Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.


495
Key points
(1)
In
the case of incomplete oxidation of the sample, the absorber liquid will have a characteristic
taste or odor, which can be easily detected by the operator when drawing air through the absorber during
titration.
In
such cases, the test shall be discarded.
(2)
Weighmg should be done rapidly in order to prevent evaporation losses.
(3)
Flame must be maintained at a point just below smoking with a steady, symmetrical appearance.
Questions
conditions?
(1)
Describe the effect of sulfur upon nickel alloys. What is the significance of oxidizing and reducing
(2)
What
is
the relationship between the sulfur content and coke deposition (turbine type fuels)?
Corrosion (Copper strip,
212
OF)
(ASTM
D
130-83)
The corrosion test for gasoline is intended primarily to prevent corrosion of metals in the fuel and
induction systems of engines. Inasmuch as the most likely corrosive substance present in gasoline and
kerosine is sulfur, which readily attacks copper, it
is
customary to require that products of this type shall
pass a test involving contact with polished copper for a specified time, at a specified temperature.
The test involves determination of discoloration produced when a strip
of
sheet copper is immersed in
the gasoline for three hours at a temperature of
212"
F.
The degree of discoloration is first examined in
the center
of
the copper strip and the relative proportions of areas of different degree
of
discoloration are
determined before assigning a discoloration number from the standard chart. For example,
6+
would
indicate that the greater portion of the copper strip has a discoloration number of
6
with some areas
around the edges having a discoloration number
of
7.
Questions
(1)
How are the copper strips polished?
(2)
How long does the copper strip remain in the bath at test temperature?
(3)
What is the specified bath temperature?
(4)
Under what conditions is the fuel reported as passing the corrosion test?
FUNCTIONS OF LUBRICATING OILS
The primary purpose of any lubricant is to reduce friction, and thereby eliminate metal-to-metal
contact. Lubricating oil provides a film which permits surfaces
to
glide over each other with less friction.
Lubrication is essential, therefore, to prevent wear in any mechanical device where there are surfaces
rubbing against each other. The selection of the proper lubricating oil for a given application depends
upon the design of the equipment and the conditions under which the equipment is to be operated.
In
internal combustion engines, lubricating oils must perform four basic functions:
(1)
lubrication,
(2)
cleaning,
(3)
cooling, and
(4)
sealing.
(I)
Lubrication
In order to lubricate properly, an oil must have the following characteristics:
(a) It must be of low enough viscosity to flow readily between closely-fitted, rapidly moving parts,
but
of
sufficient viscosiy to prevent metal-to-metal contact between these parts.
(b) It must be tough enough
so
that it will not break down or fail under high temperatures and
pressures.
(c) It must have a low enough pour point to enable it
to
flow readily when starting under extremely
low temperatures.

496
(d) It must have high enough flash and fire points
so
that it will not burn, vaporize, or otherwise be
(e) Its carbon content must be low enough
so
that it will not deposit excessive amounts of carbon.
(f)
The oxygen absorption of the oil must be low enough
so
that varnish and gum do not form.
(g) The neutralization number must be as low as possible. (Low neutralization number indicates that
the amount
of
acid present is small). Inasmuch as acid is detrimental to engine parts, the acid content
must be very low.
consumed under high heat.
(2)
Cleaning
A major function
of
a lubricating oil is cleaning or carrying off dirt, road dust, small carbon and steel
particles, gum, varnish, etc. This function has become particularly important because of increased
compression ratios, higher speed engnes,
high
operating temperatures, and closer tolerances. Filters have
been developed to filter out part of the dirt, and ventilation systems have been designed to carry off
vapors and moisture. These devices, however, perform only part
of
the job. Additives or detergents,
therefore, are blended with lubricating oils. The detergent, which is soluble in the oil, cleans dirt, gum,
and other impurities from the engine and moving parts. It then holds these impurities in suspension. As
the oil, carrying the particles in suspension, is circulated, the dirt is removed by the filter. Gum and
varnish, however, are not removed by the filter. Consequently, the
oil
must be changed at intervals. If
not, the amount of gum and varnish held in suspension by the oil increases to the point where these
substances are deposited thoughout the engine, causing poor performance.
(3)
Cooling
A lubricant must cool moving parts by carrying off waste heat.
This
is especially true in aircraft
engines where the lubricants must carry off approximately one-third
of
the total waste heat.
