Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.

485
Solution (approximate):
Reid vapor pressure of a mixture
=
(30%
X
4)
+
(70%
X
2)
=
1.2
+
1.4
=
2.6 psi.
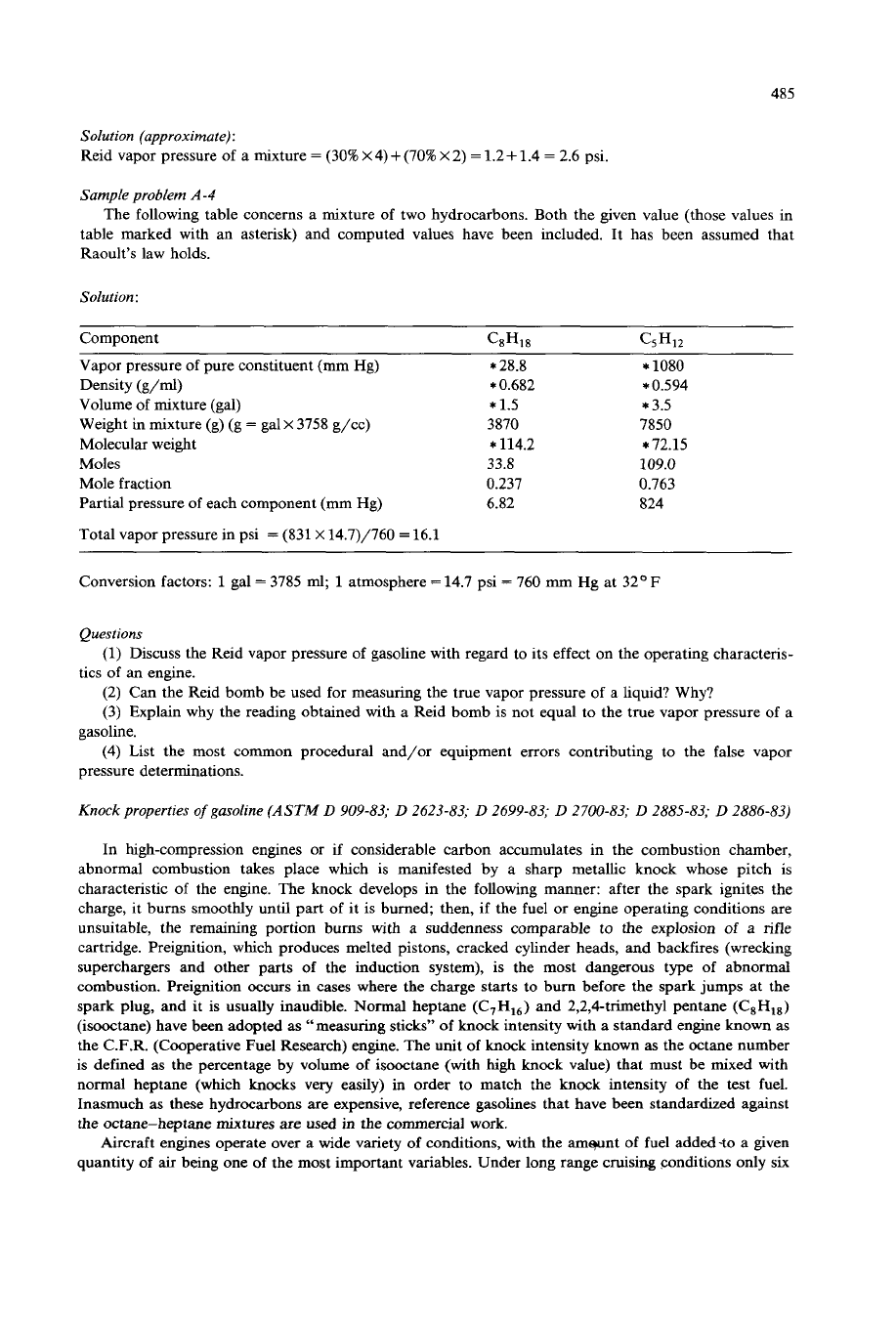
Sample problem
A-4
The following table concerns a mixture of two hydrocarbons. Both the given value (those values in
table marked with
an
asterisk) and computed values have been included. It has been assumed that
Raoult’s law holds.
Solution
;
Component CaH,a
C5H12
Vapor pressure of pure constituent (mm Hg)
Density
(g/ml)
Volume of mixture (gal)
Weight in mixture (g)
(g
=
galx 3758 g/cc)
Molecular weight
Moles
Mole fraction
Partial pressure
of
each component
(mm
Hg)
Total vapor pressure in psi
=
(831
X
14.7)/760
=
16.1
*
28.8
*
0.682
*
1.5
3870
*
114.2
33.8
0.237
6.82
*
1080
*
0.594
*
3.5
7850
*
72.15
109.0
0.763
824
Conversion factors:
1
gal
=
3785
nd;
1
atmosphere
=
14.7 psi
=
760
mm
Hg at 32O
F
Questions
tics of an engine.
(1)
Discuss the Reid vapor pressure of gasoline with regard to its effect on the operating characteris-
(2) Can the Reid bomb be used for measuring the true vapor pressure of a liquid? Why?
(3) Explain why the reading obtained with a Reid bomb is not equal to the true vapor pressure of a
(4) List the most common procedural and/or equipment errors contributing to the false vapor
gasoline.
pressure determinations.
