Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.


505
(2)
Soda-base grease has a high melting point.
It
is soluble, however, in water.
(3)
Aluminum-base grease is water resistant. It has a comparatively low melting temperature;
(4) Lithium-base grease is water resistant and has a wide range of operating temperatures.
(5)
Barium-base grease is
also
water resistant and has high heat stability.
however, it changes texture (becoming leathery) on heating and cooling.
Penetration
of
lubricating greases
(D
21
7-82)
The penetration number indicates the consistency
of
greases, petrolatums, and similar plastic
petroleum products. Consistency of finished greases is affected primarily by the kind of soap used in
their manufacture. It is also affected by the amount
of
soap, method of manufacture, water content, and
rate of cooling. The penetration number is determined by grease manufacturers to control uniformity in
production and by users to compare greases. The penetration number, however, is not a true value
of
the
ability
of
a grease to perform in service.
To
predict performance, other characteristics must be known.
In testing grease, an instrument known as a
penetrometer
is used under prescribed conditions
of
temperature, load, and time.
This
instrument measures the depth to which a pointed cone penetrates the
grease when the cone is dropped into a sample from a given height. The depth
of
penetration, in tenths
of
a millimeter, is read
on
the scale
of
the penetrometer and reported as the penetration number. Low
penetration numbers indicate a stiff grease, whereas higher numbers are indicative
of
softer greases.
Key points
erroneous results, because it is equivalent
to
overworhng the grease beyond the specified
60
strokes.
(1) In performing this test, a manipulation should be used. Excessive manipulation will result in
(2)
Entrapped
air
should be removed prior to the test (especially large
air
bubbles).
(3)
This test should be performed as rapidly as possible.
Dropping point
of
grease
(D
127-63)
The dropping point is the temperature at which grease passes from the semisolid to the liquid state
under certain test conditions.
The dropping point indicates the resistance
of
greases to heat. The test method is used, for example,
to distinguish conventional lime-base cup greases from high-melting-point types, such as soda-base
greases.
Key points
(1)
Rate
of
heating is
of
prime importance.
(2)
Working of the grease should be avoided as far as possible.
ORSAT GAS
ANALYSIS
Method
of
analysis
The Orsat method
of
gas analysis consists of taking a measured volume of gas sample, removing
various components (one at a time by suitable reactions), and measuring the decrease in gas volume after
removal
of
each component. The results are then reported on a percentage basis.
Carbon dioxide
Carbon dioxide is removed by absorption in a solution containing about
500
g
of potassium
hydroxide per liter of solution; however, solutions having lower concentrations may be used. Sodium
hydroxide can be used instead
of
potassium hydroxide although it deposits carbonate more readily.
Reference: M.P. Matuszak, 1954.

506
Unsaturated hydrocarbons
Unsaturated hydrocarbon gases which include ethylene, propylene, acetylene, butylene, and benzene
are removed by absorption in fuming sulfuric acid containing 15-20% sulfur trioxide. Unsaturated
hydrocarbons (olefins and diolefins) may also be removed with bromide water.
Carbon monoxide
Carbon monoxide may be removed by direct absorption or it may first be oxidized to carbon dioxide.
Conversion
of
CO to
CO,
can be achieved by passing the gas over copper oxide at 300
C
or by slow
combustion in oxygen in the presence
of
a glowing platinum coil. The carbon dioxide is then absorbed
using potassium hydroxide.
A solution of cuprous chloride in hydrochloric acid or a suspension of cuprous
sulfate and
beta-naphthol in sulfuric acid may be used for direct absorption of carbon monoxide. Absorption is more
complete and reaction is somewhat faster in the latter solution. In the case of cuprous chloride, two
absorption pipettes should be used for accurate work: one for removing the bulk of carbon monoxide
and the other for removing the last traces of monoxide from the gas. It is better, however, to use one
pipette with cuprous chloride and the other pipette with cuprous sulfate-beta-naphthol. It is preferable
to use acid cuprous chloride rather than ammoniacal cuprous chloride because it does not lead to alkaline
conditions in the burette. Inasmuch as cuprous sulfate-beta-naphthol is a suspension in sulfuric acid and
settles at the bottom of the stock bottles to a depth
of
approximately one inch, the solution should be
vigorously shaken and the solids suspended prior to transfer to a gas pipette.