In
order to
perform its cooling function, a lubricant must have sufficiently low viscosity to flow readily at all
temperatures of operation.
(4)
Sealing
Another function
of
lubricating oil is to seal the space between the piston rings, cylinder walls, and
pistons to prevent blowby (leakage of combustion gases from the combustion chamber, past the rings,
into the crankcase). When the space between piston rings, cylinder walls, and pistons is properly sealed,
the full force of the burning and expanding fuel gases is exerted on the head of the piston, and none of
the force
of
combustion is lost.
TESTS ON LUBRICATING OILS
Viscosity
Viscosity or “body” is the measure of the oil’s fluidity, or rather its resistance to flow.
Viscosity (kinematic) can be measured with glass viscometers, immersed in a constant-temperature
bath. (See
D
445-83.)
The time necessary for the oil to flow between two notched areas (on the outside)
of the capillary tube multiplied by the experimentally determined constant for the individual capillary is
termed the kinematic viscosity (centistokes), which can be converted to Saybolt viscosity (Saybolt
Universal seconds) by means
of
formula
or
tables.
The Saybolt type of viscometer was the original apparatus used to measure the viscosity
of
petroleum
oils and the unit for expressing viscosity given by this apparatus is used thoughout the petroleum
industry. The Saybolt viscometer contains a carefully machined tube and orifice meeting definite
measurement specifications. The tube, which is surrounded by a constant temperature bath, is filled with
491
test oil and is allowed to flow by gravity through the orifice into a calibrated flask. The time in seconds
necessary to fill the flask up to a definite volume is termed the Saybolt seconds viscosity.
The viscosity
of
lubricating oils is the best single index which indicates the uses for which the oils can
be recommended.
In a bearing, the viscosity of the lubricating oil at the operating temperature, determines the bearing
friction, heat generated, and the rate of
oil
flow under the particular conditions
of
load, speed, and
bearing design. Lubricating oil prevents direct contact
of
metal surfaces through adhesion or ability
of
the oil to stick to the surface of the metal. The oil must be viscous enough, however, in order not to be
squeezed out by the bearing pressure. With increasing viscosity, the ability
of
the oil to stick also
increases. Generally, the lower the pressure and greater the speed, the less viscous must be the oil used.
Although a reasonable factor
of
safety is essential, excessive viscosity means unnecessary friction and
heat generation. Inasmuch as the rate of change of viscosity with temperature varies with different oils,