Knock
properties
of
gasoline
(ASTM
D
909-83;
D
2623-83;
D
2699-83;
D
2700-83;
D
2885-83;
D
2886-83)
In high-compression engines or if considerable carbon accumulates in the combustion chamber,
abnormal combustion takes place which is manifested by
a
sharp metallic knock whose pitch is
characteristic of the engine. The knock develops in the following manner: after the spark ignites the
charge, it burns smoothly until part of it is burned; then, if the fuel or engine operating conditions are
unsuitable, the remaining portion burns
with
a suddenness comparable to the explosion
of
a rifle
cartridge. Preignition, which produces melted pistons, cracked cylinder heads, and backfires (wrecking
superchargers and other parts of the induction system), is the most dangerous type of abnormal
combustion. Preignition occurs
in
cases where the charge starts to bum before the spark jumps at the
spark plug, and it is usually inaudible. Normal heptane (C7HI6) and 2,2,4-trimethyl pentane (C,H,,)
(isooctane) have been adopted as “measuring sticks” of knock intensity with a standard engine known as
the C.F.R. (Cooperative Fuel Research) engine. The unit of knock intensity known
as
the octane number
is
defined as the percentage by volume of isooctane (with high
knock
value) that must be mixed with
normal heptane (which knocks very easily) in order to match the knock intensity of the test fuel.
Inasmuch
as
these hydrocarbons are expensive, reference gasolines that have been standardized against
the octane-heptane mixtures
are
used
in
the commercial work.
Aircraft engines operate over a wide variety of conditions, with the amqunt of fuel added
to
a given
quantity of
air
being one of the most important variables. Under long range cruising conditions only
six
486
TEL IN ISOOCTANE,
ml
/gal
OCTANE NUMBER
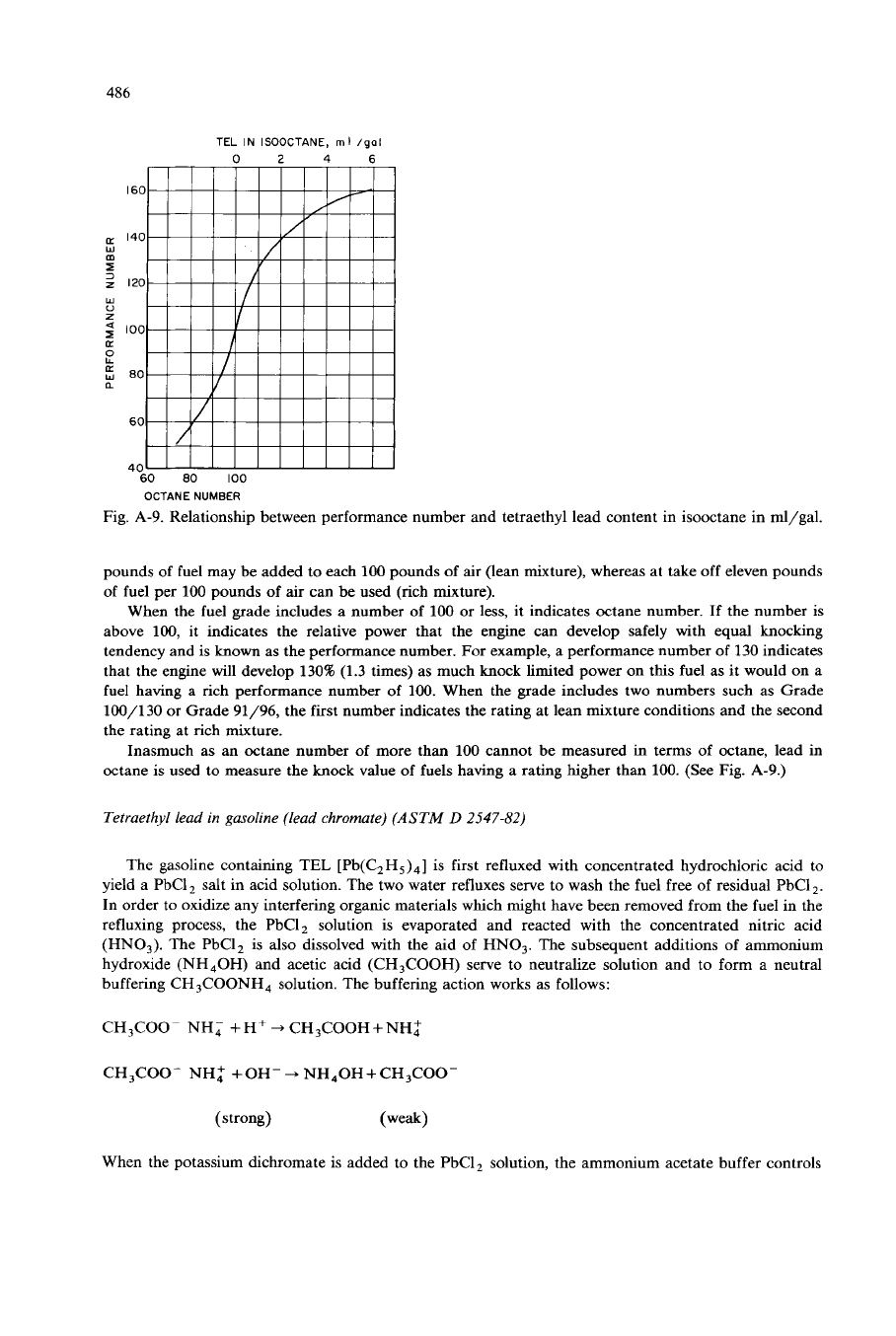
Fig.