Hydrogen
Hydrogen may be determined by oxidation to water. This is accomplished by passing the gas over a
copper oxide at 250-300 C or by slow combustion
of
the
gas
in oxygen in the presence
of
a glowing
platinum coil. Inasmuch as the volume of water formed is negligible, the amount
of
hydrogen present in
the gas sample is equal to the reduction in gas volume
of
the sample caused by the oxidation.
Methane and ethane
Methane and ethane are determined by slow combustion in oxygen in the presence
of
a glowing
platinum coil. The reduction of gas sample volume after combustion must be determined in addition to
the volume of carbon dioxide formed.
Nitrogen
gas contains all inert components.
Combinations
of
combustibles
The choice of methods to determine combustible gases (hydrogen, carbon monoxide, methane, and
ethane) is dependent upon the combination of combustibles present in the sample. Any one or two
of
these gases may be determined by a slow combustion method. Methane and ethane are always
determined by a slow combustion method.
If
both methane and ethane are present in the gas sample,
slow combustion cannot be used to determine the volume of hydrogen and carbon monoxide. Carbon
monoxide may be determined by absorption; however, if hydrogen is also present, oxidation over copper
oxide at
300
O
C should be used.
Simultaneous determination of more than two combustible gases by slow combustion
is
not
recommended by Fisher Scientific Co.
After removal of all reactive components, the remaining gas is assumed to be nitrogen. The remaining
Introduction
of
gas
sample
The manifold and copper oxide tube should be flushed with nitrogen. After the level of the liquid in
the burette is raised to the uppermost graduation mark (marked either
100
ml or
0
ml),
the manifold is
closed off from the atmosphere. The zero point on the burettes usually corresponds to the upper end of
the capillary just below the upper stopcock. A capillary glass tube bent into an
L-
or U-shape, is used if
necessary. The connection may be purged by drawing two to three successive
10-
to
15-ml
portions of the

SO7
sample gas into the burette and then discarding them into the atmosphere. A three-way stopcock in the
connections permits a quick and easy execution of this process.
The sample to
be
analysed is drawn into the burette after the connection has been filled with the gas.
This is done without mixing the gas with nitrogen. In
a
portable apparatus, however, the nitrogen in the
manifold is displaced into the burette by the entering gas. The manifold must not
be
filled with the gas
before drawing in the gas sample.
Size
of
gas sample
It is convenient to analyze exactly
100
ml of gas
so
that percentages of various components may be
read directly on the burette. More than
100
ml
of gas is first drawn in and then the excess gas is
discarded
so
that the volume
of
gas analyzed is exactly
100
ml.
The leveling bottle is raised
so
that the
bottom of its meniscus is level with the lowest burette graduation mark. Then, the excess gas is allowed to
escape slowly to the atmosphere. This occurs by way
of
(1)
the stopcocks on the burette and sampling
bulb, or
(2)
the intake stopcock provided at the end
of
the manifold in some Orsat models. When the
meniscus in the burette also reaches the lowest gradation, the stopcock connected to the gas sample
source should be closed.
Number
of
passes
The number of passes required for the absorption of any particular gas component is dependent upon
the design
of
the pipette, the reagent used, the age
of
reagent, etc. Three to four passes are usually
considered sufficient for
CO,,
whereas six or more passes may
be
required for other components,
e.g.,
0,
and
CO.
After all of a particular component has been removed, i.e., when there is no further contraction
of the sample gas volume, the gas is passed once again into the pipette. When more than
12
passes for
complete absorption are required for
0,
and more than
S
passes are required for
CO,,
the reagent should
be replaced with fresh solution.
Sample problem
A-6
(ORSAT
analysis)
Orsat analysis is used to analyze a gas sample that is
50%
saturated with water vapor, and contains
TABLE
A-I
Given and computed values
of
Sample problem
A-6
Initially After passing through After passing through
p yrogallol conc.