the viscosity test should be made at that standard temperature which approximates most closely the
temperature of the oil in use.
In the case
of
lubricating oils for automotive equipment, the viscosity
of
the crankcase oil at low
temperature also indicates the ease of starting in cold weather.
In
transformer oils, or oils for circulation
in heat-carrying systems, where the rate
of
circulation
of
an oil is important, viscosity becomes a factor
of
practical significance.
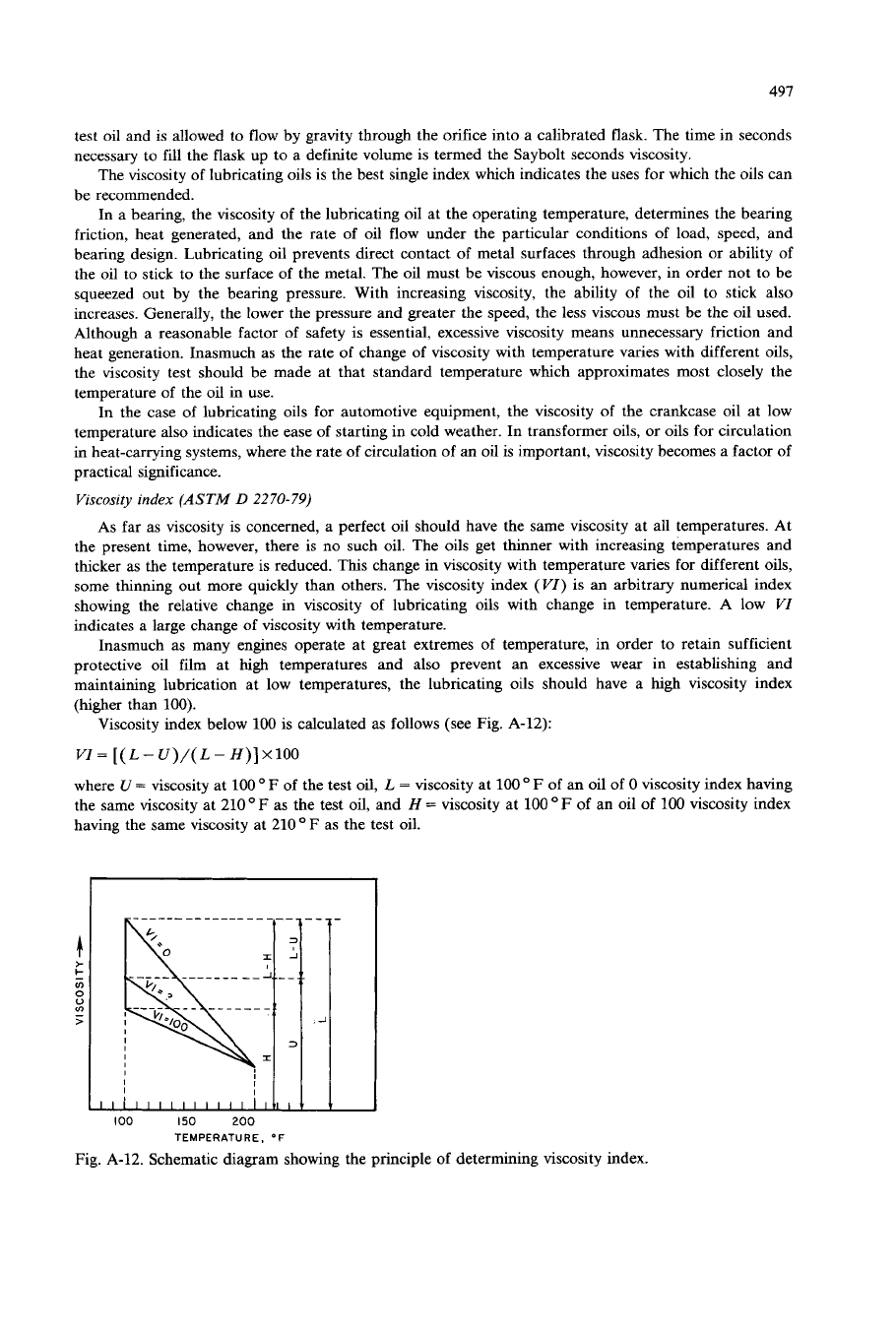
Viscosity
index
(ASTM
D
2270-79)
As far as viscosity is concerned, a perfect oil should have the same viscosity at all temperatures. At
the present time, however, there is no such
oil.
The oils get thinner with increasing temperatures and
thicker as the temperature is reduced. This change in viscosity with temperature vanes for different oils,
some thinning out more quickly than others. The viscosity index
(VI)
is
an arbitrary numerical index
showing the relative change in viscosity
of
lubricating oils with change in temperature. A low
VI
indicates a large change of viscosity with temperature.
Inasmuch as many engines operate at great extremes
of
temperature, in order to retain sufficient
protective oil film at high temperatures and also prevent an excessive wear in establishng and
maintaining lubrication at low temperatures, the lubricating oils should have a high viscosity index
(higher than
100).
Viscosity index below
100
is calculated
as
follows (see Fig. A-12):
VI
=
[(
L
-
U)/(
L
-
H)] X100
where U
=
viscosity at
100
OF
of the test
oil,
L
=
viscosity at
100
F
of an oil
of
0
viscosity index having
the same viscosity at 210
F
as the test
oil,
and
H
=
viscosity at
100
F
of
an oil
of
100
viscosity index
having the same viscosity at 210
F
as
the test oil.
I00
150
200
TEMPERATURE,
OF
Fig. A-12. Schematic diagram showing the principle of determining viscosity index.

498
Thus, viscosity index can be calculated from the viscosity at
100
F
and 210
O
F. The flatter the
viscosity temperature curve (that is, lower the rate of change of viscosity with temperature), the higher is
the viscosity index.
Flush
and
fire
tests
(1) Cleveland Open Cup (ASTM
D
92-78), (2) Pensky-Martens Closed Tester (ASTM
D
93-80), and
(3) Tag Closed Tester (ASTM
D
56-82).
The flash point of
a
petroleum product is the temperature at which sufficient vapors are given
off
to
form an inflammable mixture with
air.
The fire point may be defined as the temperature to which a
product must be heated in order to bum continuously after the flammable air-vapor mixture is already
ignited. The flash-point test is more frequently used, because the fire-point test usually does not give any
additional information.
