mol) ,
0
L
β
Be,Fe:Be
=
0
L
β
Be:Be,Fe
=−4T (J/mol) ,
0
L
β
Fe:Be,Fe
=
0
L
β
Be,Fe:Fe
=−380−4T (J/mol) .
The calculated Gibbs energies of mixing in the α bcc
solid solution at 227 and 727
◦
CaredrawninFig.20.5
using the solid lines for the ordered state (bcc−B2) and
the disordered state (bcc−A2), respectively. The con-
vex curvature of the free energy in the vicinity of the
equiatomic composition corresponds to the formation
of the B2 structure.
20.2.4 Construction of Stable
and Metastable Phase Diagrams
The information on the experimental data on the phase
boundaries and other thermodynamic quantities are
thermodynamically analyzed together with the esti-
mated metastable quantities of the bcc phase described
in the foregoing section.
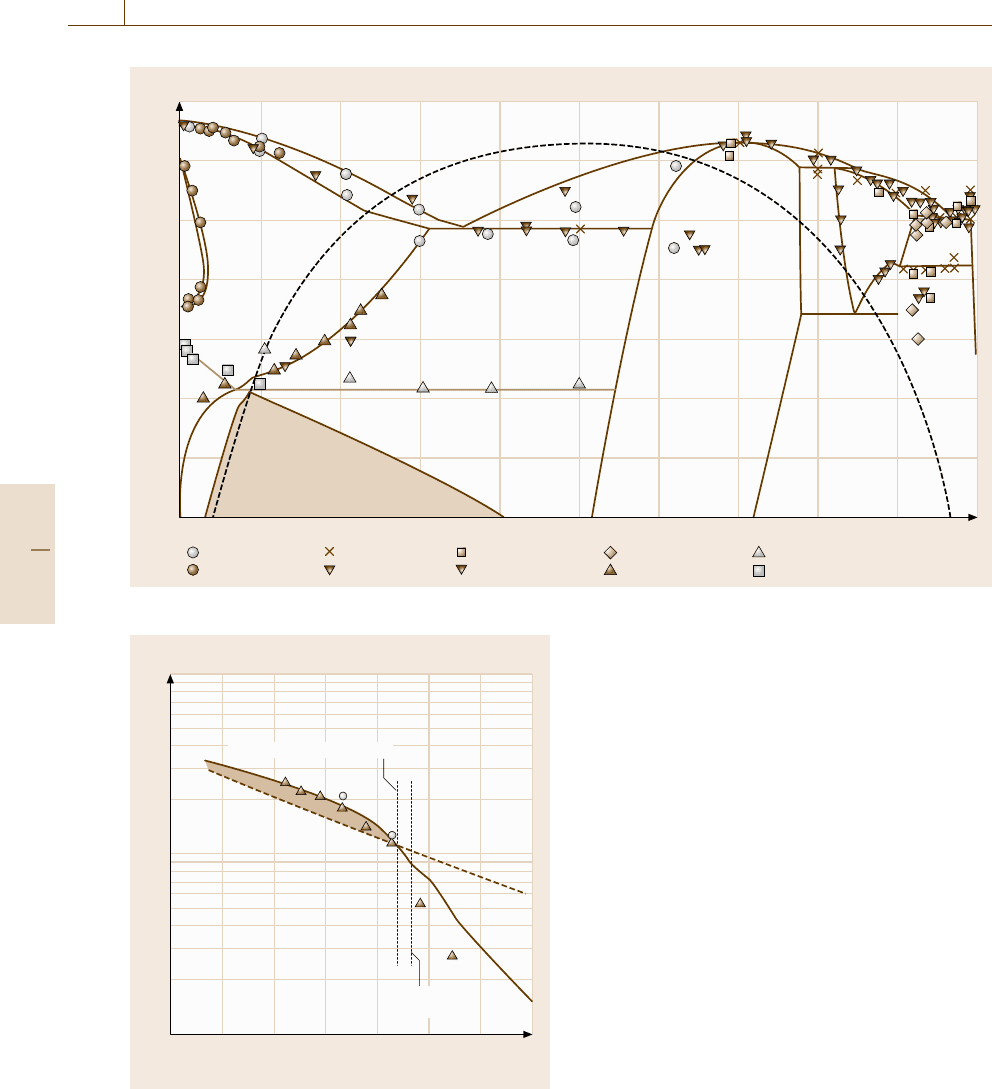
The calculated results of the Fe
−
Be phase diagram
are compared with the experimental data in Fig. 20.6.