H,SO,
Degree
of
saturation with
water
(X)
50.0
*
100.0
*
0.0
Total volume
of
gases
(ml)
100.0
*
70.2
*
45.9
*
0.0
45.9
-
0.0
=
45.9
22
-
Volume
of
H,O
vapor
(ml)
%
X
100
=
1.5
100
-
1.5
=
98.5
750
X
70.2
=
2.1
70.2
-
2.1
=
68.1
Volume
of
dry gases (ml)
Type
of
gas removed
-
4
CZH,
Volume
of
gas removed
(ml)
-
98.5
-
68.1
=
30.4
68.1
-
45.9
=
22.2
Volume
X
of gas removed,
on dry basis
(a)
-
22.2
-
X
100
=
30.9
98,5
X
100
=
22.5
Volume
of
N,
in sample
(ml)
98.5
-
30.4
-
22.2
=
45.9
-
-
Volume
X
of
N,
in original
sample, on dry basis
(%)
=
46.6
-
-
508
N,,
CO,,
and
C,H,.
Mercury was used to transfer the gases, and concentrated
H,SO,
was used to
remove the
C,H,.
At the temperature
of
the test, the vapor pressure of
H,O
is
22
mm
Hg.
Atmospheric
pressure was
750
mm
Hg.
Table A-I lists the given and computed values (values marked with
an
asterisk
have been given, whereas the others have been computed).
Sample problem
A-7
of
this gas at
75
F and a pressure of
20
psig, if it is saturated with water vapor?
A
gas contains
22%
C,H,,
16%
CO,,
and
8%
0,.
The remainder is
N,.
What is the weight of
1
cu ft
Solution
:
Component
Vol.
MW
Vol%XMW
(a)
26= 6.16
44
=
7.05
32
=
2.56
28
=
14.76
18=
0.2215
30.75
AMW
-4verage molecular weight
=
30.75.
p
=
20
+
14.73
=
34.73
psia.
Vapor pressure
of
H,O
at
75
OF
=
22
mm.
Partial vapor pressure
of
water vapor
=
(22
X
14.73)/760
=
0.426
psi.
Volume percent
of
water
=
0.426/34.73
=
1.23%.
Volume percent
of
N,
=
54- 1.23
=
52.77%.
One Ib-mole occupies
379
cu ft at standard conditions
(14.7
psia and
60
F).
60°
+460° =520°R
75O
+460° =535OR
Specific weight
of
gas,
y,
in Ib/cu ft, at standard conditions
=
(30.75
lb/mole)/(379
cu
ft/lb mole)
Correcting to new pressure and temperature
(34.73
psia and
535 R).
y,
=
(30.75/379) X(520/535)X (34.7/14.7)
=
0.186
Ib/cu ft.
FREEZING
POINT
TESTS
Freezing point of
fuels
(ASTM
D
2386-67)
The freezing point test is important because it shows the temperature at which solid particles begin to
form in the fuel. In the case of aviation fuels, the freezing point shall not be higher than minus
76
F.
The fuel usually becomes cloudy before formation of solid particles, but the clouding due to the freezing
of
the very small amount of dissolved water is disregarded.
Kqv point
into the test bottle. Formation of finely divided ice crystals will obscure the results.
Lowering
of
freezing point
(1)
Great care must be exercised in avoiding introduction of moisture, even in very small amounts,
Inasmuch as the solvent in a solution has a lower vapor pressure than the pure solvent, a hgher
tcrnperature must be reached before the vapor pressure of solution will reach any specified pressure.

509
-
ATf
TEMPERATURE
Fig.
A-13.
Schematic diagram showing freezing point depression and boiling point elevation upon
addition of solvent
to
a solution.
AT,
=
increase of the boiling point temperature,
AT,
=
lowering of the
freezing point temperature,
A
=
boiling point of the solvent,
B
=
boiling point of the solution,
C
=
freezing
point of the solvent, and
D
=
freezing point of the solution.
Thus, dissolving a non-volatile solute in a solvent will raise the boiling point. In the case of freezing a
solution, the solution must be cooled down to some temperature below the freezing point
of
the pure
solvent, until the vapor pressure curve for the solution intersects that for the
solid
solvent (see Fig.
A-13).