A-9.
Relationship between performance number and tetraethyl lead content in isooctane in ml/gal.
pounds of fuel may be added to each
100
pounds
of
air (lean mixture), whereas at take off eleven pounds
of fuel per
100
pounds of air can be used (rich mixture).
When the fuel grade includes a number of
100
or
less,
it indicates octane number. If the number
is
above 100, it indicates the relative power that the engine can develop safely with equal knocking
tendency and is known as the performance number. For example, a performance number of 130 indicates
that the engine will develop 1301% (1.3 times) as much knock limited power
on
this fuel as it would
on
a
fuel having a rich performance number of 100. When the grade includes two numbers such as Grade
100/130 or Grade
91/96,
the first number indicates the rating at lean mixture conditions and the second
the rating at rich mixture.
Inasmuch as an Octane number of more than 100 cannot be measured
in
terms of octane, lead in
Octane is used to measure the knock value of fuels having a rating higher than
100.
(See Fig.
A-9.)
Tetraethyl lead in gasoline (lead chromate) (ASTM
D
2547-82)
The gasoline containing TEL [Pb(C,H,),] is first refluxed with concentrated hydrochloric acid to
yield a PbCl
,
salt in acid solution. The two water refluxes serve to wash the fuel free of residual PbCl
,.
In order to oxidize any interfering organic materials which might have been removed from the fuel in the
refluxing process, the PbCl, solution is evaporated and reacted with the concentrated nitric acid
(HNO,). The PbCl, is also dissolved with the aid of HNO,. The subsequent additions of ammonium
hydroxide (NH,OH) and acetic acid (CH,COOH) serve to neutralize solution and to form a neutral
buffering CH,COONH, solution. The buffering action works as follows:
CH,COO- NH; +H++CH,COOH+NH:
CH,COO- NH: +OH-+ NH,OH+CH,COO-
When the potassium dichromate is added to the PbCl, solution, the ammonium acetate buffer controls

487
the precipitation of the Pb as PbCrO, (MW
=
323.2) rather than as the PbCr20, (MW
=
423.2), which
would precipitate in acid solution. This reaction is as follows:
2PbC1,
+
K2Cr20,
+
H20+2CH3COONH4
+
2PbCr0,
+
2KC1+
2NH,Cl +2CH3COOH
The lead is gravimetrically determined as PbCrO,.
Lead is the most powerful antiknock additive, and reduces "knock" in chain paraffins, cyclic
paraffins, aromatics, and olefins (not equally with all, however). For aviation use it is blended with
ethylene dibromide and this mixture is identified by a distinctive dye.
Lead bromide in the presence of water and
of
metals in the cylinder unit, particularly aluminum, at
atmospheric temperatures produces corrosive liquids which cause rusting of steel or cast iron if the
surfaces are not protected with
an
oil film. In view of the corrosiveness
of
lead bromide, the amount of
ethylene dibromide which can be added is limited. It should be exactly equal to the theoretical amount
necessary to convert all the lead to lead bromide during combustion of the fuel-air mixture.
Key points
0.05-0.10
ml per gallon.
usually sufficient.
(1)
With highly volatile gasolines, omission
of
the distillate may lead to results which are low by
(2)
The boiling flask must be cleaned after each use. Refluxing
50
ml
of
acetone for five minutes is
Fisher TEL-meter
The polarographic method is fundamentally one of electrodeposition on a micro-scale. The dropping
mercury electrode is used as the primary electrode (cathode), and the large
pool
of mercury at the bottom
of the cell serves as the secondary electrode. As the mercury droplets fall through the refluxed fuel, the
positive metal ions in the solution plate out on the droplets, picking up electrons from the mercury. Thus,
mercury at the bottom of the cell becomes more positive than that in the capillary tube, and a current
passes through the cell. The greater the concentration of metal ions in the solution, the greater the current
through the cell.
In order to standardize the dropping electrode, the current passed by a solution of known metal ion
concentration is measured. It is not necessary to use
a
separate standard solution, because a different
metal ion can be used for the standardization. The voltage at which the standardizating metal ion (pilot
ion) plates out on the drop must be different from the voltage needed to plate out the metal ion, the
concentration of which is to be determined. If a known amount of the pilot ion is added to the unknown
solution, and the mixture diluted to a definite volume, the currents due to the plating out of each metal
can be measured and their ratio used to determine the concentration
of
the unknown.
Making a reading involves only three steps:
(1)
Apply voltage great enough to exceed the cadmium (pilot ion) deposition voltage (use designated
button). This current is then recorded in the TEL-meter by zeroing the galvanometer with this knob.
(2) Apply a voltage too small to plate out cadmium ion but great enough to plate out the lead (use
other designated button). This current is then recorded by zeroing the galvanometer with ths knob.
(3) Apply
a
voltage too small to deposit either metal. This determines the residual current that flows
even when no metal is plating
out.
The galvanometer is zeroed with knob "TEL", which controls the dial
reading.
