Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.


475
Appendix
A
TECHNOLOGY
OF
TESTING PETROLEUM PRODUCTS
AND SAMPLE EXPERIMENTS
GEORGE
V.
CHILINGARIAN,
JOHN
0.
ROBERTSON
Jr.
and C.M. BEESON
SOME THEORETICAL CONSIDERATIONS
OF
DISTILLATION
Distillation is used as a general research tool and as a control and specification test for petroleum
products. Consequently, the basic principles and theory behind distillation are
of
considerable impor-
tance. The distillation test for crude petroleum products is indicative of the approximate yield and
quality of some finished products which may be obtained from the crude oil.
In a distillation test, the liquid under test is vaporized and a set of figures recorded which indicate the
relationshp between temperature in the distilling vessel and quantities of liquefied distillate. The
distillation test is applicable to products which are vaporized in the course
of
their use; however, it also
serves for identification and classification of other products.
Petroleum products are complex mixtures
of
hydrocarbons containing large numbers of individual
compounds. When these mixtures are boiled, the vapor given off at any instant is only slightly less
complex than the liquid and is usually subjected to some sort
of
treatment, which simplifies its
composition, before it passes into the condenser. This treatment (referred to as fractionation, rectifica-
tion, dephlegmation, etc.) involves decreasing the proportion of the less volatile constituents in the vapor.
Fractionation may be achieved
(1)
by partial condensation, which liquefies more
of
the high-boiling than
of the low-boiling hydrocarbons,
(2)
by allowing the vapor to come in contact with a countercurrent
of
distillate maintained at its boiling point, or
(3)
by a combination of the foregoing two processes.
A large proportion
of
the more common laboratory distillation methods involves a certain degree
of
fractionation, which is in general incidental and is due to the cooling
of
the vapors in the necks of the
distilling flasks. This fractionation is achieved chiefly by partial condensation; however, there is also a
certain amount of contact between the reflux current
of
the condensed distillate and the rising current of
vapor. This general method of distillation does not furnish exact information concerning the true boiling
points of the constituents comprising a petroleum product. If properly standardized as to details of
apparatus and procedure, however, it can be made to yield results that are closely reproducible. These