For dilute aqueous solutions, the elevation of the boiling point
(AT,)
and the depression
of
the
freezing point
(AT,)
are proportional to the molality of the solute molecules (or ions). The
AT,
and
AT,
do not depend upon the nature of the solute, unless it controls ionization. In the more concentrated
solutions encountered in petroleum testing, the elevation
of
the boiling point and lowering of the freezing
point are proportional to the mole percent of the solute.
The reason that these properties are proportional to the molality for dilute solutions is that the
number of moles of solute/1000
g
of solvent ratio is practically proportional to the mole percent
of
solute for dilute solutions.
Sample problem
A-8
One
ml
of kerosine has been added
to
10
ml of benzene. If the freezing point of benzene has been
lowered
2.2"C,
what is the molecular weight of kerosine? Molecular weight of benzene is
78.1,
the
specific gravity of benzene is
0.879,
specific gravity of kerosine is
0.799,
and one mole of impurity lowers
the freezing point of
1000
g
of benzene
5
"
C.
Solution
:
Mole percent impurity for
5OC
lowering
of
freezing point
=
100
X
[l/(l
+
(1000/78.1)]
=
7.25%.
78.1
=
molecular weight
of
benzene and
(1000/78.1)
=
moles of benzene in
1000
g.
Mole percent impurity for
2.2"
C
lowering
of
freezing point
=
[7.25
X
(2.2/5)]
=
3.19%.
3.19%
=
[
N/( N
+
moles of solvent)]
X
100,
where
N
=
moles
of
impurity and the moles
of
solvent (benzene)
=
(10
X
0.879)/78.1
=
0.1125.
Thus:
3.19
=
[N/(N
+
0.1125)]
X
100;
N
=
0.00371.
Inasmuch as
1
cc kerosine
=
0.799
g
+
0.00371
moles kerosine,
molecular weight
of
kerosine
=
(0.799
x
1)/0.00371
=
215
g/mole.
If formula
of
kerosine is
CnHZn+,,
then
n
=
15:
12
X
n
+
l(2n
+
2)
=
215.
Thus,
the formula of kerosine
in
this case is
C,,H,,.
Sample problem
A-9
kg of H,O
1.6
O
C.
One mole
of
impurity lowers the freezing point
1.86
O
C
in
1000
g
of
H20.
What
is
the fraction dissociation
a
of
H,C204,
if
0.6
moles
of
H2C,04
lowers the freezing point of
1

510
.
*\
Solution
;
H,C,O, +2H20+C202-
+2H30f
0.6
-
(0.6
X
o()
0.6
X
a
2(0.6
X
a)
1
DRYER
I/
PUMP
METER
:/63/4"
PIPE
VACUUM
/I
Moles of material present
=
(0.6-0.6a)+0.60(
+
1.2a
=
0.6+1.2a
(0.6+1.2a)/l= 1.6/1.86
and
a=
(0.86-0.6)/1.2
=
(0.26/1.2)
=
0.22.
How could one refine the above solution? Show calculations.
CHARCOAL TEST FOR GASOLINE CONTENT
OF
NATURAL GAS
The theory
of
charcoal test for gasoline content of natural gas is based upon selective adsorption
of
hydrocarbons by charcoal. Although this test was not used after the advent
of
the gas chromatograph, it
is
a very good experiment demonstrating basic principles to the students. It should be done in
conjunction with the gas chromatograph experiment. This test usually measures iC, content
of
natural
gasoline. The amount
of
gasoline is recorded as gal/Mcf
of
gas.
Primary
charcoal is prepared by heating
wood in a closed space. Activated charcoal is then prepared by heating the primary charcoal in the
presence of CO, to
900
OF. This creates a tremendous surface area:
20
acres/lb
of
charcoal in capillaries.
One cc of activated charcoal can adsorb 60 cc
of
methane or
80-100
cc
of
NH,.
After opening the rate valve (see Fig. A-14), temperature is plotted against the volume of natural gas
passed through the meter. Four maximums can be observed
on
this graph (Fig. A-15). Upon reaching the
fourth maximum, the flow of natural gas is shut off, i.e., the test is carried only to the fourth temperature
rise.