The shaded area shown in this figure is the metastable
(bcc+B2) two-phase region, which is accompanied by
the ordering of the bcc structure on formation. The dot-
ted line shows the order–disorder transition line, along
which the two-phase field expands into the higher tem-
perature range. The age hardening of this alloy has been
investigated experimentally and the results are summa-
rized in [20.15], and a brief outline of the ageing process
of this alloy is as follows. The disordered bcc structure
forms in the initial stage, and consequently, the B2-type
ordered structure separates in the bcc phase. A 100
modulated structure with changes in concentration was
observed in some samples using electron microscopy.
This ordering behavior of the bcc structure is possibly
explained by the metastable equilibria in Fig. 20.6.
It is well known that the solubility of Be in bcc
Fe (α) deviates significantly from the Arrhenius equa-
tion; i. e., a proportional relationship exists between the
logarithm of the solubility and the reciprocal of the tem-
perature. The solubility of Be in the α phase is shown
in Fig. 20.7. The solubility would be denoted by the
broken line if there were neither an order–disorder tran-
sition nor a magnetic transition in the bcc Fe phase.
This is approximated by the straight line following the
Arrhenius law for dilute solutions. Thus, the deviation
of the solubility from the ideal Arrhenius law is repre-
Part E 20.2
1072 Part E Modeling and Simulation Methods
1600
1400
1200
1000
800
600
400
200
Fe 10 20 30 40 50 60 70 80 90 Be
Be (mol. %)
Temperature T (°C)
L
α
ζ
ε
Oesterheld
Wever
Geles
Heubner
Hammond
Ko
Oesterheld
Wever
Gordon
Teitel
bcc + B2
δ
γ
Fig. 20.6 Calculated Fe
−
Be phase compared with experimental data
10
2
10
1
10
0
6 7 8 9 10 11 12 13
Reciprocal temperature 10
4
/T (K
–1
)
Order–disorder transition
Magnetic
transition
Solubility of Be in α-Fe (mol. %)
Fig. 20.7 Solubility of Be in the α phase
sented by the shaded areas. The dashed lines show the
order–disorder transition temperature and the magnetic
transition temperature of the bcc Fe phase. The solu-
bility of Be decreases in the lower temperature region
below 650
◦
C, while it increases in the higher temper-
ature region. The decrease in solubility in the lower
temperature region can be explained from the viewpoint
of the change in magnetism [20.16]. On the other hand,
the solubility increases owing to the order–disorder
transition of the bcc phase in the higher temperature
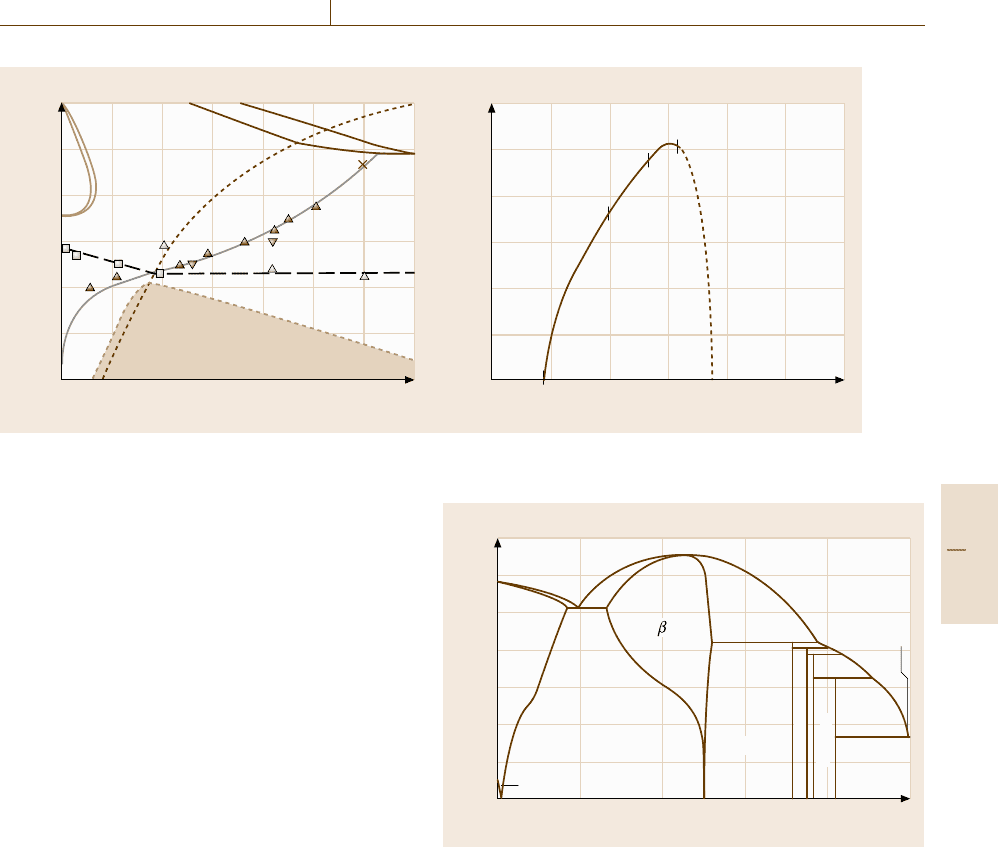
range. Figure 20.8 shows the change in the order pa-
rameter along a solubility line for the α phase. The order
parameter was defined by
η = y
1
Be
−y
2
Be
. (20.34)
The solubility line in Fig. 20.8a intersects the order–
disorder transition line indicated by the dotted line at
about 650
◦
C. As can be seen in Fig. 20.8b, the order
parameter along the solubility line increases at a higher
temperature than 650
◦
C, yielding the progress of the