Flash-point test
in
addition to indicating fire hazard, is also related somewhat to the consumption
of
the oil. A large number
of
“flash testers” have been devised and used and can be subdivided into two
general classes: (1) closed-cup testers, and
(2)
open-cup testers.
The closed-cup tester is preferable in determining the flash point as an index of fire hazard, because it
comes closer
to
paralleling actual conditions than does the open-cup tester. In handling or storage
of
petroleum products, an explosive ignition of vapors can occur in the unfilled portions of tanks, drums, or
other containers. The closed-cup tester permits measuring the temperature which the oil must reach
before it gives
off
enough vapor to create
an
explosive mixture in a closed system. In addition, the
closed-cup tester is more accurate than the open cup.
The open-cup testers yield sufficiently accurate results to meet almost all of the practical require-
ments. In addition, the main advantages of the open-cup testers are cheapness and simplicity in
operation.
The flash points
of
petroleum products vary over a wide range. The majority of lubricating oils have
flash points between 275
O
and 650
OF,
whereas the flash points
of
more volatile gasolines and naphthas
are considerably below
0
OF.
The normal closed-cup flash point range for kerosine is 100-160 F. Gas
oils and fuel oils generally have flash points between
110
and 300
F.
The naphthas used as paint
thinners and solvents have a closed-cup flash point ranging from 80 to 110
OF.
A
closed-cup tester with a water bath and without a stirrer in the cup is best for the naphtha-kerosine
group
of
products. For the testing of fuel oils and gas oils, a closed-cup tester without a waterbath but
with a stirrer in the cup is preferred. Lubricating oils are best tested with a simple open-cup tester.
For a large number
of
petroleum products, including lubricating oils, the flash point is determined for
the purposes
of
identification and classification. Flash point
of
an oil may serve as a rough indication of
its tendency to vaporize. The Occurrence
of
foaming in the course
of
a flash-point determination is a
sensitive qualitative indication
of
the presence of moisture in the test sample. In general, the interpreta-
tion
of
results is not simple, because the flash points bear no direct relation to the usefulness
of
oils.
Flash point is regarded
as
the most important index
of
fire hazard. For kerosine
and
napththa, the
fire hazard is a very important consideration. There are wide variations in the flash-point requirements
for kerosine enforced by different counties, states, and countries.
This
is not done because there is a
particular temperature limit differentiating between safety and danger. Kerosine with a flash point of
90
O
F
is just as safe
as
a kerosine having
a
flash point of 100
OF,
if
users are familiar with the precautions
necessary in handling it. Safety is measured by the habits
of
the user rather than by the physical and
chemical properties of the product. The user, however, must know the exact properties
of
the product in
order to know how to handle it.
Key
points
(1) The flash and fire tests should be performed in a room free of drafts.
(2)
Careless breathing or unnecessary movements near the flash cup, which disturb the vapors over
the cup. should be avoided.

499
(3)
The true flash point must not be confused with the bluish halo that sometimes surrounds the test
(4)
Rate of temperature increase is of prime importance.
(5)
The test should not be repeated on the same portion of sample once used. Fresh portions of the
flame.
sample should be used for each test performed.
Questions
closed-cup testers?
(1)
What relationship exists between the results obtained by open-cup and those obtained by
(2)
List all the methods for determining the flash points.
(3)
Can one set gasoline afire with a match? Kerosine? Motor oil? Explain.
(4)
What is the definition of kerosine and gasoline?
(5)
Why does the petroleum industry bother with the flash and fire points?
(6)
Suggest improvements in the open-cup method
of
testing.
(7)
What relationship exists between the flash and fire points
of
petroleum products and their boiling
(8)
Enumerate the conditions that must exist at the time a flash occurs above a liquid petroleum
points?
product.
Color
of
lubricating
oils
and petroleum
The ASTM Union Colonmeter test describes the determination
of
the color
of
lubricating oils and
petroleum. Measurement
of
color depends upon matching the color
of
a given depth or thickness
of
oil
with various color standards. (Also see Saybolt Chromometer Method,
D
156-82;
ASTM Color Scale,
D
By transmission
of
light, the color
of
oils varies from a light yellow to a deep red, whereas by reflected
light the oil may exhibit a blue or yellow-green appearance at the surface, regardless
of
the oil’s color.