Upon adsorption on charcoal, the gas changes to a liquid, releasing the latent heat
of
vaporization.
This
heat raises the temperature of the charcoal and its surrounding media.
Upon completion
of
the test, the tube containing charcoal is subjected to
(1)
steam distillation or (2)
distillation with diethylene glycol, having a boiling point
of
472OF, or glycerine, with a boiling point of
554OF. These media have low boiling points
so
that the heavy ends
of
gasoline are not cracked.
In
addition, they are immiscible with gasoline.
CHARCOAL
RATE VALVE
THERMOMETER-
Fig.
A-14. Schematic diagram of charcoal test for gasoline content
of
natural gas.
'
CNGA
Bul.
TS-351. Standard Procedure for the Charcoal Test for the Determination
of
the Gasoline
Content of Natural Gas.
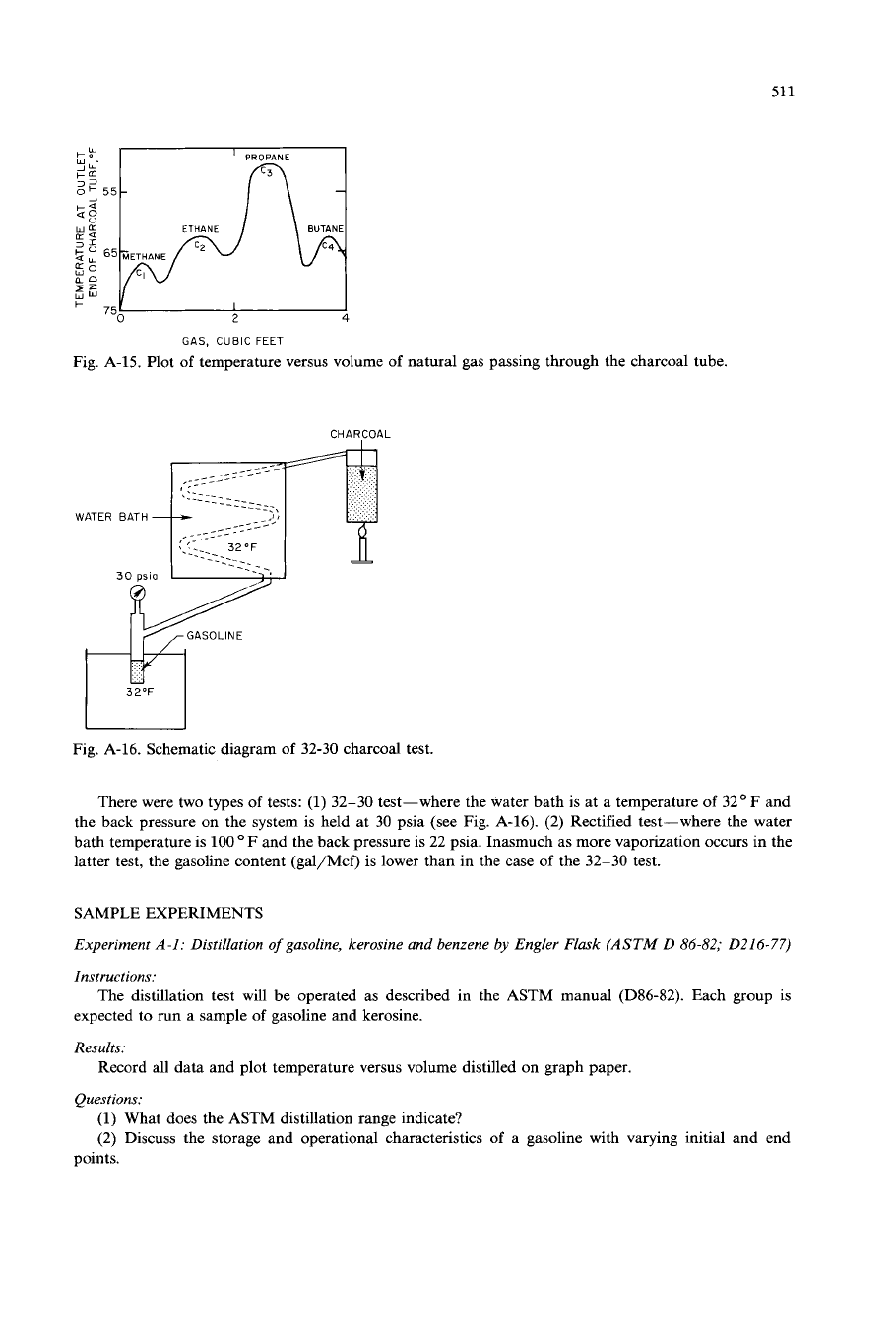
GAS, CUBIC
FEET
Fig. A-15. Plot
of
temperature versus volume
of
natural gas passing through the charcoal tube
CHARFOAL
WATER
BATH
I
32OF
30
psi0
8.