B2 ordering in the bcc disordered phase.
Part E 20.2
The CALPHAD Method 20.2 Incorporation of the First-principles Calculations into the CALPHAD Approach 1073
1400
1200
1000
800
600
400
200
Fe 5 101520253035
Be (mol. %)
0.6
0.5
0.4
0.3
0.2
0.1
0
Be contenet in α phase (mol. %)
Fe 10 20 30 40 50 60
a) Temperature T (°C)
bcc+B2
γ
α
b) Order parameter
1190 °C
1000 °C
830 °C
650 °C
Fig. 20.8 (a) Enlarged Fe-rich portion of the calculated Fe
−
Be binary phase diagram. The dotted line shows the order–
disorder transition line. (b) Change in the order parameter along the solubility line for the α phase
20.2.5 Application to More Complex Cases
In the foregoing Sect. 20.2.3, two-sublattice formalism
was applied to describe the Gibbs energies derived
from the first-principles calculations. However, it is
generally true that a more complex thermodynamic for-
malism such as a four-sublattice model is appropriate
so as to reflect directly the results of the ab initio
values in phase diagram computations, since several
stoichiometric compositions are selected for superstruc-
tures, i. e., A
3
B–D0
3
, AB-B2, AB-B32, AB
3
–D0
3
etc.
in the bcc phase. Then in this section, an analysis of
metastable phase separation of bcc phase in the Co
−
Al
and Ni
−
Al binary systems is presented [20.17] using
the four-sublattice model. The more complex thermo-
dynamic modeling brings a detailed knowledge about
the metastable phase equilibria in these binary systems.
The Co
−
Al phase diagram consists of liquid, two
fcc solid solutions γ (Co) and γ (Al), hcp Co(ε), β with
CsCl structure, and the intermetallic phases Co
2
Al
5
,
CoAl
3
,Co
4
Al
13
,andCo
2
Al
9
, as shown in Fig. 20.9.
There exists one eutectic reaction concerning the li-
quid phase and six invariant reactions between the solid
phases. The B2 (β) phase has a large homogeneity
range at higher temperatures, but the range decreases
remarkably in the lower temperature region. It has been
confirmed experimentally that the two-phase region for
the A1(γ )/B2(β) phases extends over a wide com-
position range with decreasing temperature. Owing to
a sudden drop in the homogeneous region, these exper-
imental values were debatable.
2000
1800
1600
1400
1200
1000
800
600
Co 20 40 60 80 Al
Temperature T(K)
Al (mol.%)
γ(Co)
ε
(hcp)
γ (Al)
L
Co
2
Al
5
CoAl
3
Co
4
Al
13
Co
2
Al
9
Fig. 20.9 Outline of the Co
−
Al binary phase diagram
Formation Energies of Superstructures
for the Co–Al System
The formation energies to the segregation limit for
the Co
−
Al system in the D0
3
, B2, B32 structures in
the ground state are summarized in Table 20.7, where
the corresponding values for the Ni
−
Al system are
included. The Gibbs free energies of the metastable bcc-
based phase are evaluated using the effective cluster
interaction energies up to the tetrahedron cluster, in-
cluding the second pair interactions. Using the same
procedure as in Sect. 20.2.2, the Gibbs energy of mix-
ing can be obtained as a function of composition at
a constant temperature T .