This appearance is due to the fluorescence and is called the “cast” or “bloom”
of
the oil. It may be noted
by looking at the oil at an angle, or by viewing it in thin layers on a black background.
Properly refined paraffin-base oils and even some mixed-base oils have a yellowish-green cast,
whereas naphthene oils appear deep blue. The natural yellowish cast
of
the paraffin-base or mixed-base
oils, however, may be spoiled by improper acid treatment.
The chief significance of color as applied to lubricating oils lies in the fact that it is a generally
accepted index
of
the uniformity
of
a given grade of oil. Color requirements
of
lubricating oils are
frequently overemphasized because color does not necessarily indicate quality. For example, it is
erroneously believed by some that pale color is indicative
of
low viscosity. Color requirements should not
be made any more stringent than service demands.
The color is of definite importance, however, in the case of dry-cleaners’ naphtha, because it must be
free from materials whch might discolor fabrics.
1500-82.)
Key points
(1)
Color
of
oils which are intermediate to the standard colors must be expressed in terms
of
the
darker standard
as
“minus”. For example,
an
oil having
a
color between
8
and
9
is to be expressed as
“9
minus”.
Questions
(1)
What does the color
of
lubricating oil indicate?
(2) Name other methods for determining the color
of
petroleum products.
(3)
How would one determine the color
of
a
“green” lube oil?
Cloud
and pour points
(ASTM
D
97-66;
also see
D
2500-81)
Because
of
partial separation
of
wax and congealing
of
the hydrocarbons composing the oil,
petroleum oils become plastic solids when cooled sufficiently.

500
The cloud point of a petroleum oil is the temperature at which paraffin wax or other solid substances
begin to crystallize out or separate from solution. The pour point is the lowest temperature at which the
oil
will pour or flow when chilled without disturbance, under definite prescribed conditions.
The cloud point cannot be determined for
oils
in which wax does not separate prior to solidification,
or in which separation is not visible. The cloud and pour points depend upon the source of the crude oil
from which they are made, upon the grade or kind, and upon the method
of
manufacture.
The cloud point is useful when the haze or cloud in the oil above
a
given temperature is objectionable
for some reason. It has a more limited value and narrower range
of
application, however, than the pour
point. The test may also give erroneous results if the oil
is
not dry and water separates out.
The pour point gives an indication
of
the temperature below which it might be dangerous to use oil in
gravity-lubricating systems and where the head tending to produce flow is small. The pour point also
shows at what temperature the oil may not be possible to pour or remove from its container. The
tendency to flow, however, is also affected by the sue and shape of the container, the head or force
exerted upon the oil, and the nature
of
its physical structure when solidified.
The cloud test involves cooling the oil in a specified test jar from at least 25
F
above the cloud point.
The cooling bath is held between 15
"
and
30
"
F
below the cloud point
of
the oil. At intervals, the test jar
is
removed from the brine bath without disturbance to the oil, and the temperature at which a distinct
cloudiness or haziness first appears in the bottom of the test jar is recorded as the cloud point. The pour
test is conducted in the same manner. The oil, however,
is
first heated to 115
"
F
to be sure that all the
wax has dissolved, and then cooled to
90"
before the test. As in the cloud test, the bath is held at
15-30
OF
below the estimated pour point. At intervals
of
5
"
F,
the test jar
is
removed from the bath and
tilted to determine if the oil will flow or move. If there is
no
movement when the jar is held horizontal for
five seconds, the recorded temperature is called the "solid point". The pour point temperature is taken to
be 5
"
F
above the solid point temperature.
Inasmuch as there is no single test which can be taken
as
a positive and direct measure
of
the
performance of an oil under
all
conditions of service, cloud and pour points should be interpreted in light
of actual performance under the particular conditions
of
use.
Key
points
(1)
Great care must be exercised in order not to disturb the mass of oil after the formation of paraffin
wax crystals. Any disturbance of the spongy network of wax crystals will result in low and erroneous
pour points.
(2) The test may give misleading results if the oil is not dry, due to the separation of water. Thus, the
test should always be interpreted with this fact in mind.
Questions
principles.
(1) What does the cloud point indicate? Name one other test method that depends
on
similar
(2) What physical property
of
an oil does the pour point indicate? How else could it be determined?
(3)
Explain how the "thermal history" of a sample might affect the pour point.
(4)
Discuss dewaxing. How does it affect the cloud and pour points?