-
Fig. A-16. Schematic diagram
of
32-30 charcoal test.
There were two types of tests:
(1)
32-30 test-where the water bath is at a temperature
of
32O
F
and
the back pressure on the system is held at 30 psia (see Fig. A-16). (2) Rectified test-where the water
bath temperature is 100
OF
and the back pressure is 22 psia. Inasmuch as more vaporization occurs in the
latter test, the gasoline content (gal/Mcf) is lower than in the case
of
the 32-30 test.
SAMPLE EXPERIMENTS
Experiment
A-I:
Distillation of gasoline, kerosine
and
benzene by Engler
Flmk
(ASTM
D 86-82; 0216-77)
Instructions:
expected to run a sample
of
gasoline and kerosine.
Results:
The distillation test will be operated as described in the ASTM manual (D86-82). Each group is
Record
all data and plot temperature versus volume distilled on graph paper.
Questions:
(1) What does the ASTM distillation range indicate?
(2) Discuss the storage and operational characteristics
of
a gasoline with varying initial and end
points.

512
(3)
Considering this distillation as fractionation, how many theoretical plates are present in this
(4)
Given an
ASTM
distillation curve, can one tell what compounds are present in a gasoline? How?
(5)
What corrections are to be applied to (a) the temperature data obtained in the experiment, and
(6)
Butane (boiling point
=
31
F)
is known to be present in gasoline, yet the IBP obtained is
58
F.
(7) Draw three general type curves
of
temperature-composition diagrams for two-component sys-
experiment?
Why?
(h) the liquid volume data recorded for the experiment?
Why?
tems. Use solid lines for the liquids and broken lines for the vapors.
Experiment
A-2:
Distillation
of
crude oil with Hemple Column
(ASTM
D
285-62)
Instructions:
Apparatus and procedures should conform to the
ASTM
standards. Distillation shall be discontinued
at
460
OF.
Each group will run a minimum
of
two distillations on the same sample
of
crude oil. Exercise
great care to obtain a representative sample.
Results:
Volume of water, cc
=
-
Volume percent of naphtha,
'%
=
-
Questions:
(1)
What does the
ASTM
distillation of a crude oil indicate?
(2)
With the information collected from this experiment, plot a distillation curve: temperature versus
percentage of sample distilled. From this curve, determine the content
of
naphtha having a boiling point
range
of
-
to
-.
(3)
What other fractions can
one
determine from this distillation curve? Explain! (See Nelson,
1950;
Chapter
MI).
Experiment
A-3:
Graviiy by hydrometer and vapor pressure by the Reid Bomb
Part I-Gravity by the hydrometer
(ASTM
D
287-82)
Instructions:
Procure, assemble and operate the gravity test
as
described in the
ASTM
manual. Each group is
expected to run determinations at three temperatures on samples
of
gasoline, kerosine, and lubricating
oil.
Duta:
Questions:
(1)
Report the obtained values in terms
of
specific gravity, Ib/cu ft, and Ib/gal.
(2) What is the API gravity of water? Can a negative
API
gravity exist?
(3)
Name three different instruments that may be used to determine liquid densities. Explain how
each would work, directly or indirectly.

513
(4)
Correction tables apply to petroleum products. How would one proceed in the case of benzene,
toluene, and various nonpetroleum products?
