Chilingarian G.V. et al. Surface Operations in Petroleum Production, II
Подождите немного. Документ загружается.

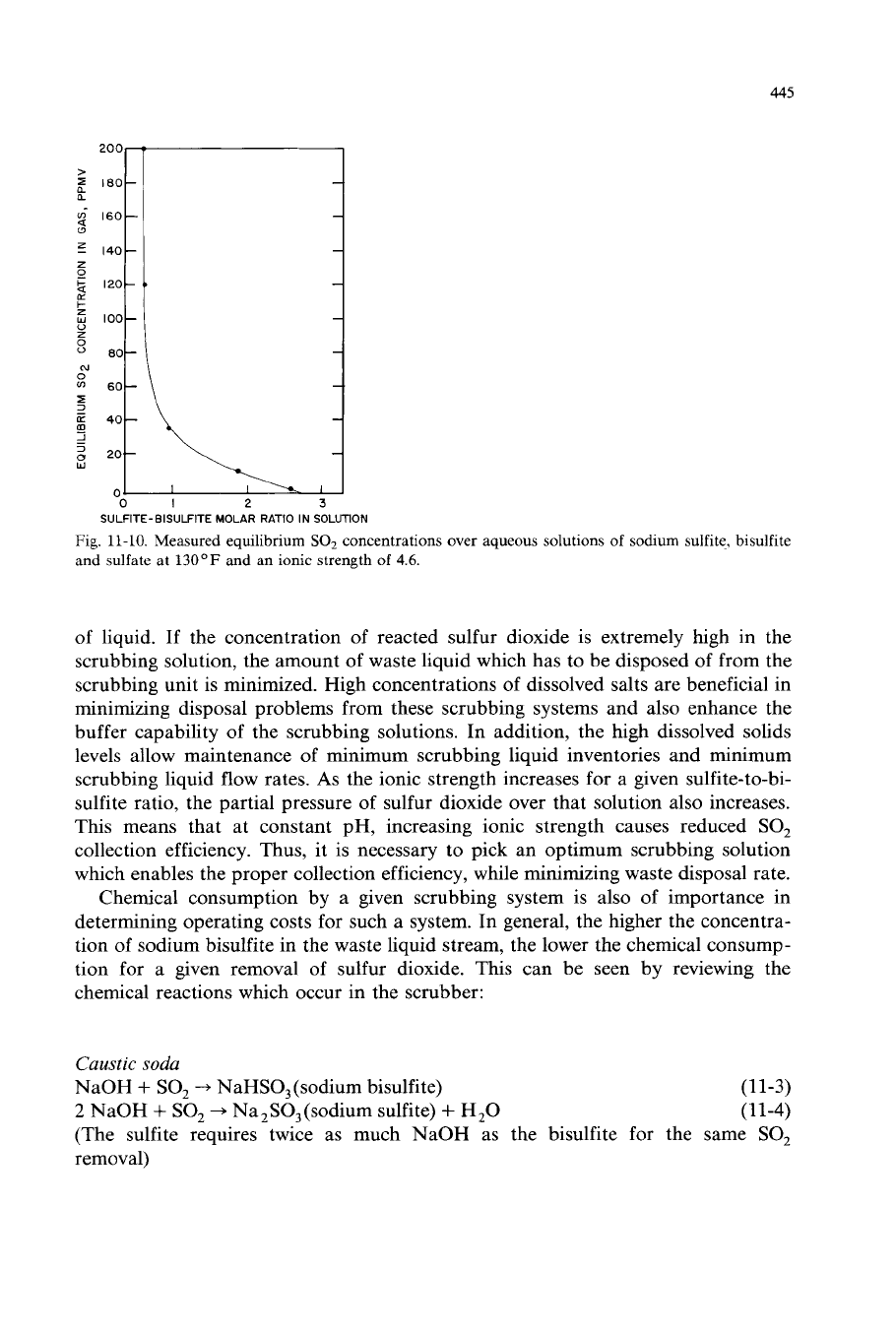
445
SULFITE-BlSULFlTE
MOLAR RATIO
IN
SOLUTION
Fig.
11-10.
Measured equilibrium
SO,
concentrations over
aqueous
solutions of sodium sulfite, bisulfite
and
sulfate
at
130°F
and
an ionic strength
of
4.6.
of liquid. If the concentration of reacted sulfur dioxide is extremely high in the
scrubbing solution, the amount of waste liquid which has to be disposed
of
from the
scrubbing unit is minimized. High concentrations of dissolved salts are beneficial in
minimizing disposal problems from these scrubbing systems and also enhance the
buffer capability of the scrubbing solutions. In addition, the high dissolved solids
levels allow maintenance of minimum scrubbing liquid inventories and minimum
scrubbing liquid flow rates.