The TEL-meter in effect (a) subtracts the residual current, (b) calculates the lead-to-cadmium ratio,
and finally (c) translates this ratio into ml-of-TEL/gal on the dial face.
Contamination of fuels
The contamination test can be briefly outlined as follows:
(1)
A four-gallon
representative
sample shall be used for this test.
(2) Some clear,
unleaded gasoline or naphtha is filtered through a 200-mesh sieve to insure

488
cleanliness.
nearest milligram.
mixed thoroughly at room temperature and filtered through the weighed sieve.
and the washings filtered through the same weighed sieve.
(212OF)
to remove the naphtha or gasoline.
(3)
A suitable 200-mesh sieve is dried at
100
O
C
(212O
F),
cooled in a dessicator, and weighed to the
(4)
The entire oil sample (4 gal), together with
an
equal amount
of
filtered naphtha or gasoline is
(5)
After filtration of the oil, the oil container
is
thoroughly rinsed with clean naphtha or gasoline
(6)
The sieve is then thoroughly washed with filtered naphtha or gasoline and dried at
100°C
(7)
The sieve is again cooled in a dessicator and weighed to the nearest milligram.
(8) The increase in weight
of
the sieve in milligrams per gallon and the presence of fibrous material in
the residue is reported.
The content of relatively large foreign solid particles must not exceed
15 mg/gal and should not
contain significant quantities
of
fibrous material.
Inasmuch as the foreign material, such as rust, scale, sand particles, and fiber, settles out on standing,
the importance
of
taking
representative
samples cannot be overemphasized. These contaminants clog the
filters and screens in the lubrication system. Inasmuch as fibrous material settles very slowly and has
a
great tendency to clog filters and screens, its presence is of greater concern than that
of
scale or rust.
Questions
(1)
What type of samples can be used for the contamination test?
(2) A thousand-barrel tank is equipped with three bleeders spaced equally over the height
of
the tank.
If
one-third of the sample is taken from each bleeder, would the sample be representative? Explain!
Doctor test
The doctor test is a very sensitive test for mercaptans and hydrogen sulfide. The doctor test, however,
fails
to
indicate the total quantity of sulfur in
an
oil, and the presence
of
free sulfur. The doctor test is
also inferior to the copper-strip test for detection of corrosive sulfur compounds. Although it does
indicate the presence of mercaptans, it is debatable whether or not these compounds are harmful at low
concentration normally existing in the majority
of
refined petroleum products. High concentration
of
mercaptans gives a characteristic and unpleasant odor to the fuels.
The test is made by shaking the test fuel with a sodium plumbite solution, which is prepared by
dissolving litharge in caustic soda. Upon shaking, a pinch
of
sulfur is added and the mixture is shaken
vigorously again. If the yellow color
of
the sulfur film is noticeably masked or sample is discolored, the
fuel is reported as “sour”.
If the sulfur film is only slightly discolored (grey) or flecked with black specks, the sample is reported
as “sweet”. In the presence of much hydrogen sulfide, a black precipitate forms before addition of sulfur
and usually masks the mercaptan precipitate. In the absence of hydrogen sulfide, and presence of
mercaptans, the first precipitate will be yellow or orange in color. After addition of sulfur it will darken
slowly and eventually will become black. The chemical reactions are as follows:
2
RSH+Na2Pb02
+
Pb(RS),+2NaOH
Mercaptan
+
Sodium plumbite
+
Lead mercaptide +Sodium hydroxide
(orange)
(RS),Pb+S+ PbS+(RS)2
Lead mercaptide +Sulfur
+
Lead sulfide
+
Disulfide
(orange) (black) (oil soluble)
The lead mercaptides may be sufficiently soluble in the case
of
heavier petroleum products, such as

489
kerosine, and instead of forming a precipitate, will give a yellow or orange tinge to the oil layer. On
addition of sulfur, the oil layer will darken slowly with eventual formation of a dark precipitate.
Mercaptan sulfur test
(D
1323-62)
The mercaptan sulfur test should be run on samples which show a “sour” doctor test. The
specification limit
on
mercaptan sulfur is required in jet fuels because mercaptans corrode certain metals
of the jet aircraft fuel system, cadmium-plated parts being particularly sensitive.
Inasmuch as the specification limit on mercaptan sulfur in jet fuel
(0.005%)
is half the maximum
allowable concentration for the use of
a
100-cc sample, this size sample can be used in all but research or
special fuels. The step requiring removal of HIS from the sample is unnecessary in all fuels where H,S
has been shown to be absent by the negative copper strip corrosion.
A 100-cc sample is shaken with
15
cc of standardized silver nitrate solution and approximately
15
cc
of alcohol. The alcohol serves as an emulsion breaker and is not critical as to volume. A portion of the
known quantity of silver nitrate present precipitates the mercaptans as insoluble silver mercaptide:
RSH
+
AgNO,
-+
AgSH
+
RNO, (R may be any organic radical in the mercaptan)
A large amount of shaking is required to insure complete reaction between the mercaptans in the fuel
layer and the silver nitrate in the alcohol-water layer. The excess
of
silver nitrate is then titrated back
with standardized ammonium thiocyanate and the amount of mercaptans is determined by the difference
between the silver originally added and that remaining in the solution (which reacts with the thiocyanate):
AgNO, +NH,SCN
+
AgSCN+2(NH4),S04
The indicator used is femc
alum
which reacts with thiocyanate to form
a
brick-red femc thiocyanate:
FeNH,(SO,),
+
3NH,SCN
+
Fe(SCN),
+
2(NH,),S04
The NH4SCN reacts preferentially with AgNO, until the silver ion is exhausted. After that the red
Fe(SCN), begins to form. Thus, any perceptible darkening
of
the yellow water layer during the titration
indicates exhaustion of AgNO, and consequently an end point. Titrating to a brick red end point as the
method implies requires the use of more NH,SCN than that actually needed to react with the excess
silver ion and will give an erroneous end point.