Part E 20.2
1074 Part E Modeling and Simulation Methods
Table 20.7 Formation energies to the segregation limit for Co
−
Al and Ni
−
Al in the D0
3
, B2, B32, and A2 structures in
the ground state
Alloy system Molar fraction of Al Structure Formation energy (kJ/mol)
Co
−
Al 0.25 D0
3
−12.7
0.5 B2 −62.3
0.5 B32 −23.7
0.75 D0
3
−18.3
Ni
−
Al 0.25 D0
3
−40.7
0.5 B2 −69.5
0.5 B32 −34.0
0.75 D0
3
−18.2
Expression of Gibbs Energy of the B2 Phase
Using the Four-Sublattice Model
The β phase was described using a four-sublattice
model, so as to reflect the results of the ab initio cal-
culations on the phase diagram computation as
Al
1
y
1
Al
M
1
y
1
M
0.25
Al
2
y
2
Al
M
2
y
2
M
0.25
Al
3
y
3
Al
M
3
y
3
M
0.25
Al
4
y
4
Al
M
4
y
4
M
0.25
. (20.35)
The number of each sublattice site is 0.25, and the four
sites are therefore equivalent. The disordered state is de-
scribed when the site fractions of the different species
are the same in the four sublattices. For the ordered
structures, if two sublattices have the same site frac-
tions, as do the two others, but are different, then the
0
–10
–20
–30
–40
–50
–60
–70
Co Al0.2 0.4 0.6 0.8
Gibbs free energy ΔG (kJ/mol)
Molar fraction x
Al
ab initio calculation
Thermodynamic analysis
Fig. 20.10 The calculated Gibbs free energy of the bcc
phase in the Co
−
Al binary system at 727
◦
C compared
with the results from the ab initio energetic calculations
model describes the B2 and B32 phases. If three sublat-
tices have the same site fractions, and are different from
the fourth, then D0
3
ordering is described.
The Gibbs energy of the β phase is expressed by
G
m
=
i
j
k
l
y
1
i
y
2
j
y
3
k
y
4
l
0
G
i:j:k:l
+
RT
4
4
s=1
i
y
s
i
ln
y
s
i
+
4
s=1
i
j>i
k
l
m
y
s
i
y
s
j
y
r
k
y
t
l
y
u
m
L
i, j:k:l:m
,
(20.36)
where
0
G
i:j:k:l
denotes the Gibbs energy of a compound
i
0.25
j
0.25
k
0.25
l
0.25
, and terms relative to the same stoi-
chiometry are identical, whatever the occupation of the
sublattice. L
i, j:k:l:m
is the interaction parameter between
unlike atoms on the same sublattice.