As
the ionic strength increases for a given sulfite-to-bi-
sulfite ratio, the partial pressure of sulfur dioxide over that solution also increases.
This means that at constant pH, increasing ionic strength causes reduced
SO,
collection efficiency. Thus, it is necessary
to
pick an optimum scrubbing solution
which enables the proper collection efficiency, while minimizing waste disposal rate.
Chemical consumption by a given scrubbing system is also of importance in
determining operating costs for such a system. In general, the higher the concentra-
tion of sodium bisulfite in the waste liquid stream, the lower the chemical consump-
tion for a given removal of sulfur dioxide. This can be seen by reviewing the
chemical reactions which occur in the scrubber:
Caustic
soda
NaOH
+
SO,
-+
NaHSO,(sodium bisulfite)
2
NaOH
+
SO,
+
Na,SO,(sodium sulfite)
+
H,O
(11-3)
(11-4)
(The sulfite requires twice as much NaOH as the bisulfite for the same
SO,
removal)

446
Soda
ash
Na,CO,
+
2
SO,
+
H,O
+
2
NaHSO,
+
CO,
Na,CO,
+
SO,
+=
Na,SO,
+
CO,
(11-5)
(11-6)
Unfortunately, high sodium bisulfite concentrations result in relatively low sulfur
dioxide collection efficiencies. A caustic consumption rate of
0.5
Ib NaOH per Ib
SO,
removed may be achieved, but if the exhaust stack is tested, it will not be in
compliance with regulation
SO,
levels. One technique which has been widely used
by scrubbing system suppliers in sulfur dioxide removal applications other than
oilfield burners, is a two-stage scrubbing system where the gas stream is first
contacted with a solution containing substantial concentrations of sodium bisulfite
at pH’s in the range of
4-6.
The gas stream is then passed into a second stage where
it is contacted with a higher pH scrubbing solution. In the first stage, only a small
amount of sulfur dioxide is removed, but this sulfur dioxide is absorbed by sodium
sulfite, which would have otherwise been disposed of without being reacted.
Therefore, chemical utilization is maximized. When the gas is then contacted with
the more alkaline scrubbing solution in the second stage, it is still possible to
acheve the same collection efficiency which would result in a single stage system
utilizing a higher pH scrubbing solution. Unfortunately, the two-stage process is not
particularly attractive for oil-fueled systems in the oilfields for three reasons. First,
the acidic waste liquid is difficult to dispose
of
because it attacks mild steel tanks
and even corrodes stainless steel tanks. Second, the waste liquid has a sufficiently
high sulfur dioxide vapor pressure to create secondary
SO,
emissions at its point of
disposal. These secondary emissions will be regulated in the future. Finally, and of
greatest importance, is the fact that virtually all of the makeup water used in the
scrubbing systems contains some soluble chlorides. Chlorides are corrosive to the
300
series stainless steels, even in neutral solutions. The corrosion rates increase
exponentially as the acidity
of
the scrubbing solutions increase. Acidic sodium
bisulfite solutions, containing chlorides, therefore, will cause accelerated corrosion
of the scrubber, the piping and the liquid recirculation system. The increased
chemical utilization is seldom
of
sufficient benefit to compensate for these negative
factors. Furthermore, if the system is operated with a two-stage absorption config-
uration, the operator’s attention required to maintain optimum operating conditions
is greatly increased and system’s reliability is compromised.
A
variety of scrubbing systems are available for use on oil-fired heaters. The
mechanical operation of these scrubbers differs greatly.