Part 11-Vapor pressure by the Reid Bomb (ASTM D 323-82)
Instructions:
Determine the vapor pressure of a given sample of gasoline according to the standards and
procedures set up in the ASTM manual. Each group is required to run one sample.
Questions:
(1)
What is the Reid vapor pressure of (a) butane and (b) pentane?
(2) What is the Reid vapor pressure of
5%
(by weight) addition of butane to a gasoline, the Reid
(3)
Can the Reid bomb be used for measuring the true vapor pressure of a liquid? Why?
vapor pressure of which is 0.2 psi?
Experiment
A-4:
Water in petroleum
by
distillation and water and sediments in petroleum
by
centrifugation
(ASTM
D
1796-83;
D
4007-81;
D
4006-81)
Part I-Water in petroleum by distillation (ASTM D 95-83)
Instructions:
Set up the equipment as instructed in the ASTM manual.
Each group is expected to obtain a reliable check on the sample furnished as to percentage of water.
Results:
Run
No.
1
Run No. 2
Questions:
(1) To what type of samples can this method be applied?
(2) Why are distillation specifications
of
the solvent
used
given in great detail?
(3)
A 1000-bbl tank is equipped with three bleeders spaced equally over the height
of
the tank. A
sample of the crude
is
taken, one-third
of
the sample from each bleeder. Would this be a representative
sample? Explain!
(4)
What would happen
if
calcium carbide were placed
in
(a) wet petroleum and (b) dry petroleum?
Write the chemical equations.
Part 11-Water and sediments in petroleum by centrifugation (ASTM D 96-73)
Instructions:
Each group will make two determinations of the water and sediment content of the given crude oil
(emulsion). Results should compare within the limits specified by the ASTM manual. Take a representa-
tive sample.
Results:
Run
No.
1
Run
No.
2
Aug.
Questions:
manual?
(1) What is the purpose of the benzene addition and the heating procedure described in the ASTM

514
(2) The volume percentage
of
sediment is desired for
a
very dark-colored gas oil. Outline in tabular
(3)
What force
of
gravity (number
of
“g’s”) was exerted by the centrifuge in this experiment? How
(4)
Results are given in volume percentage
of
water. If the emulsion has an API gravity
of
30
O,
what
from
(1)
an approximate method, and (2) an exact method.
much would you weigh under these conditions? (Refer to Perry and Chilton Handbook.)
is the weight percent water?
Experiment
A-5:
Viscosity by Saybolt uiscosimeter
(ASTM
D
88)
Instructions:
not compare, make an additional determination. Be sure to obtain a representative sample.
Two samples will be tested. Each sample will be run twice at 100
O
and twice at 212O F. If results do
Results:
Viscosity of sample
No.
1
Viscosity of sample
No.
2
sus
sus
Questions:
Refer to the method of conversion presented in ASTM D 446. (Also see ASTM
D
2161-82.)
(1)
Report the values obtained in both kinematic and absolute units (centistokes and centipoises).
(2) Check the above conversions using the following equations:
M/d
=
0.226
f
-
195/t
(for oils above 100 sec Saybolt or less)
and
M/d
=
0.220
t
-
135/f
(for oils above 100 sec Saybolt)
where:
M
=
absolute viscosity, cP,
d
=
density
of
oil at temperature
of
the test
(100”
-212O), and
t
=
Saybolt seconds (Universal).
(3)
Convert the values obtained at 212O
F
to temperatures of
100
OF,
140
O
F, and 180
O
F
(see ASTM
(4)
Determine the viscosity index
of
each
oil
tested (see ASTM
D
567).
For the low-viscosity oil, it
(5)
Define the term viscosity.
(6) Describe two other methods of determining viscosity.
D 341-77).
may be necessary to use the centistoke table.
E.uperiment
A-6:
Flash and fire points by fag closed tester, by open cup, and by Pensky-Martens tester
Part I-Flash point by tag closed tester (ASTM D 56)
Instructions:
must be conducted under a firehood.
Each group is expected to obtain and check results on a sample
of
kerosine and motor oil. All work
Results:
Kerosine flash point
Run
no.
1
Run
No.
2
Lubricating oil flash point
Run
No.
1
Run
No.
2