The titration is best performed by a rough addition of NH,SCN from the burette to a definite red
end point. This can then he back-titrated to the original yellow color with standard AgNO, solution. The
final fine titration can be accomplished by the dropwise addition
of
NH,SCN until the first perceptible
color change occurs in the water layer.
Key points
AgNO, and consequently an end point.
It should not be titrated to the brick red end point.
(1)
Any perceptible darkening of the yellow water layer during titration indicates exhaustion of the
(2) Care must be exercised in standardizing the solutions.
Water-tolerance of aviation fuels
The water-tolerance test determines the degree of solubility of water in aviation gasoline and jet fuels.
It
is
also indicative
of
the amount of alcohol or other water-miscible constituents in the gasoline.
The water-tolerance test involves addition of
80
cc of aviation fuel to 20 cc of distilled water and
shaking the mixture vigorously for two minutes. The samples are then allowed to stand for five minutes
before taking readings.

490
One hundred thousand gallons of average aviation fuel wil dissolve from
3
to
6
gal
of
water at
75
F.
High aromatic content and temperature can increase water solubility. On cooling from
75
to
32OF,
around 40-50% of the dissolved water is thrown out of solution.
Although
3
gal of water per
100,000
gal
of fuel does not seem significant, it may form considerable
volumes of finely divided ice crystals which may plug the fuel filter or fuel screen. The water may also
cause freezing and plugging of a fuel line.
The specifications
of
many users require that fuel shall neither lose nor gain in volume when shaken
with water. This prevents the addition of components which would separate out on contact with water,
such as wood and grain alcohols. It also ensures that the fuel will not dissolve excessive quantities
of
water.
In addition, the water-tolerance test of the fuel should not show a lace in the water layer and/or
sediment or scum at the interface between layers. These are indicative of the tendency of fuels
to
plug
micronic filters on fuel servicing units and aircraft at time of use. The repeated occurrence
of
bad water
tolerance in cases where filters were plugged with soapy material, indicates a definite relationship
between the two.
Mechanism
of
filter
plugging
In the case of JP-4 fuel, there were a number of situations in the past in which fuels, although
conforming to specification requirements at the point of manufacture, nevertheless caused micronic
filters on fuel servicing units and aircraft
to
plug at time of use. This problem was more common for
California-produced jet fuels. It was also observed that all cases where filter clogging has occurred, the
water tolerance test has shown a lace in the water layer and/or sediment or scum at the interface
between layers.
Several theories have been proposed
to
explain the mechanism
of
filter plugging, including the
following:
(a) Collection at the filter of uniformly dispersed colloidal particles which form in the fuel during or
after the refinery treatment.
(b) Collection at the filter
of
a stable fuel-water emulsion formed upstream from the filter. This can
possibly occur during agitation in storage tanks
or
aqua systems where there is high throughput relative
to available storage and settling facilities.
(c) Formation at the filter of an emulsion with the water which filtered from the fuel.
The complex nitrogen and sulfur polymerization products, which were probably responsible for the
dark color of jet fuel in certain areas, could form a colloidal suspension in the fuel. This suspension in
addition to collecting at the filter, could also act as an emulsifying agent. Metallic naphthenates, which
form through reaction of the naphthenic acids with caustic or possibly iron rust, could be very good
emulsifying agents and, possibly, precipitate
out
as a colloidal suspension.
The naphthenic acids, which are present in high concentration in untreated jet fuels from some crude
oils, could also act as emulsifying agents. They probably will not be as detrimental, however, as the soap
of the acids.
No
single theory, however, can account for all cases of filter plugging and bad water tolerances, and
the filter problem is the result of some combination of these potential offenders.
The water tolerances are influenced by the source
of
crude oil, amount of heavy components in the
blend, and type and efficiency
of
refinery treatment. Although the water tolerance test is not as positive
as the flame photometer, etc., for the presence of soapy material, it is adequate enough for detection of
potential trouble. A strict interpretation of the water tolerance test should be made. Passing only fuels
with a clean and clear interface solves this problem.
Questions
(1)
List the chemicals whch accentuate emulsion-forming tendencies
of
jet fuels.
(2)
Describe the purpose and procedure for determining the water tolerance test.
(3)
Upon completion of the water tolerance test, what do the drops
of
water clinging
to
the sides of
(4) Under what conditions would dissolved water in aircraft fuel cause malfunctioning
of
aircraft
the stoppered graduate indicate?
engines?

491
Thermal value of fuel oil
(D
240-76;
D
1405-64;
D
2382-83)
The thermal (calorific) value
of
a fuel is the amount
of
heat generated as a result of its complete
combustion. Results are usually expressed in “calories per gram” or “British thermal units (Btu) per
pound”. The calorie is the amount
of
heat necessary to raise the temperature of a gram
of
water one
degree Centigrade, whereas the British thermal unit is the amount of heat required to raise the
temperature
of
one pound
of
water one degree Fahrenheit. Inasmuch as one pound is equivalent to
453.59
g
and a degree Centigrade is equivalent to 1.8OF,
1
Btu
is
equal to 251.99 cal(1 cal/g=1.8
Btu/lb).