A
common characteristic of
all sulfur dioxide removal scrubbers is that they must make available to the gas
stream a sufficient surface area of scrubbing liquid to allow adequate mass transfer
for removal of the sulfur dioxide from the gas stream. The most common techniques
are to distribute the liquid
(1)
in the gas stream as small liquid droplets
or
(2)
over
extended surfaces in the scrubber. This can be done by forcing the scrubbing liquid
through a high-pressure nozzle, atomizing the scrubbing liquid with steam or air
pressure, atomizing the scrubbing liquid by introducing it into a high-velocity gas
stream, or cascading the scrubbing liquid across baffles and/or packing material
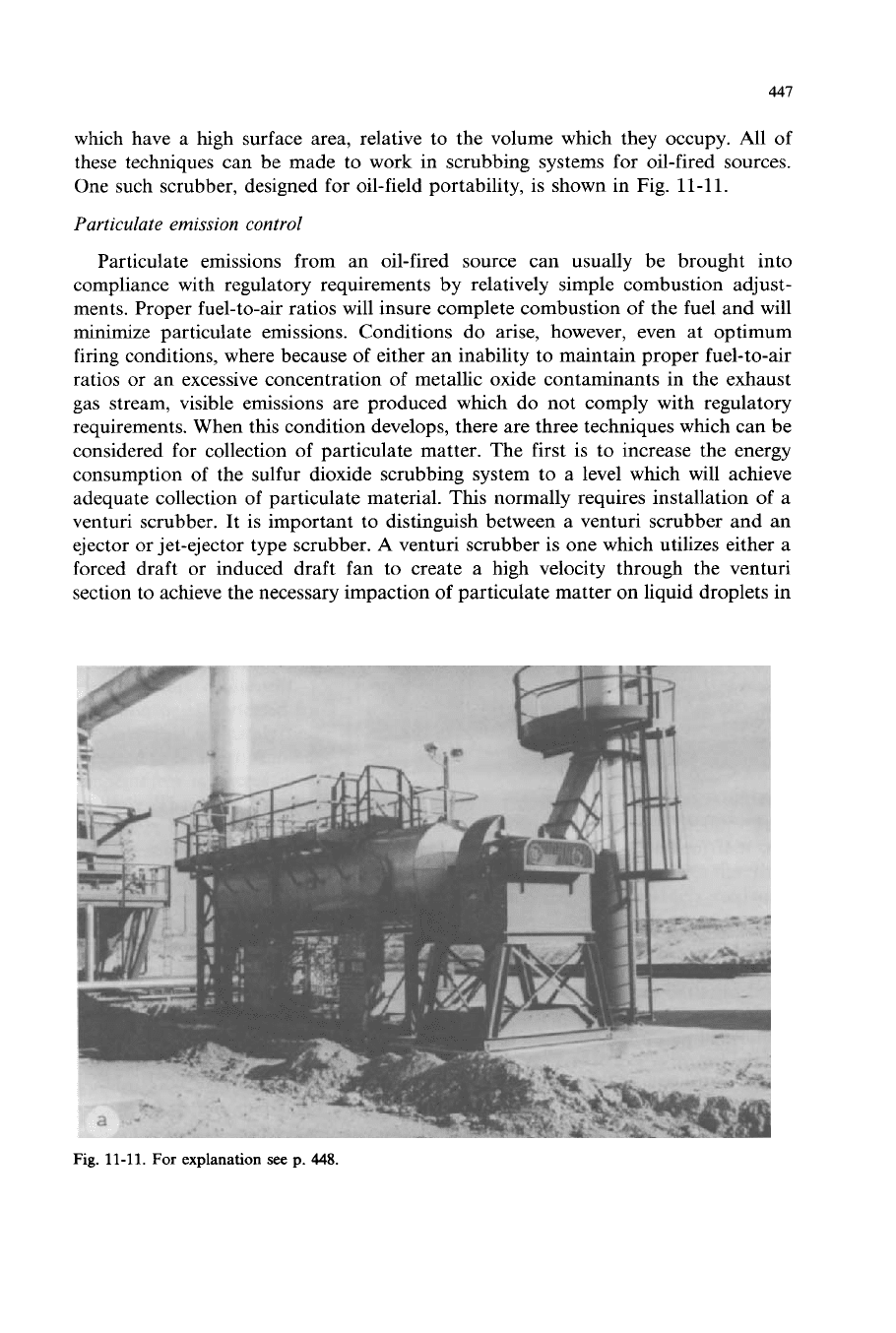
447
which have a high surface area, relative to the volume which they occupy. All
of
these techniques can be made to work in scrubbing systems for oil-fired sources.
One such scrubber, designed for oil-field portability, is shown in Fig.
11-11.
Particulate
emission
control
Particulate emissions from an oil-fired source can usually be brought into
compliance with regulatory requirements by relatively simple combustion adjust-
ments. Proper fuel-to-air ratios will insure complete combustion of the fuel and will
minimize particulate emissions. Conditions do arise, however, even at optimum
firing conditions, where because of either an inability to maintain proper fuel-to-air
ratios or an excessive concentration of metallic oxide contaminants in the exhaust
gas stream, visible emissions are produced which do not comply with regulatory
requirements. When this condition develops, there are three techniques which can be
considered for collection of particulate matter. The first is to increase the energy
consumption of the sulfur dioxide scrubbing system to a level which will achieve
adequate collection of particulate material. This normally requires installation of a
venturi scrubber. It is important to distinguish between a venturi scrubber and an
ejector or jet-ejector type scrubber.