Dilution
of
crankcase
oil
(ASTM
D
322-80)
All fluids possess viscosity and, therefore, show certain frictional phenomena when motion occurs.
Inasmuch as friction releases heat, the temperature
of
a
cold engine-oil rises with use. This causes the
viscosity of the oil to decrease, giving rise to an important problem for the internal combustion engine.
When the engine is run at low temperatures, or the piston rings are worn, unburned gasoline may be
forced into the crankcase causing dilution of lubricating oil. With a lowered viscosity from both dilution
and increase in engine temperature, the oil cannot perform the lubrication necessary to prevent
overheating and breakdown
on
the moving parts. Lubricating oils are made to compensate for the
dilution in small amounts and, therefore, no harm
is
done to the engine until the dilution becomes
excessive. Excessive dilution is caused mainly by short running periods
of
the engine, or worn-out parts

501
of
the engine, allowing “blow-by” of the gasoline. If, however, the engine is kept hot for a long period
of
time, some of the gasoline will be distilled from the oil, tending to restore the normal viscosity.
Precipitation number
of
lubricating oils
(ASTM
D
91
-81)
The method entitled “Precipitation Number
of
Lubricating Oils” is intended for the determination
of
the precipitation number of steam cylinder stocks, black oils, and other lubricating oils. A definite
volume of oil and precipitation naphtha are heated in a prescribed calibrated tube and then centrifuged.
The volume
of
sludge or asphalt present in oil, which is packed at the bottom of the tube due to the
centrifugal force, can be read from the calibrations on the tube.
Crude oils contain non-volatile constituents which by distillation are concentrated in the residual
products. If the latter are used as lubricants, it is necessary, therefore, to determine the amount
of
this
“asphaltic” material. Inasmuch as the “asphaltic material” is sparingly soluble in naphtha, which is
composed
of
low-boiling paraffin hydrocarbons, this characteristic forms the basis for the precipitation
test.
The precipitation tests are successfully used for classifying the several groups of residual lubricating
oils. Although some oils which contain appreciable quantities of asphaltic constituents are unsuitable for
certain types of service, the importance of moderate variations is exaggerated by many users.
Black oils, which are used for the lubrication of gears and car journals, usually have moderately high
precipitation numbers. Another group, which is characterized by a moderate percentage
of
asphaltic
material, is steam-refined cylinder stocks. Filtered products such as “bright stocks”, which are largely
used as constituents of motor oils, contain negligible quantities of material that is insoluble in the
precipitation naphtha.
One disadvantage of this method is the fact that the compactness
of
the precipitate is not the same for
all types of oil; therefore, there is
no
relationship between the precipitation number and the percentages
of “asphalt” as defined by gravimetric methods.
Questions
(1)
What is the significance
of
the precipitation number test?
(2)
What is the relative amount of “asphaltic” material in black oils, cylinder stocks, and “bright
stocks”?
Carbon residue
(D
189-81;
D
524-81)
Some oils may be vaporized at room pressure and in the absence of air without leaving an appreciable
residue, whereas other oils, upon distillation, leave a non-volatile carbonaceous residue.
The ASTM method involves destructive distillation
of
a weighed quantity
of
oil in an apparatus
so
designed as to exclude air, to permit proper control of rate
of
heating, and to eliminate possible
condensation
of
distillates in or on the oil contained.
This method throws some light on the relative carbon-forming propensity of the oil. The quantity
of
carbon deposited in the combustion chamber should be proportional to the carbon residue
of
the oil.
Other factors such as the viscosity
of
the oil, the mechanical condition
of
the engine, and the conditions
of
carburation of the fuel, however, may control the carbon deposition. The results
of
this test, therefore,
must be considered in connection with other tests and the use for which the oil is intended.
Key point
residue.
Ash
conteni
(ASTM
D
482-80;
D
874-82)
(1)
Petroleum products containing ash-forming constituents will have an erroneously high carbon
The ash content method
is
used for determining the ash content
of
fuel oils and other petroleum oils.
The test
is
made by
burning
a weighed quantity of oil in a platinum or porcelain crucible and weighmg
the remaining
ash.

502
If a petroleum oil contains any inorganic foreign material, an ash or residue will be obtained after
burning and ignition. This foreign material can then be identified by examination
of
the ash. This
method also presents a quick means for determining the presence of clay that might have been left in the
oil during clay refining. The ash test used for lubricating oils shows the amount
of
silt, dust, sand grains,
and metallic particles resulting from the wear of the motor. All these inorganic substances are destructive
to the operating or moving parts of the engine and should not be present in the oil.