methods have the added advantage of simplicity and convenience, and though subject to theoretical
objections, are the most important types used by the petroleum industry. For products encountered in the
petroleum industry, the
A.S.T.M.
Standard Method
of
Test for Distillation
of
Gasoline, Faphtha,
Kerosine, and Similar Petroleum Products
(D
86)
is almost universally used in the United States. This
method involves a moderate degree
of
fractionation because
of
the condensation and reflux in the neck of
the flask.
The operation of the distillation depends upon the fact that materials differ in their vapor pressure at
a given pressure. Consequently, when a solution or a mixture of two or more volatile substances is boiled,
the vapor coming off first has a greater proportion
of
the substance
of
high vapor pressure than the
proportion of the same component in the original solution. The distillate (material whch has been
vaporized and condensed) is richer in the more volatile component, whereas the residue (liquid left in the
flask) is poorer in that same component. As distillation proceeds, the composition of the residue becomes
progressively lower in the more volatile material and, therefore, richer in the higher boiling point
components.
476
Solution type equilibrium diagrams
The basic data of any distillation problem are the equilibria between the liquid and vapor phases
of
the system to be subjected to distillation. The examples presented in this section are only for
two-component systems. If the original charge in the flask has only two components, it is called
a
“binary” mixture; if there are three components,
a
“ternary” mixture;
and if more than three
components, a “multicomponent” system. A boiling-point diagram may be obtained experimentally for a
binary system.
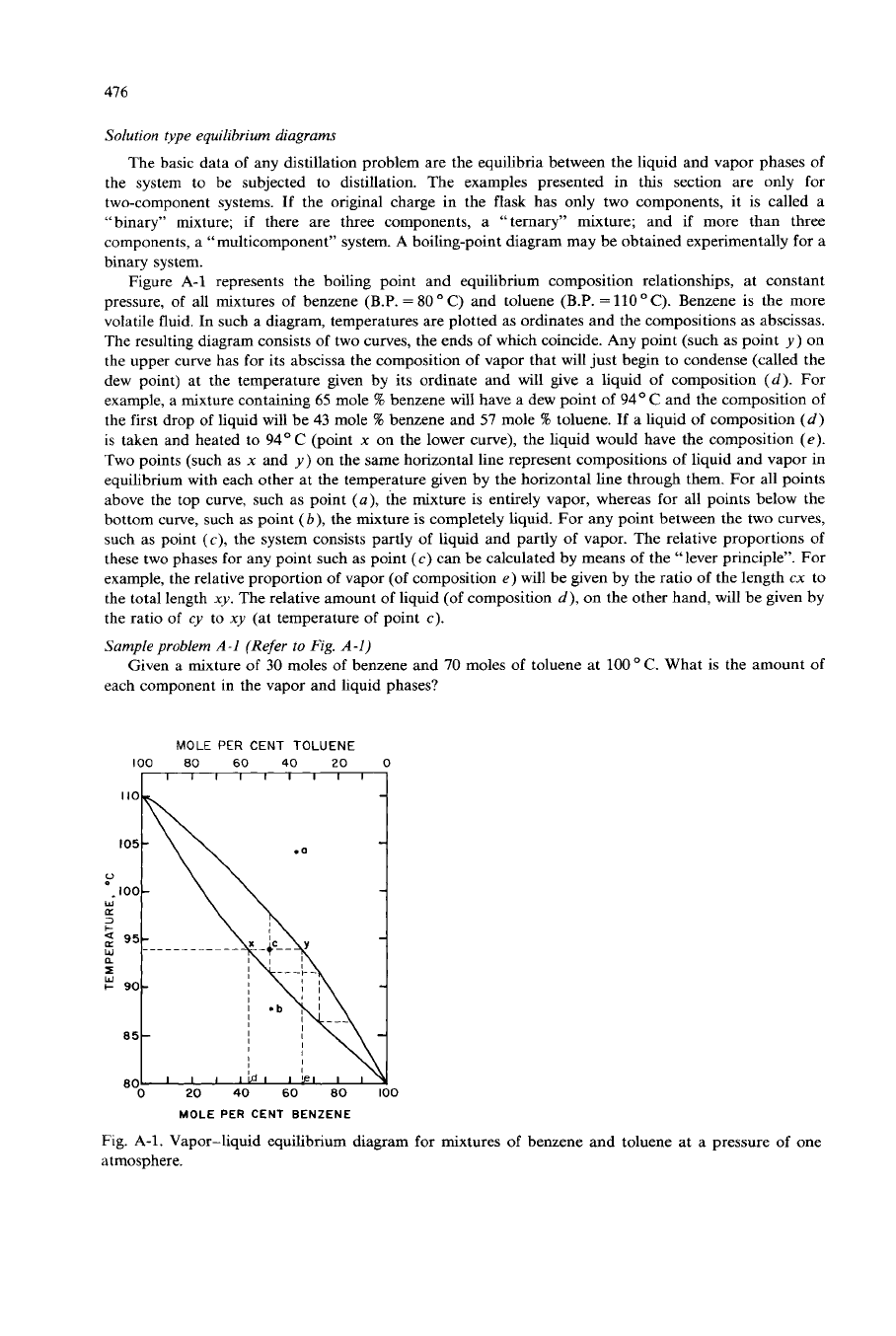
Figure A-1 represents the boiling point and equilibrium composition relationships, at constant
pressure, of all mixtures of benzene (B.P.
=
80
”
C) and toluene (B.P.
=
110
C). Benzene is the more
volatile fluid. In such a diagram, temperatures are plotted as ordinates and the compositions as abscissas.
The resulting diagram consists
of
two curves, the ends
of
which coincide. Any point (such as point
y)
on
the upper curve has for its abscissa the composition
of
vapor that will just begin to condense (called the
dew point) at the temperature given by its ordinate and will give a liquid
of
composition
(d).
For
example, a mixture containing
65