Thermal values are reported as “gross” (higher) or “net” (lower). The heat liberated when
1
Ib
of
a
fuel at
60
F
is burned and the products
of
combustion are cooled to
60
O
F is called the net heating
value. If in addition the H20 vapor in the flue gas is condensed, the
gross (high) heating value is
obtained. The
gross
heating value is measured directly by use of the conventional bomb calorimeter and
is
generally used in the United States, whereas in Europe the net heating value is used.
The thermal value
of
gasoline can be determined in an oxygen bomb calorimeter. A weighed quantity
of
gasoline is burned in a steel bomb placed in a definite quantity
of
water. The thermal value of the fuel
can be calculated from the rise in temperature
of
the water due to the burning of a definite quantity
of
fuel.
Key points
(1)
Sample of fuel must be weighed rapidly to minimize evaporation.
(2) Apparatus must be assembled immediately after weighmg and placing the sample in the cup.
(3)
Temperature
of
water in jacket must be regulated to agree with the temperature of water in the
(4) It is necessary to determine the correct oxygen pressure to insure complete combustion.
bucket.
Sample problem
A-5
What is the net heating value
of
methane expressed in Btu per cu ft? The gross heating value
of
methane is given as
1009
Btu per cu ft and the heat evolved when
1
cu ft
of
H20 vapor is condensed at
60
OF
is equal to
50.3
Btu. (The heat evolved
on
condensing
1
lb of H20 is equal to 1058.2 Btu).
Solution
:
CH,
+
202
+
COZ
+
2 HzO
One cu ft
of
methane
on
burning produces
2
cu ft of water vapor; therefore, the net heating
value
=
1009
-
(2
x
50.3)
=
908.4
Btu/cu ft.
Question
value (total heat of combustion)?
(1)
How does the net heating value (net heat of combustion) of a fuel differ from the total heating
Gum content
(ASTM
381-80)
During
cracking, unsaturated hydrocarbons, such as the olefins C,H,,, diolefins C,H,,_,, and
acetylene, are produced, which upon exposure to air during storage result in the formation
of
so-called
“gum” in the gasoline. Diolefins cause the formation
of
tars, a
loss
in color, and the formation
of
gum
during storage. They tend to polymerize and combine with other unsaturated molecules, forming
high-molecular weight, gum-like solids. These compounds are soluble in the gasoline unless a marked
degree of aging has taken place. Upon evaporation of the gasoline, however, they form resinous materials
insoluble in the gasoline. This gum content is usually expressed in milligrams of
gum per
100
ml
of
sample.

492
Excessive gum content in gasoline is attended by stickiness of the valve stems and carburator
mechanism, clogging
of
screens and filters in the fuel lines, and formation
of
deposits in the intake
manifold. All these effects result in uncertain engine performance. If the motor gasoline contains
dissolved non-volatile lubricating oil, it will be also obtained as a residue along with the gum. Such
gasolines obviously require special treatment depending upon the nature of the non-volatile material.
Freshly manufactured gasoline does not normally have an appreciable gum content. A more
significant property of the gasoline, therefore, is its tendency to form gum in storage (gum stability).
Gum stability depends upon the nature of gasoline and refining. The gum forms at varying rates and
different gasolines have varying gum contents after a given period of aging. The rate
of
gum formation
also depends upon the temperature of storage, access of air, and presence
of
catalytic materials.
Method
of
testing:
(1) One method
of
testing involves rapid evaporation
of
a sample of the gasoline at an elevated
temperature in a current
of
heated air or dry steam. The results indicate the amount of gum deposition
which may
occur
if the fuel is used immediately, but do not indicate the stability
of
the product toward
gum formation upon storage.
(2) The second method involves accelerated oxidation in closed systems at elevated temperatures. The
amount of gum and lead precipitate after a specified period
(5
or
16
hours) of oxidation may be used as
an indication of the tendency of the fuel to form deposits on storage.
Key
points
(1)
The flowmeter should be calibrated to give required flow rate
of
dry steam
or
air.
(2)
Prior
to
weighmg, the beakers must be allowed
to
cool in the vicinity
of
the balance for at least
two hours.
Questions
(1)
What is the purpose of the oxidation stability test?
(2) What
is
the purpose of the existent
gum
test?
(3)
What is the specified bath temperature for conducting existent gum test on jet fuel? Reciprocating
fuel?
Aniline point
(D
611
-82)
and aniline-gravity constant
The aniline-gravity constant, which is calculated from the aniline point and API gravity values, can
be used in both the aviation gasoline and jet fuel specifications as a substitute for the heat
of
combustion
test. The aniline-gravity constants, however, must have high values before they are considered as a rough
measure of the heating values. (The amount of heat liberated when a unit quantity of a fluid is burned is
called the heating value or heat
of
combustion.)
The aniline-gravity constant is the product
of
the gravity in degrees API and the aniline point in
degrees Fahrenheit. The high-API gravity fuels (light fuels), therefore, will have higher aniline-gravity
constants.