Key
points
method is not recommended for oils containing metallo-organic addition agents.
attack platinum at high temperatures.
of alcohol before heating.
Question
(1)
Inasmuch as large portions of the metal compounds may be lost by vaporization, the ash content
(2)
A
platinum dish should not be used when the sample contains lead, zinc or other metals, which
(3)
In order to avoid foaming and loss of samples containing moisture, it is advisable to add
1-2
ml
(1)
List the materials which can be present in the ash.
Neutrulizntion number
(D
664-81)
The procedures described in “neutralization number” method enable determination
of
organic
constituents having acid characteristics in petroleum products and compounded products. They also
show the presence of contamination by alkalies and mineral acids.
The majority of petroleum lubricating oils undergo treatment with mineral acid and caustic alkali in
the course
of
refining. Small quantities of these undesirable chemicals may remain in the finished oil in
case the refining operations are not properly conducted.
The “neutralization number” of an oil is defined as the weight in milligrams
of
potassium hydroxide
required to neutralize one gram of oil. Inasmuch as the mineral acid or alkali is usually not present in the
oil, the neutralization number is directly proportional to the “organic acidity”.
The “alkali neutralization number”
is
defined as the weight in milligrams
of
potassium hydroxide
equivalent to the acid required to neutralize one gram of oil, whereas the
“
mineral-acid neutralization
number” is defined as the milligrams
of
potassium hydroxide necessary to neutralize the mineral acid
content in one gram
of
oil.
Inasmuch as the effect
of
alkah or mineral acid in a petroleum oil is deleterious, it is usual to specify
that the mineral-acid neutralization or alkali neutralization number shall be either zero or extremely low.
The practical significance of “organic acidity”, however, is complicated by the following considerations:
(1)
In
some cases, the presence of organic acids somewhat improves the friction-reducing qualities of
oil.
(2)
The organic acids are usually not corrosive and do not have direct harmful effects.
(3)
Oils derived from different crude oils show a wide range in the neutralization number, unless
treated.
The organic acids are mainly natural constituents of crude oils and their presence does not necessarily
indicate improper refining
or
poor quality.
Thus, it is obvious that the general quality
of
lubricating oil cannot be evaluated on the basis of
neutralization number, and the specifications should not include limits on this property unless an oil of
low organic acidity is required.
Questions
(1)
Describe the procedure for determining “alkali neutralization number”.
(2)
What is the difference between the neutralization and saponification numbers?
Saponification number
(ASTM
D
94-80)
The saponification number is the best obtainable index
of
the percentage
of
fat
or
fatty oil in a given
product. The method is applicable to new or used petroleum oils including electrical insulating oils, and

503
to mixtures of fats and mineral oils. It cannot be used on oils containing compounds of sulfur,
phosphorus, the halogens, or other elements that consume both free and combined fatty acids.
The saponification number is the number of milligrams
of
potassium hydroxide required to saponify
one gram
of
the oil, and is a measure of both free and combined fatty acids.
The saponification numbers
of
some commercial fats and fatty oils are shown below:
Blown cottonseed oil
Blown rapeseed oil
Tallow
Lard oil
Neat’s foot oil
Cottonseed
oil
Peanut oil
Degras
Soya bean oil
Castor oil
Rapeseed oil
Fish oil
Sperm oil
210-225
195-216
193-198
192-198
193-204
191-197
186-197
110-210
189-197
176-187
170-179
140-193
120- 140
In cases where the saponification number
of
the fat or fatty oil is not known, a value
of
195
is used in
commercial practice.
Key points
(1)
The presence
of
inorganic and certain organic acids, most nonalkali soaps, free sulfur, and other
substances which consume alkali will increase the saponification number above that
of
fatty saponifiable
materials for which the method is primarily intended.
(2)
The odor of hydrogen sulfide near the end of back-titration is an indication for presence of
certain types of reactive sulfur compounds. A gravimetric determination of the actual amount
of
fatty
acids is a more reliable method for such compounds.
(3)
The glassware must be chemically clean. The flasks must be cleaned with chromic acid cleaning
solution and rinsed with distilled water.
Questions
(1)
What are the permissible differences in results for highly colored oils?
(2)
How is the percentage of fatty oil or fat in a compounded petroleum product determined?