mole
%
benzene will have
a
dew point
of
94O
C
and the composition of
the first drop of liquid will be
43
mole
B
benzene and
57
mole
%
toluene.
If
a
liquid
of
composition
(d)
is taken and heated
to
94°C
(point
x
on the lower curve), the liquid would have the composition
(e).
Two points (such as
x
and
y)
on
the same horizontal line represent compositions of liquid and vapor in
equilibrium with each other at the temperature given by the horizontal line through them. For all points
above the top curve, such as point
(a),
the mixture is entirely vapor, whereas for all points below the
bottom curve, such as point
(b),
the mixture is completely liquid. For any point between the two curves,
such as point
(c),
the system consists partly
of
liquid and partly
of
vapor. The relative proportions
of
these two phases for any point such as point
(c)
can be calculated by means
of
the “lever principle”. For
example, the relative proportion
of
vapor (of composition
e)
will be gwen by the ratio
of
the length
cx
to
the total length
xy.
The relative amount of liquid
(of
composition
d),
on the other hand, will be given by
the ratio of
cy to
xy
(at temperature
of
point
c).
Sampie problem
A-l
(Refer
to Fig.
A-1)
each component in the vapor and liquid phases?
Given a mixture
of
30
moles
of
benzene and
70
moles
of
toluene at
100
”
C.
What is the amount
of
MOLE
PER
CENT
TOLUENE
100
80
60
40
20
0
-
85
-
0
20
40
60
80
100
MOLE
PER
CENT
BENZENE
Fig. A-1. Vapor-liquid equilibrium diagram for mixtures
of
benzene and toluene at
a
pressure of one
atmosphere.
477
MOLE PER CENT BENZENE
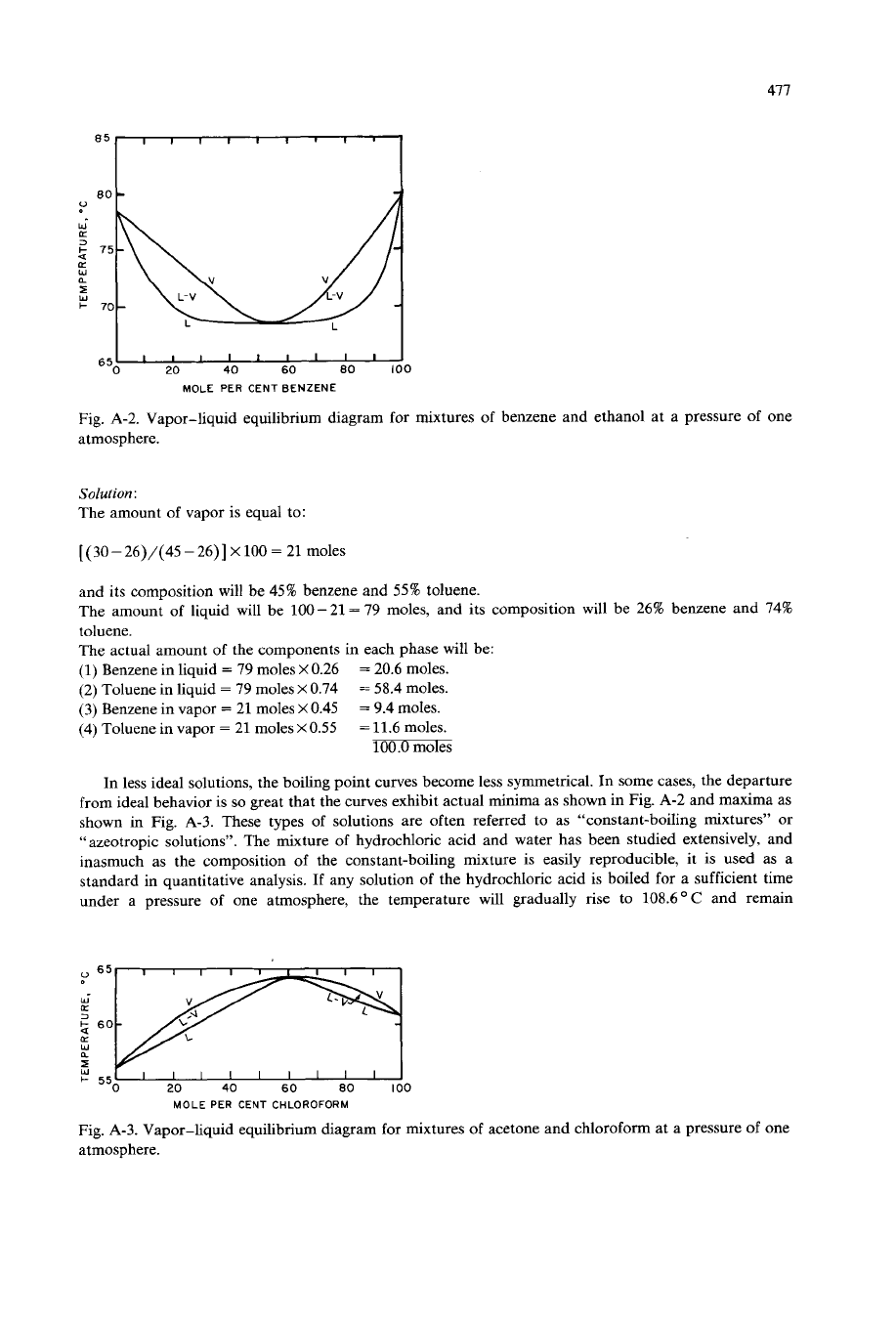
Fig.
A-2.
Vapor-liquid equilibrium diagram for mixtures of benzene and ethanol at a pressure
of
one
atmosphere.
Solution
:
The amount
of
vapor is equal to:
[
(30
-
26)/(45
-
26)]
x
100
=
21
moles
and its composition will be
45%
benzene and
55%
toluene.
The amount of liquid will be
100-21
=
79
moles, and its composition will be
26%
benzene and
74%
toluene.
The
actual amount
of
the components in each phase will be:
(1)
Benzene in liquid
=
79
moles
X
0.26
(2)
Toluene in liquid
=
79
moles
X
0.74
(3)
Benzene in vapor
=
21
moles
X
0.45
(4) Toluene in vapor
=
21
moles
X
0.55
=
20.6
moles.
=
58.4
moles.
=
9.4
moles.
=
11.6
moles.
100.0
moles
In less ideal solutions, the boiling point curves become less symmetrical. In some cases, the departure
from ideal behavior is
so
great that the curves exhibit actual minima as shown in Fig.
A-2
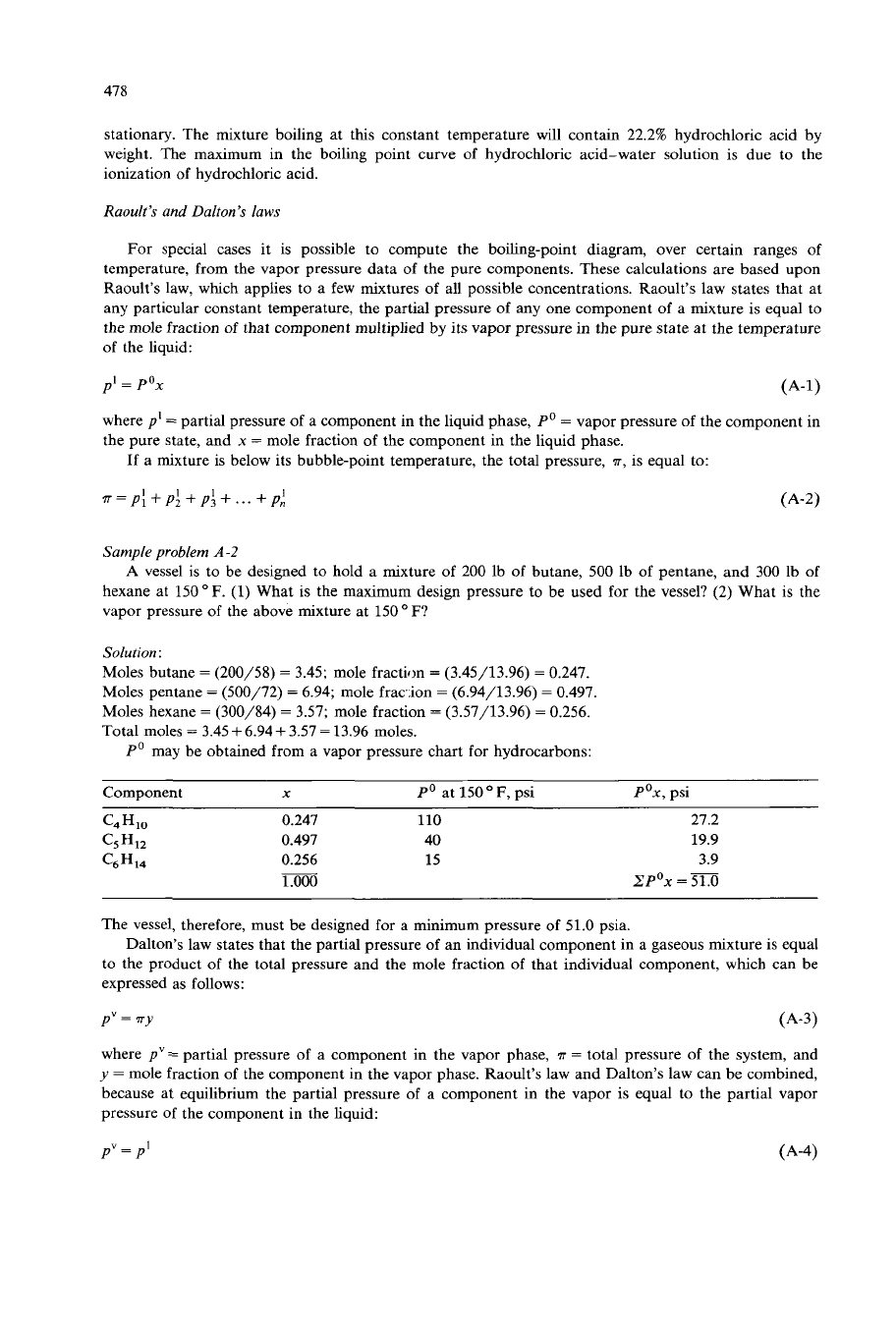
and maxima as
shown in Fig.