This
is of great importance because with the higher-API gravity fuels a greater quantity
of
fuel
can be carried in the aircraft before reaching the limiting fuel weight. In addition, there
is
a
direct
correlation between the API gravity and heating value
of
fuels.
The aniline point is the temperature in
O
F
at which an equivolume mixture of fuel and aniline are no
longer soluble in one another. The principle involved in this test is the fact that when two immiscible
liquids are heated together, they become increasingly soluble in one another until a point is reached
where they are completely miscible.
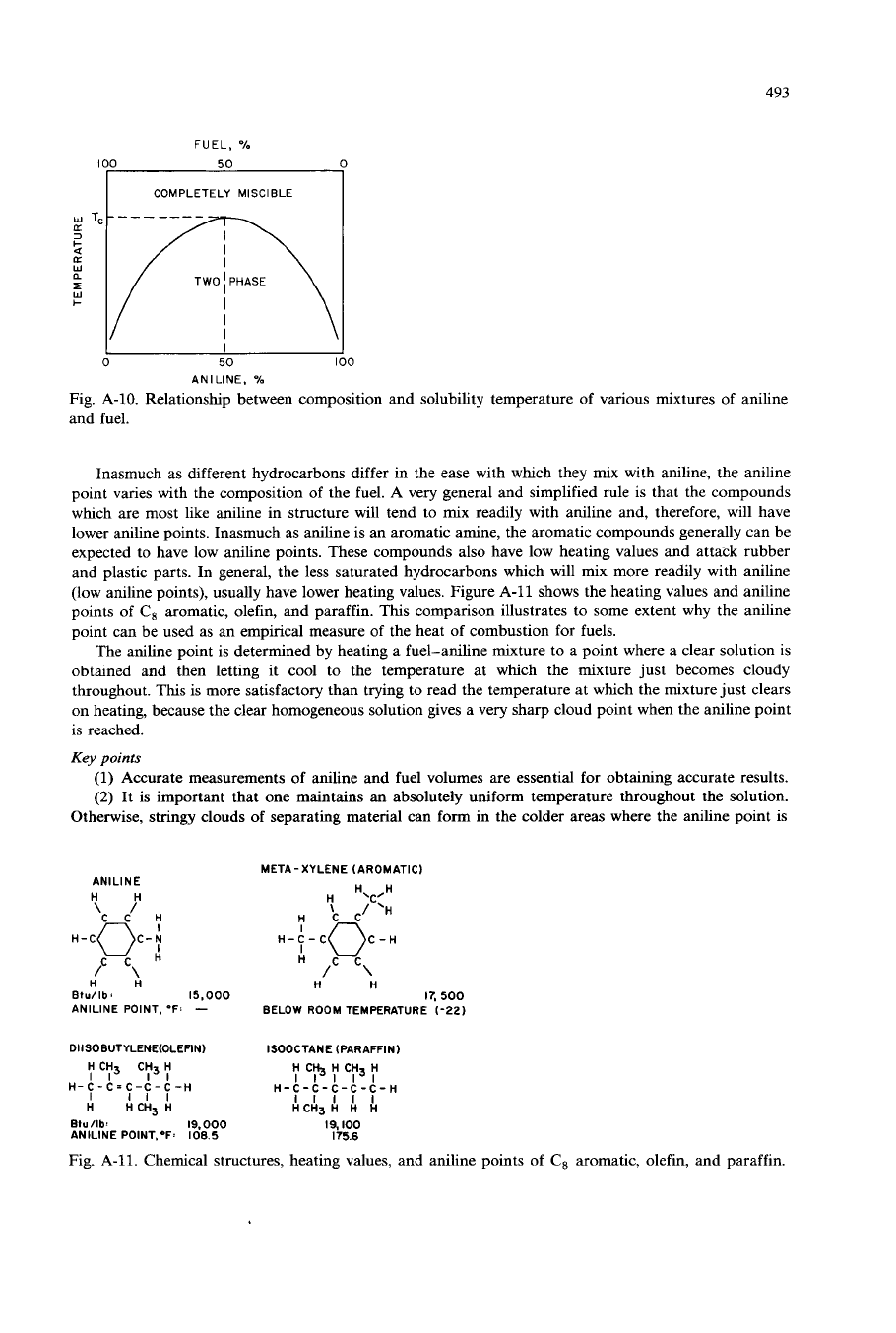
Figure A-10 shows the solubility temperature of different mixtures
of
aniline fuel. At any given
composition
of
fuel and aniline there is one temperature at which complete solubility first occurs on
heating. For a
50-50
mixture, this temperature, shown as
T,
in Fig. A-10, is defined as the aniline point.
Should the composition, however, be changed even slightly, for example to a 49-51 mixture through
careless measurement, then the temperature at which mixing occurs would differ from the true aniline
point. Accurate measurements of aniline and fuel volumes, therefore, are essential for obtaining good
results in this test.
493
nw
W
COMPLETELY
MISCIBLE
Tc--------
TWO
I
PHASE
Inasmuch as different hydrocarbons differ in the ease with which they mix with aniline, the aniline
point varies with the composition of the fuel.
A
very general and simplified rule is that the compounds
which are most like aniline in structure will tend to mix readily with aniline and, therefore, will have
lower aniline points. Inasmuch as aniline is an aromatic amine, the aromatic compounds generally can be
expected to have low aniline points. These compounds also have low heating values and attack rubber
and plastic parts.