Sulfur
(bomb)
(ASTM
D
1552-83)
The sulfur content of lubricating oil is determined by burning a definite weight of oil in a steel bomb
filled with oxygen. All the sulfur in the oil
is
thus converted to sulfuric acid, whch is then quantitatively
determined as barium sulfate.
The lubricating oil fractions (boiling range of
600-850
OF)
of different crude oils vary in their sulfur
content, some having high values and others low values. It is very expensive to remove the high-boiling
sulfur compounds in this fraction.
The heat generated by an engine may break down the sulfur compounds in the oil and the
newly-formed sulfur compounds may be corrosive to the metal parts. Consequently, it is important to
determine the sulfur content of lubricating oils.
Key points
(1)
Admit the oxygen slowly to avoid blowing the oil from the cup.
(2)
Do
not add the oxygen or ignite the sample if the bomb has been jarred, dropped, or tilted.

504
Corrosion test of lubricating oils (copper strip at
212OF)
Lubricating oils should not contain any material which tends to corrode the metal parts of the engine.
The test is carried out by immersing a strip
of
polished copper in the
oil
contained in a test tube. The
reason for using a copper strip is the fact that it is the most sensitive material to corrosive materials such
as sulfur and chlorine compounds. After the test tube is kept in
a
water bath (212OF) for a definite
period of time, the copper strip is examined for corrosion by comparing the color change
to
a standard
corrosion chart.
CLASSIFICATION AND REQUIREMENTS OF LUBRICATING GREASES
Lubricating grease is a blend of lubricating agent, soap, and stabilizing agents. The oil is the
lubricating agent, the soap is
a
thickener, and the stabilizing agent keeps the finely divided particles of
soap suspended in the oil. The hardness
of
a grease depends upon the amount
of
soap used-the more
soap, the harder the grease. Properties
of
the grease, such as appearance, texture, melting point, and
oxidation characteristics, are dependent primarily upon the type of soap used in the manufacture of the
grease.
Soap is made by the reaction of a fixed oil and an alkali such as hydrated lime, caustic soda,
aluminum salts, or lithium. Soaps are classified according to the alkali used in their preparation.
Similarly, greases are classified according to the type of soap employed in their manufacture, the
common types being calcium-base, soda-base, aluminum-base, lithium-base, and mixed-base greases. The
latter contain soaps made from two alkalies.
Most greases are made by batch processes which normally involve the following steps:
(1)
saponifica-
tion, (2) soap processing,
(3)
mixing, and
(4)
milling.
(1)
Saponification.
Fat or fatty acid, the alkali, and the petroleum oil are placed into a kettle which
may be either open-type or pressure-type. When the kettle is heated, the fat or fatty acid reacts with the
alkali, forming soap.
(2)
Soap processing.
The soap and the petroleum oil mixture is dehydrated and more petroleum oil is
added to cool the mixture. In the case of calcium-base greases, water must be added to plasticize the
grease.
(3)
Mixing.
More petroleum is added until the desired consistency is reached.
(4)
Milling.
The mixture is either milled in the kettle
or
processed by a homogenizer in order to
produce a smooth product.
In general, grease should not be used where oil can perform the necessary lubrication. There are
conditions, however, under which grease is a more suitable lubricant. For example, grease is used in
bearings which, because
of
their nature, are unable to retain oil. Grease is also used in inaccessible
bearings where grease is applied by grease cups. Use of oil in such bearings, would necessitate shutting
down the machinery. In addition, under dirty atmospheric conditions, the use
of
grease is advisable as it
seals the ends of the bearings and thus prevents dust and dirt from entering the bearings.
The most important requirements of greases are:
(1)
Stability.
A grease must be stable both during storage and when in use. It must be free from
(2)
Water resistance.
In some cases, a grease which is insoluble in water is required. In others, the
(3)
Satisfactory performance in operation.
Inadequate lubrication will result if a grease does not
(4)
Noncorrosiueness.
The grease must not chemically attack the various metals and other materials
Properties of greases vary with the type
of
soap used:
(1)
Lime-base grease is water resistant and has good pumpability. Lime-base grease, however, has a
bleeding (separation of oil), oxidation, and changes in consistency.
grease must only be resistant to the weathering or washing action
of
water.
perform satisfactorily in the equipment for which it was intended.
with which it comes in contact.
low melting point and, therefore, should be applied only where temperatures do not exceed
175
F.