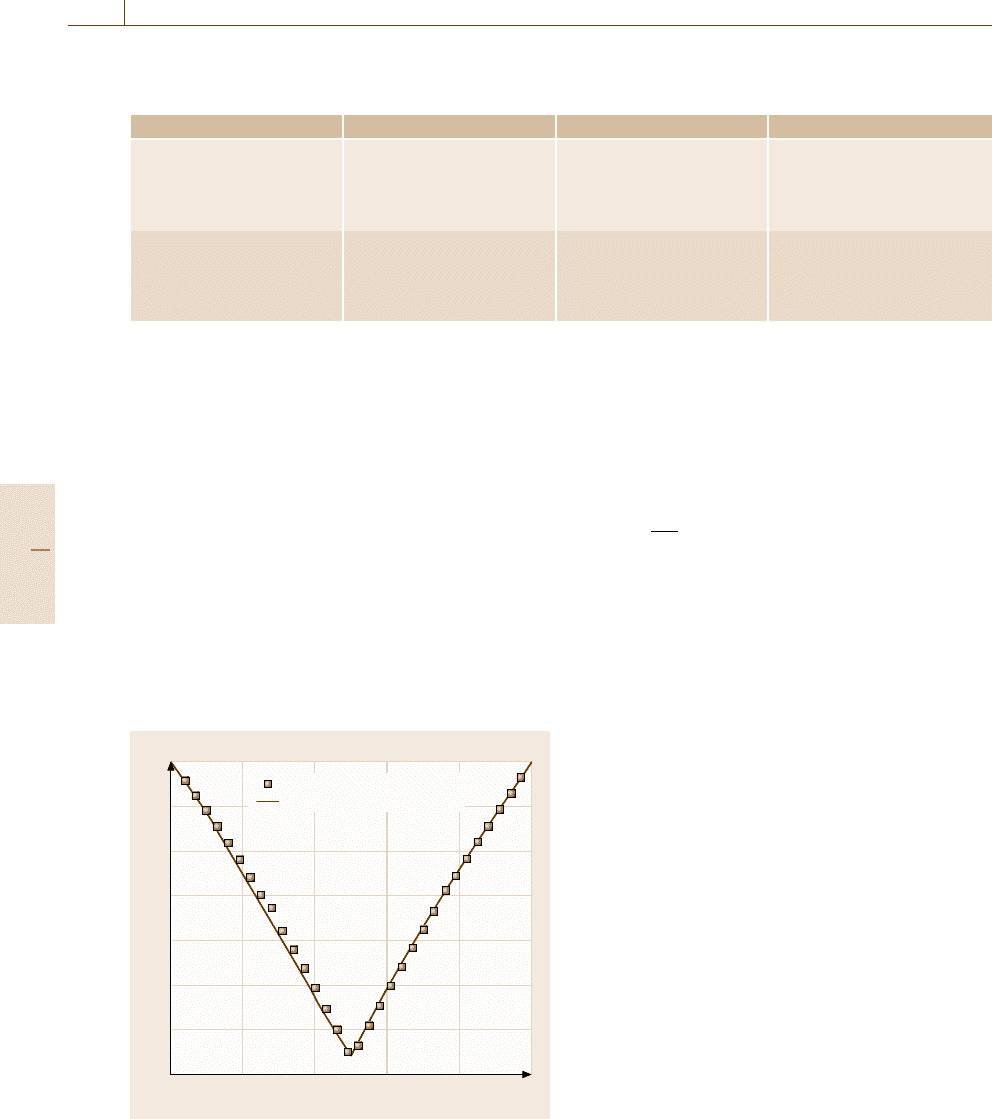
Calculated Phase Equilibria
in the Co–Al Binary System
The calculated Gibbs free energy of the bcc phases
at 727
◦
C was compared with the results from the ab
initio energetic calculations in Fig. 20.10. The calcu-
lated Co
−
Al binary phase diagram is compared with
the experimental data in Fig. 20.11. The characteristic
features of the binary phase diagram are well repro-
duced by the calculations. The dotted line shows the
metastable two-phase separated region between the A2
(bcc-Co) and the B2 (β) phases. The two-phase sepa-
ration, based on the bcc structure, closely relates the
anomaly in the phase boundaries in the Co
−
Al bi-
nary system. This situation is schematically illustrated
in Fig. 20.12, where the Gibbs free energies of the B2
(β)andtheA1(γ (Co)) phases are drawn. The two-
phase separation of the β phase does not occur at higher