In
general, the less saturated hydrocarbons which will mix more readily with aniline
(low aniline points), usually have lower heating values. Figure A-11 shows the heating values and aniline
points of
C,
aromatic, olefin, and paraffin. This comparison illustrates to some extent why the aniline
point can be used as an empirical measure of the heat of combustion for fuels.
The aniline point is determined by heating a fuel-aniline mixture to a point where a clear solution is
obtained and then letting it cool to the temperature at which the mixture just becomes cloudy
throughout.
This
is more satisfactory than trying to read the temperature at which the mixture just clears
on heating, because the clear homogeneous solution gives a very sharp cloud point when the aniline point
is reached.
Key
points
(1)
Accurate measurements
of
aniline and fuel volumes are essential for obtaining accurate results.
(2)
It
is
important that one maintains
an
absolutely uniform temperature throughout the solution.
Otherwise, stringy clouds
of
separating material can form in the colder areas where the aniline point is
ANILINE
'C
?
H
H-COC-t!4
d
/c c\
META-XYLENE (AROMATIC)
HH H
Btu/
Ib
:
15,000
17, 500
ANILINE POINT, 'F:
-
BELOW
ROOM
TEMPERATURE
1-22]
DIISOBUTYLENE(0LEFIN) ISOOCTANE (PARAFFIN)
H CHS CH H H C% H CH3 H
I I
1~1
IIIII
H-C
-
C
=
C-C -C -H
H-C-C-C-C-C-H
I
Ill
IIIII
H
H CH3 H HCH3 H H H
EtuIlb:
19.000
19,100
ANILINE P0INT;F:
108.5
175.6
Fig. A-11. Chemical structures, heating values, and aniline points of
C,
aromatic, olefin, and paraffin.

494
reached. The temperature at which the entire solution is uniformly cloudy should be regarded as the true
aniline point.
Questions
(1)
Define the aniline point.
(2) What is the purpose
of
this test?
(3)
How is the aniline point of fuel related to the Btu value? Why?
(4)
Give an actual correlation between the 'API gravity and heating value for some fuel.
Fluorescent- Indicator Adsorption (FIA) test
(D
1319.83;
also
see
D
936-83)
The fluorescent-indicator adsorption test is used for determining the amounts
of
aromatics, olefins,
and saturates in fuels. It
is
desirable to limit the amount of aromatics in fuels because
of
their tendency
to leave carbon deposits in the combustion chambers, their relatively low heating values, the ease with
which they attack rubber and plastic parts in the fuel-handling systems, and their tendency in some cases
to have poor freezing points. The compounds containing sulfur, nitrogen, or oxygen are also determined
as aromatics. On the other hand, olefins tend to produce gums and other undesirable polymerization
products on storage and are limited for this reason. Inasmuch as the
high
olefin content is characteristic
of
cracked stocks in fuels, a tight limit on olefins virtually restricts the refinery to the use
of
the more
stable straight-run blending stocks. The FIA is
a
rapid substitute for the two laborious chemical analyses
for
(1)
total unsaturates by acid absorption and
(2)
olefins by bromine number. The FIA method is based
upon the principle that liquid organic compounds adsorb
on
the surface of finely divided silica gel with
varying degree of attraction, which depends upon their molecular size, weight and arrangement
(structure), heat of wetting, polarity, and solubility. Silica gel has a greater affinity for alcohols than for
hydrocarbons. Among the hydrocarbons, silica gel has the greatest affinity for the aromatics and a
greater affinity for olefins than for paraffins or cycloparaffins.
Thus,
a
small sample can be washed down a column
of
packed silica gel with isopropyl alcohol and
the fuel will separate into its paraffinic, olefinic, and aromatic components. The aromatics, which have
the greatest affinity for silica gel, will be the last to be displaced by alcohol and, therefore, will
concentrate at the top of the fuel layer. The olefins will form the next band and the saturated
hydrocarbons will be in the bottom layer. This
is
due to the chasing
of
molecules down the column, each
molecule displacing another type more loosely held by the gel.
The dye used in the
FIA
test has olefinic and aromatic components which will concentrate in
respective layers.
On
using ultraviolet light, the various components of the fuel appear as color bands in
the packed column. The percentage of different components is determined from the ratios of color band
lengths to the overall length of the fuel sample (sum
of
lengths).
Key points
sealing, and (c) incomplete elution of the hydrocarbons by alcohol.
(1)
Erratic results can occur frop (a) improper packing
of
the silica gel in the tube, (b) improper glass
(2)
Isoamyl alcohol is recommended in the case
of
higher-boiling hydrocarbon samples.
Sulfur
content
(Lamp)
(ASTM
D
1266-80)
(also
see
D
129-64)
The sulfur lamp test is intended
for
the determination
of
the total amount
of
sulfur
in fuels, without
attempting to separate this value into quantities
of
various classes of sulfur compounds. The sample is
burned in a closed system using a wick-type lamp and in sulfur-free air. The oxides of sulfur are absorbed
in water, oxidized to sulfuric acid by means of hydrogen peroxide, and determined gravimetrically as
barium sulfate. The volumetric method involves titration with NaOH.
Many fuels are not permitted to contain more than
0.05%
sulfur by weight, because the sulfuric acid
which can form on combustion is highly corrosive.