A-3.
These types
of
solutions are often referred to as “constant-boiling mixtures” or
“azeotropic solutions”. The mixture of hydrochloric acid and water has been studied extensively, and
inasmuch as the composition
of
the constant-boiling mixture is easily reproducible, it is used as a
standard in quantitative analysis. If any solution
of
the hydrochloric acid is boiled for a sufficient time
under a pressure of one atmosphere, the temperature will gradually
rise
to
108.6OC
and remain
Lz
W
MOLE PER
CENT
CHLOROFORM
Fig.
A-3.
Vapor-liquid equilibrium diagram for mixtures
of
acetone and chloroform at a pressure
of
one
atmosphere.
478
stationary. The mixture boiling at this constant temperature will contain 22.2% hydrochloric acid by
weight. The maximum in the boiling point curve of hydrochloric acid-water solution is due to the
ionization of hydrochloric acid.
Raoult's and DaltonS
laws
For special cases it is possible to compute the boiling-point diagram, over certain ranges
of
temperature, from the vapor pressure data of the pure components. These calculations are based upon
Raoult's law, which applies to
a
few mixtures
of
all possible concentrations. Raoult's law states that at
any particular constant temperature, the partial pressure of any one component of
a
mixture is equal to
the mole fraction
of
that component multiplied by
its
vapor pressure in the pure state at the temperature
of the liquid:
PI=
POX
(A-1)
where
pl
=
partial pressure
of
a component in the liquid phase,
Po
=
vapor pressure
of
the component in
the pure state, and
x
=
mole fraction of the component in the liquid phase.
If
a
mixture is below its bubble-point temperature, the total pressure,
T,
is equal to:
n
=
pi
i
p;
i
p:
+
...
i
p;
(A-2)
Sample problem
A-2
A vessel is to be designed to hold a mixture
of
200 lb of butane, 500 Ib of pentane, and 300 lb
of
hexane at
150
OF.
(1)
What is the
maximum
design pressure to be used for the
vessel?
(2) What is the
vapor pressure of the above mixture at 150
F?
Solution
:
Moles butane
=
(200/58)
=
3.45; mole fractbm
=
(3.45/13.96)
=
0.247.
Moles pentane
=
(500/72)
=
6.94; mole fracion
=
(6.94/13.96)
=
0.497.
Moles hexane
=
(300,434)
=
3.57; mole fraction
=
(3.57/13.96)
=
0.256.
Total moles
=
3.45
+
6.94i 3.57
=
13.96 moles.
Po
may be obtained from
a
vapor pressure chart for hydrocarbons:
Component
X
Po
at
150
F,
psi
POX,
psi
0.247 110
0.497 40
0.256 15
1.000
~
27.2
19.9
3.9
XPOX
=
51.0
The vessel, therefore, must be designed for a minimum pressure of 51.0 psia.
Dalton's law states that the partial pressure of an individual component in a gaseous mixture is equal
to the product of the total pressure and the mole fraction of that individual component, which can be
expressed
as
follows:
p"
=
ny
('4-3)
where
p"==
partial pressure of
a
component in the vapor phase,
T
=
total pressure
of
the system,
and
y
=
mole fraction of the component in the vapor phase. Raoult's law and Dalton's law can be combined,
because at equilibrium the partial pressure of a component in the vapor is equal to the partial vapor
pressure of the component in the liquid:
(A-4)
p"
=

479
Consequently,
y
=
(POX)/?r
(‘4-5
)
This equation expresses the equilibrium between the vapor and the liquid of an ideal solution at any
temperature and pressure. By means
of
this equation, the dew point and the bubble point of any ideal
solution can be calculated. From data obtained, a boiling point diagram may then be constructed.
Fractionation
On vaporizing a mixture of two liquids, that component which has the higher vapor pressure tends to
concentrate in the vapor, thus producing a difference in composition between the liquid and the vapor
phases which are in equilibrium. The vapor may then be condensed. Vapor coming from this condensate
is still enriched in the more volatile component. This successive vaporization and condensation is called
fractional distillation.
Figure A-1 shows the composition of the vapor gwen off at
98.5
O
C, when boiling a mixture of
benzene and toluene at atmospheric pressure. From a charge containing
30%
benzene and
70%
toluene,
the vapor will contain
51%
benzene and
49%
toluene.