temperatures T
1
mainly owing to the effect of the en-
Part E 20.2

The CALPHAD Method 20.2 Incorporation of the First-principles Calculations into the CALPHAD Approach 1075
2000
1800
1600
1400
1200
1000
800
600
400
40 60 80 AlCo 20
Temperature T(K)
Al (mol. %)
γ(Co)
L
ε
γ(Al)
Co
2
Al
5
Co
2
Al
9
Co
4
Al
13
CoAl
3
Gwyer
Fink
Koester
Schramm
Ettenberg
Takayama
Fig. 20.11 A comparison of the calculated Co
−
Al binary phase diagram with previous work
2000
1800
1600
1400
1200
1000
800
600
400
20 40 60 80Co Al
Al (mol. %)
Temperature T(K)Gibbs free energy
Al (mol. %)
T = T
1
Gibbs free energy
Al (mol. %)
G
γ
G
G
γ
G
T = T
2
T = T
1
γ(Co)
T = T
2
ε
Fig. 20.12 The schematic Gibbs free energies of the B2 (β)andA2(γ (Co)) phases in the Co
−
Al system
Part E 20.2
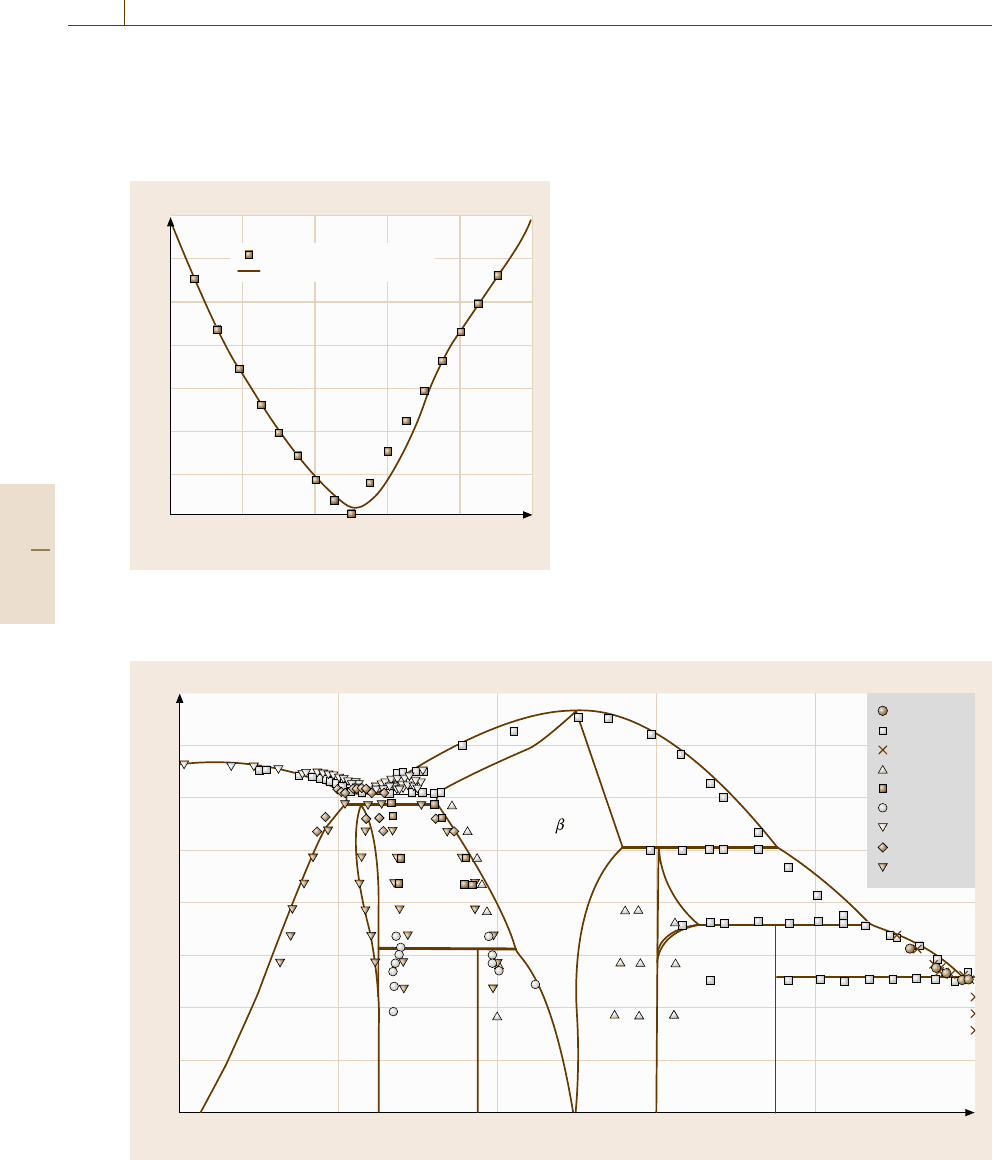
1076 Part E Modeling and Simulation Methods
tropy of random mixing on the atomic arrangement.