If
this vapor were condensed and then brought up
to boiling at
92”C,
a new vapor would be evolved having a composition
of
72%
benzene and
28%
toluene. Furthermore the new residue would contain proportionately less benzene than when first
condensed. By repeated condensations and re-evaporations, which
is
called redistillation, a final product
of substantially pure benzene could be obtained.
A similar result can be obtained by condensing a very small amount of the vapor by slight cooling,
drawing off this condensate, and cooling the vapor a little more.
This
condenses another small amount of
liquid containing a relatively large amount of toluene.
If
given sufficient time, this liquid must be in
equilibrium with the vapor at the existing temperature.
This
is the method
of
partial condensation.
If the vapors from an initial mixture
of
30%
benzene and
70%
toluene, boiling at
98.5
C,
are allowed
to
bubble into a liquid
of
the same composition as the vapor (i.e.,
51%
benzene and
49%
toluene), which
has been heated just to its boiling point
(92OC),
the vapor will condense, giving up latent heat and
thereby boiling off a new vapor containing
72%
benzene and
28%
toluene. At the same time, the liquid
residue from this second still, now reduced in benzene content by the removal
of
vapor containing
72%
benzene, could be allowed to flow into the first still. It would, therefore, be reduced again in benzene
content. Simultaneously, vapor containing
72%
benzene from the second still could be allowed to bubble
into a third still containing
72%
benzene at its boiling point
(86.5 C).
This
results in evolution
of
vapor
containing
86%
benzene and return of liquid containing
72%
benzene to the second still. Thus, supplying
heat to the first still only, the vapor from each still in the series is progressively richer in benzene and
poorer in toluene, until substantially pure benzene passes from the last still into a condenser. Providing
there has been no radiation
loss
from the stills, the amount
of
heat obtained by condensing this benzene
will be exactly equal to that introduced in the coils
of
the first still.
To
maintain the exact conditions
given, it is necessary to return all of the condensed benzene to the last still,
so
that the proper amount of
liquid could flow back to the preceding still and
so
on down the line. This operation is called “fractional
distillation”, which combines the methods of redistillation and that
of
partial condensation. Figure
A-4
shows this operation diagrammatically.
The following factors are found to be essential for the theoretical case given above:
(a) The vapors rising from each still must be in equilibrium with the liquid in the still, following the
temperature and composition conditions given on the boiling-point diagram.
(b)
The liquid flowing back into each lower still must equal in amount and composition the vapors
coming into the upper still from which that liquid is flowing.
(c) There must be no addition or loss
of
heat in the system except the introduction of heat in the
steam coils
of
the first still and the removal
of
exactly the same amount of heat in the final condenser.
Practical application of this method of vapor enrichment involves some modification of theory.
Actually, the purpose of such
an
operation is to take out as much pure benzene as possible. All the
condensate, therefore, cannot be returned to the last still. By taking
off
a portion of the benzene
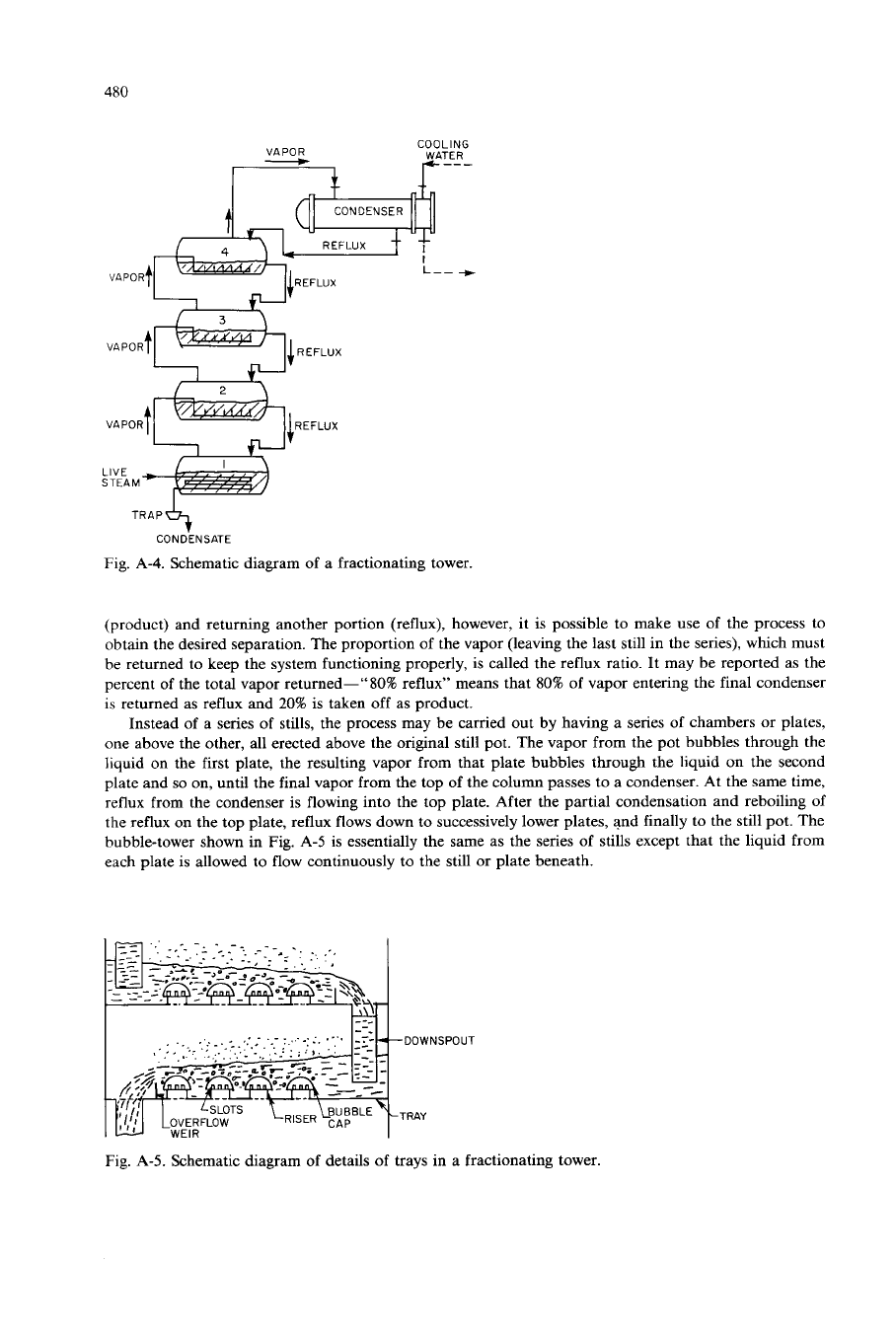
480
VAPOR
-
COOLING
WATER
VAPORt IREFLUX
STEAM
T
TRAP&+
CONDENSATE
Fig. A-4. Schematic diagram
of
a fractionating tower.
(product) and returning another portion (reflux), however, it is possible to make use of the process to
obtain the desired separation. The proportion of the vapor (leaving the last still in the series), which must
be returned to keep the system functioning properly, is called the reflux ratio. It may be reported as the
percent of the total vapor returned-"80% reflux" means that 80% of vapor entering the final condenser
is returned as reflux and
20%
is taken
off
as product.
Instead of a series of stills, the process may be carried out by having a series of chambers or plates,
one above the other, all erected above the original still pot. The vapor from the pot bubbles through the
liquid
on
the first plate, the resulting vapor from that plate bubbles through the liquid
on
the second
plate and
so
on,
until the final vapor from the top of the column passes to a condenser. At the same time,
reflux from the condenser is
flowing
into the top plate. After the partial condensation and reboiling
of
the reflux
on
the top plate, reflux flows down to successively lower plates, and finally to the still pot. The
bubble-tower shown in Fig. A-5 is essentially the same as the series
of
stills except that the liquid from
each plate is allowed to flow continuously to the still or plate beneath.
Fig.
A-5.
Schematic diagram of details
of
trays in a fractionating tower.

481
Inasmuch as complete equilibrium is not established on any actual plate and because part
of
the
product is continually withdrawn, the number of actual plates in a column
is
always greater than that
required for the theoretical conditions. The ratio
of
the fractionation actually accomplished by a plate to
that calculated for a “theoretical” plate is called the plate efficiency. It is generally equal to about
50-60%.
It can be shown mathematically that the number
of
theoretical plates required for a given
separation increases as the reflux ratio decreases. There is, however, a minimum reflux ratio for any
separation, below which even an infinite number
of
plates will not give a desired product.
Distillation
of
gasoline
The distillation curve of gasoline as shown in Fig.
A-6,
is somewhat indicative of the performance
The general requirements for volatility of a motor gasoline are as follows:
(a) The gasoline should contain enough readily volatile constituents to permit starting an engine
under reasonably unfavorable conditions without preheating. It should also be possible to operate the
engine with
a
reasonable degree
of
flexibility during the period while it is warming up to the optimum
operating temperature.
(b) The gasoline should not contain too large a percentage of highly volatile constituents, because it
might cause excessive evaporation losses and premature vaporization in carburators or fuel lines, with
attending vapor lock. It should be remembered, however, that the vapor lock may also be due to the
improper location of the fuel lines.
The gasoline should not contain any considerable percentage of relatively heavy, slightly volatile
constituents which would not usually vaporize completely enough (after atomization into the manifold)
to permit even distribution to the various cylinders of the engine. These heavy ends are also undesirable
because they tend to dissolve in the oil on the cylinder walls and increase dilution
of
the oil in the
crankcase. The design and method
of
operation of the car also have important effects upon the amount
of dilution.
The distillation curve of gasoline is somewhat indicative of its performance characteristics in the car.
This
method does not give true boiling points
of
the individual components in any given gasoline, but
does give a reliable indication
of
whether the volatility is within the required limits which have been
determined by experience through operation of full-scale engines.
In the case
of
aviation fuels, it is usually required that when the thermometer in the flask reads
167 OF, not less than
10%
nor more than
40%
of
the volume in the flask should have been boiled off. It is
also required that at 221O
F
not less than 50% and at 275
OF
not less than
90%
shall have boiled
off.
The
end point
(FBP)
should not exceed
338O
F.
In addition, the residue left at the bottom
of
the flask after it
has boiled dry must not be more than 1.5%.
The temperature at which 10%
of
the gasoline is boiled off enables a reasonable prediction
of
the
lowest atmospheric temperature at which the engine will start. The sum
of
10% and
50%
points
is
also
indicative
of
vapor locking tendency.
characteristics
of
gasoline in an automobile.
I
ENP
PT.
KNDICATIVE
OF
SPEED
I
Vo
DISTILLED-
Fig.
A-6.
Significance
of
various portions
of
a distillation curve
of
gasoline

482
The gasoline should not contain any considerable percentage of relatively heavy, only slightly volatile
constituents, which will not vaporize completely enough to permit even distribution to the various
cylinders of an engine. For an excellent treatment of distillation, the reader is referred to the classical
work
of
Nelson
(1949).
Key points
test procedures should be strictly complied with:
In order to obtain reproducible results for the distillation experiments (see ASTM D86) the following
(1)
Proper position
of
the thermometer in the flask.
(2)
The proper size of the hole in the asbestos board, depending on the type
of
fuel tested.
(3) The proper rate of distillation.
(4) The temperature of the condenser bath.
(5) There must be a tight contact between the flask and the opening
of
the asbestos board. The corks
must also be tightly fitted.