On the other hand, the tendency towards ordering be-
comes stronger at lower temperatures T
2
.IntheB2
structure, the interaction between unlike atoms strength-
0
–10
–20
–30
–40
–50
–60
–70
Ni 0.2 0.4 0.6 0.8 Al
Gibbs free energy ΔG (kJ mol)
ab initio calculation
Thermodynamic analysis
Molar fraction x
Al
Fig. 20.13 Calculated Gibbs free energy of the bcc phase
in the Ni
−
Al binary system at 727
◦
C compared with the
results from the ab initio energetic calculations
2000
1800
1600
1400
1200
1000
800
600
400
Ni 20 40 60 80 Al
Temperature T(K)
γ(Ni)
Al (mol. %)
L
γ(Al)
NiAl
3
Ni
2
Al
3
Ni
5
Al
3
γ
Fink
Alexander
Phillips
Taylor
Nash
Robertson
Hilpert
Verhoeven
Jia
Fig. 20.14 A comparison of the calculated Ni
−
Al binary phase diagram with previous work
ens around the 50% Al composition, since the degree
of order reaches a maximum at the equiatomic com-
position. The decrease in the enthalpy term due to the
attractive interaction results in the acute point in the
free energy curve in the vicinity of the 50% Al compos-
ition, and consequently, a two-phase separation in the
β phase forms. This situation yields a significant shift of
the phase boundary for the (γ (Co)+β)/β composition
to the equiatomic composition.
The procedure used for the Co
−
Al binary system
is applied to the Ni
−
Al binary system. The calculated
Gibbs free energy of the bcc phase is compared with
the results of the ab initio energetic calculations in
Fig. 20.13. The calculated Ni
−
Al binary phase diagram
is compared with the experimental data in Fig. 20.14.
The phase separation of the A2 and B2 structures can
not be observed in this binary system.
Origin of Phase Separation in the Co–Al
and Ni–Al Systems
The formation energies to the segregation limit for
Co
−
Al and Ni
−
Al in the D0
3
, B2, B32, and A2 struc-
tures in the ground state, as listed in Table 20.7, are
plotted versus Al concentration in Fig. 20.15.Incom-
paring the two systems, it can be seen that in the Co
−
Al
system, the energy of the D0
3
structure at the 25% Al
Part E 20.2
The CALPHAD Method 20.2 Incorporation of the First-principles Calculations into the CALPHAD Approach 1077
10
0
–10
–20
–30
–40
–50
–60
–70
–80
Co 0.2 0.4 0.6 0.8 Al
10
0
–10
–20
–30
–40
–50
–60
–70
–80
Ni 0.2 0.4 0.6 0.8 Al
Formation energy ΔE (kJ mol)
Formation energy ΔE (kJ mol)
Molar fraction x
Al
Molar fraction x
Al
bcc-Co bcc-Al
CoAl-B2
Co
3
Al-D03
CoAl-B32
CoAl
3
-D03
bcc-Ni bcc-Al
NiAl
3
-D03
NiAl-B32
Ni
3
Al-D03
Fig. 20.15 Variation of the formation energies to the seg-
regation limit for Co
−
Al and Ni
−
Al with concentration
of Al
composition is located slightly above the straight line
connecting the energy values at the 0% Al and 50% Al
compositions, which results in a two-phase separation
of the A2 (bcc-Co) and B2 (β) structures in Co
−
Al
that is more stable than the formation of the Co
3
Al–D0
3
structure in the ground state. On the other hand, the en-
ergy plot of the Ni
−
Al system shows the stabilization of
the Ni
3
Al–D0
3
structure compared with the two-phase
separation of the A2 (bcc-Ni) and B2 (β) structures,
since the formation energy of the D0
3
phase is lower
than the straight line connecting the energy values at the
0% Al and 50% Al compositions. This energetic analy-
sis suggests that a two-phase separation of the bcc-Co
andB2(β) phases occurs on the Co rich side in Co
−
Al
system, while such a separation is not realized in the
Ni
−
Al system.