Questions
(1)
What corrections are to be applied to the temperature data obtained? To the liquid volume data?
(2) State the significance of the 167O, 221°, 275O, and 33S°F distillation control points for
reciprocating fuels.
(3) Under what conditions is a high distillation thermometer used? Under what conditions is a low
distillation thermometer used? State the controls governing the sue of asbestos board opening to be used.
(4) Indicate the maximum and/or minimum time or temperature controls applicable to the following
distribution features of reciprocating fuels: (a) allowable time for distilling off the last 5
ml,
(b) first drop
over, (c) condenser box temperature rate, (d) distillation rate, (e) sample temperature, and
(f)
receiving
graduate temperature.
LAYER TYPE AND EUTECTIC TYPE EQUILIBRIUM DIAGRAMS
Three common types of equilibrium diagrams, very often encountered in engineering work, are the
(1)
The solution type equilibrium diagram has already been discussed and is typified by Fig. A-1.
The layer type diagram is the simplest equilibrium diagram and applies to two mutually insoluble
compounds which form neither chemical compounds nor solutions. A typical example
of
this type curve
is shown in Fig. A-7.a. Figure A-7.b represents a plot of the temperature versus time for the cooling of a
solution of constant composition from the liquid state to the solid state. The flat portions
of
the cooling
curve are caused by a large percentage of latent heat of fusion being given up at constant temperature.
The third type of equilibrium diagram, i.e., the simple eutectic type diagram as shown in Fig. A-8.a, is
obtained when two materials form a eutectic. The latter is a mechanical mixture of two materials having
the lowest freezing point
of
any combination of these materials. In order to construct a diagram covering
solution type,
(2)
layer type, and (3) simple eutectic type.
LIP
Pb
+
SOLID
AI
a
5
SOLID Pb +SOLID
AI
Y
+
TIME
0
I00
O%
Pb
COMPOSITION
Fig. A-7. Liquid-solid equilibrium diagram and cooling curves for mixtures of lead and aluminum at a
pressure of one atmosphere.
483
LL
W
u
3
+
a
LL
w
32
a
I
-7.65
50%
23.5% PURE
W
LL
a
n
W
a
5
W
TIME
50%
23.5% PURE
TIME
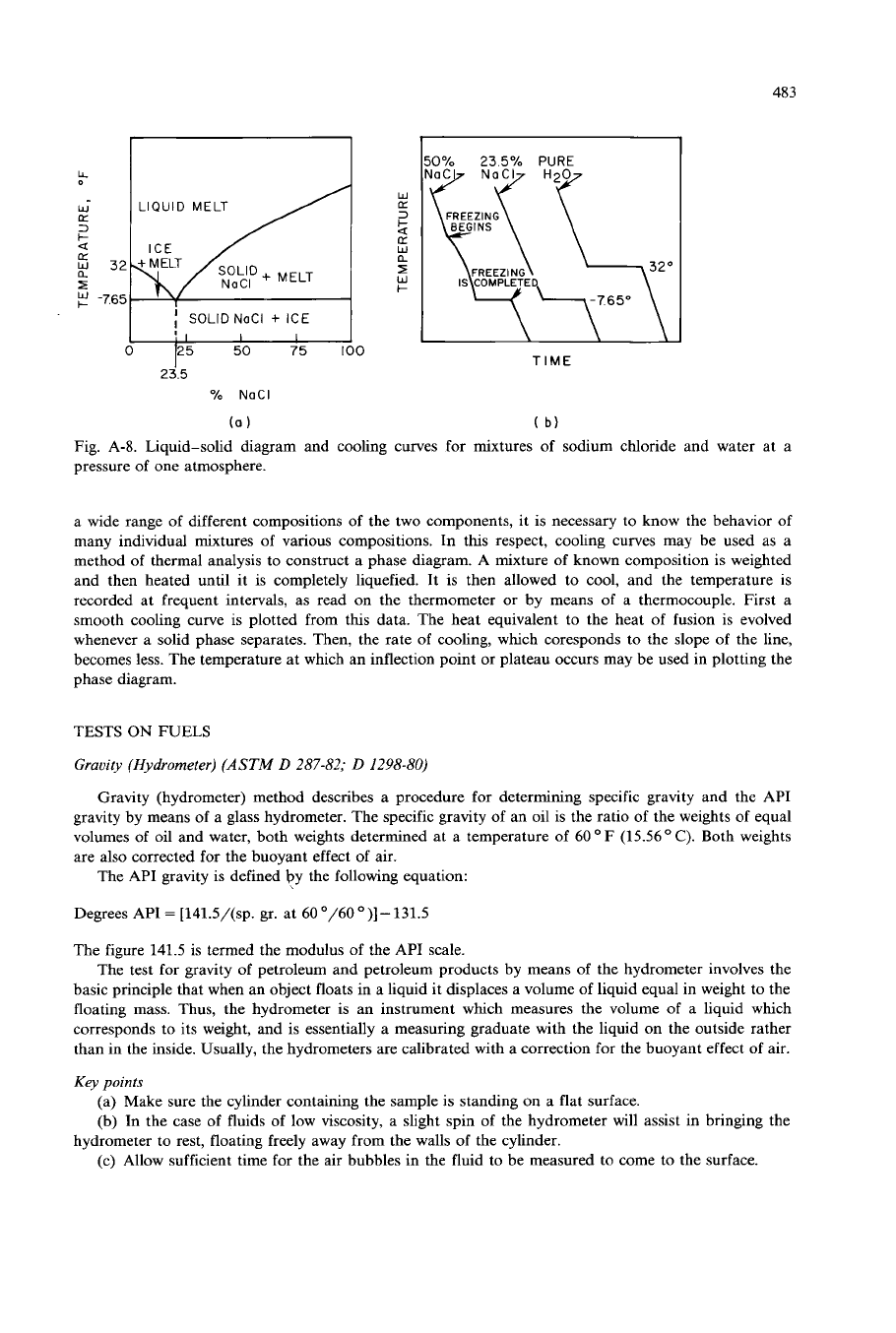
Fig. A-8. Liquid-solid diagram and cooling curves for mixtures
of
sodium chloride
pressure of one atmosphere.
and water at a
a wide range of different compositions of the two components, it is necessary to know the behavior of
many individual mixtures of various compositions. In this respect, cooling curves may be used as a
method of thermal analysis to construct a phase diagram.
A
mixture of known composition is weighted
and then heated until it is completely liquefied. It is then allowed to cool, and the temperature is
recorded at frequent intervals, as read on the thermometer or by means of a thermocouple. First
a
smooth cooling curve is plotted from this data. The heat equivalent to the heat
of
fusion is evolved
whenever a solid phase separates. Then, the rate of cooling, which coresponds to the slope of the line,
becomes less. The temperature at which an inflection point or plateau occurs may be used in plotting the
phase diagram.
TESTS
ON
FUELS
Gravity (Hydrometer)
(ASTM
D
287-82;
D
1298-80)
Gravity (hydrometer) method describes
a
procedure for determining specific gravity and the API
gravity by means of a glass hydrometer. The specific gravity
of
an
oil is the ratio of the weights of equal
volumes of oil and water, both weights determined at a temperature of 60
OF
(15.56
O
C). Both weights
are also corrected for the buoyant effect
of
air.
The
API
gravity is defined \y the following equation:
Degrees API= [141.5/(sp.
gr.
at 6Oo/6O0)]-131.5
The figure 141.5 is termed the modulus
of
the
API
scale.
The test for gravity of petroleum and petroleum products by means
of
the hydrometer involves the
basic principle that when an object floats in a liquid it displaces a volume
of
liquid equal in weight to the
floating mass. Thus, the hydrometer is an instrument whch measures the volume of a liquid which
corresponds to its weight, and is essentially a measuring graduate with the liquid on the outside rather
than in the inside. Usually, the hydrometers are calibrated with a correction for the buoyant effect of air.
Key
points
(a) Make sure the cylinder containing the sample is standing on a flat surface.
(b) In the case of fluids of low viscosity, a slight spin of the hydrometer will assist in bringing the
(c) Allow sufficient time for the air bubbles in the fluid to be measured to come to the surface.
hydrometer to rest, floating freely away from the walls of the cylinder.

484
The weight and volume relationships
of
two-component
system
and
b,
having specific weights
ya
and
yb,
respectively:
Assuming additive volumes and weights, the following relationships will hold true for components
a
(1)
Wt,
+
wt,
=
Wt,b
(2)
vol,
+
uol,
=
U0lub
(3)
(wto/Ya)+(Wtb/Yb)
=
(Wtab/Yab)
(4)
[(
wta
/
yo)
(5)
[(
wt8
a
1/70
1
+
[(
wtS&
b)/Yb
1
=
loo/
Yab
(lOO/wtab)l
+
[(
w'b/
yb)
(loo/
w'ob)l
=
[(
Wtab/Yab)
(lOO/wfab)l
(6)
wt%a=100-
wt%b
(7)
[(loo-
wt%b)/y,l
-t
wt%b/Ybl
=
loo/Yub
(8)
wr8b=
[(lOO)yb(y,-Yab)l/[Yub(Ya
-yb)l
(9)
volBa
=
[loo(yab
-
Yb)l/(Ya
-
yb)
Questions
available. How would you proceed?
(1)
You
are interested in the API gravities of various products, for which correction tables are not
(2)
What is the API gravity of water? Can a negative API gravity exist?
Vapor pressure (Reid) (ASTM
D
323-82)
The Reid vapor pressure test indicates the initial tendency
of
fuel towards vaporization. Vapor
pressure increases with temperature, and the boiling point is reached when the vapor pressure is equal to
the atmospheric pressure. There is a definite correlation between the vapor pressure, vapor-locking, and
ease
of
engine starting. The gasoline with a high vapor pressure is very volatile, which increases the
tendency to vapor lock.
The vapor pressure of a mixture
of
substances is not only a function
of
temperature but is also
dependent on the composition
of
the mixture. Each component
of
a mixture contributes to the total
vapor pressure in proportion to its mole fraction and to its vapor pressure in a pure state at the
temperature of measurement:
p'
=
POX
where p'=partial pressure of a component in the liquid phase,
Po=
vapor pressure of the pure
component, and
x
=
mole fraction of the component in the liquid phase.
Inasmuch as the composition of the liquid phase is changing through vaporization, the vapor pressure
of
the mixture will vary with the volume of vapor space.
In the Reid method, the vapor pressure is determined in the presence of a volume
of
air four times the
volume of gasoline. This standardizes the extent of evaporation, minimizes the effect of dissolved gases,
and
permits direct observation
of
the vapor pressure in terms of absolute units (e.g., in pounds per square
inch).
Although the Reid method does not measure the true vapor pressure of gasolines, it is sufficiently
accurate for commercial and transportation purposes. The true vapor pressure is about
5%
higher than
the Reid vapor pressure for gasolines.
Key points
(I)
The Bourdon gages must be carefully checked.
(2)
The whole assembly should be
thoroughly
purged.
(3)
The bath temperature should be maintained at
100k0.2°F.
(4)
Shaking
of
the unit during the test must be vigorous.
Sample problem A-3
to a gasoline with Reid pressure
of
2 psi?
What is the Reid vapor pressure of
308
by
volume
addition
of
gasoline with a vapor pressure of
4
psi