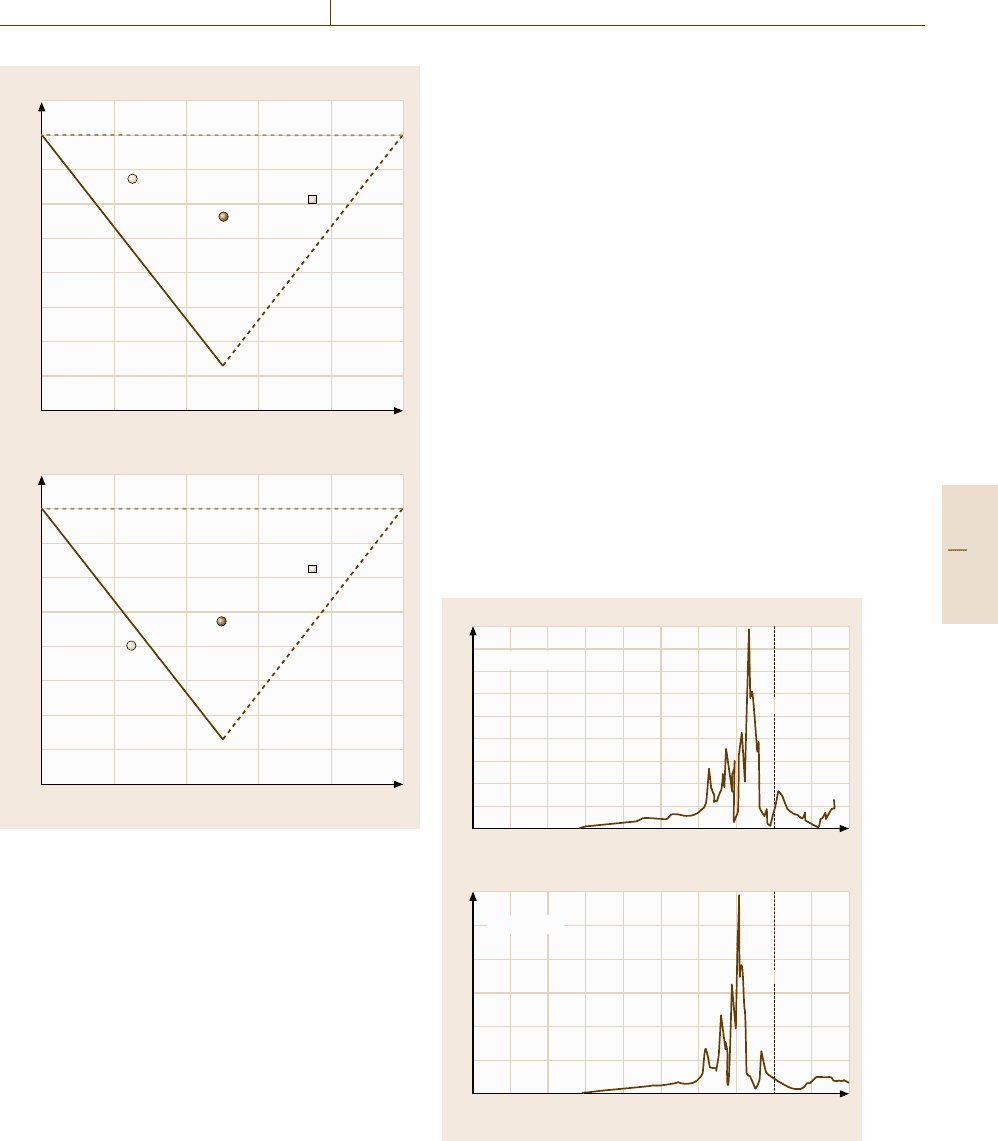
Figure 20.16 shows the density of states (DOS)of
the B2 (β) structure in the Co
−
Al and Ni
−
Al alloy
systems. In this figure, E
F
denotes the Fermi energy,
where no electrons occupy the electronic states above
this energy level. It can be seen that the distribution of
the DOS of the B2 phase in the two systems is simi-
lar, with both showing a low DOS at the Fermi level.
However, when observing the DOS of the D0
3
phase
for these two systems, as shown in Fig. 20.17, we ob-
serve a marked difference between these two systems,
in that the Fermi level is located near the peak of the
DOS for the Co
3
Al–D0
3
phase but decreases in a region
with a very low DOS in the Ni
3
Al–D0
3
phase. This fact
indicates that from an energetic point of view, the sta-
ble D0
3
structure of the Ni
3
Al phase is highly preferred,
while in the Co
3
Al–D0
3
phase is unstable with respect
to separation of the A2 and B2 phases in the Co
−
Al
system.
8
6
4
2
0
–16 –14 –12 –10 –8 –6 –4 –2 0 2 4
12
10
8
6
4
2
0
–16 –14 –12 –10 –8 –6 –4 –2 0 2 4
Total density of states
Total density of states
Energy E (eV)
Energy E (eV)
CoAl (B2)
NiAl (B2)
E
F
E
F
Fig. 20.16 The density of states of the B2 (β) structure in
the Co
−
Al and Ni
−
Al alloy systems
Part E 20.2