A
venturi scrubber is one which utilizes either a
forced draft or induced draft fan to create a high velocity through the venturi
section to achieve the necessary impaction
of
particulate matter on liquid droplets in
Fig.
11-11.
For
explanation
see
p.
448.

448
Fig.
11-11
(continued). Sulfur dioxide and particulate scrubbing system for oilfield use
on
a
50
MMBtu/hr steam generator. (Courtesy
of
Struthers-Anderson Pollution Control System.)
the scrubber. In a venturi scrubber, scrubbing liquid pressure is relatively low and
most of the energy is devoted to the induced draft or forced draft fan which
accelerates the gas. The venturi scrubbers typically operate at differential pressures
of between 6 and 60 in.
W.G.
For the most severe particulate matter collection
problems with oil-fired systems, a 60-in.
W.G.
differential pressure may, in fact, be
required.
Ejector scrubbers, using either steam or water at extremely high velocity and
pressure through a restricted throat section in the scrubber, utilize either hydraulic
energy or a combination of hydraulic and thermal energy to accelerate the collection
liquid droplets sufficiently to impact on the dust particles. Frictional losses in these

449
systems are greater than in a conventional venturi scrubber and, as a result, for the
same collection efficiency, these systems require somewhat higher total energy
consumptions. Furthermore, because of the necessity to recirculate the scrubbing
solution, particulate matter collects in the scrubbing solution and, at the very high
velocities created by the high differential pressures across the nozzles, nozzle
abrasion becomes a significant maintenance factor.
As the need for extremely high collecting particulate material efficiencies be-
comes more acute, oil-fired equipment operators can consider both electrostatic
precipitators and filtration systems as alternate collection devices.
Nitrogen oxides control
Three general techniques are available for reducing
NO,
emissions:
(1)
Catalytic reduction,
(2)
ammonia injection,
and
(3)
burner and combustion
modification.
Catalytic reduction
Catalytic converters, which have been fitted
on
automobile exhaust systems,
utilize expensive rare metal catalysts to convert
NO,
to elemental nitrogen and
elemental oxygen.
It
is necessary to use unleaded fuel to prevent catalyst poisoning
in these systems. The oil producers are not in a position to use a metallic
contaminant-free fuel for oil-fired equipment. Thus, any of the conventional cata-
lysts used for NO, reduction would be poisoned by the metallic contaminants in the
fuels for this equipment. Furthermore, there have been no selective catalytic
reduction units perfected for use in small industrial oil-fired plants. The primary
reason is that the catalyst cost would be extremely high and catalyst life would be
expected to be short. Another problem with the catalytic systems is that they require
temperatures in excess of
600"F,
and in some cases up to
1000"F,
to operate
properly. The normal convection section discharge gas temperature from oil-fired
equipment falls between
400
and
500
OF, and any higher temperature would result
in uneconomical operating conditions in the heaters. Catalytic reduction does not
hold any significant promise for oil-fired heaters in the oilfields. For internal
combustion engines in the oilfield, however, catalytic converters are widely used and
are effective.
Ammonia injection
Ammonia injection into the convective heat transfer section of an oil- or gas-fired
burner can reduce
NO,
formation. Tests have been done with ammonia injection in
the oilfields (Robinson,
1977).
Ammonia
is
injected downstream of the flame zone
and acts as a reducing agent for
NO.
The reduction reaction is a gas phase
homogeneous reaction, which takes place within a narrow temperature range of
between
1650°F
and
1900°F.
Nitric oxide is converted to elemental nitrogen and
water. Laboratory tests have resulted in
NO,
reductions of up to
80%.
The oilfield
tests indicated reduction efficiencies of about
62%
under ideal conditions. Substan-

450
tial ammonia concentrations were necessary, however, to achieve these reduction
levels. Exit gas ammonia concentrations were about half of the
NO,
concentration
in ppm (vol) at the maximum
NO,
reductions. Because the ammonia can then react
with sulfur dioxide in the convection section, there is concern about plugging of the
convection section with ammonium sulfate when this excess ammonia is present in
the gas stream. It does appear, however, that this process can operate reliably at a
50%
NO,
reduction level or greater.
Burner
modification
Low
NO,
burners are basically devices which attempt to eliminate hot spots in
the flame. Different methods of excess air injection, different methods of creating
turbulence in the flame zone, and recirculation of hot flue gas into the burner zone
have all been tried.
NO,
reductions of approximately
35-60%
can be achieved. At
present, the low
NO,
burners are probably the most reasonably priced
NO,
reduction devices.
Casing vent
gas
collection systems
Where steam injection is used for enhanced oil recovery, producing wells will
often venksteam back
to
the surface.
If
the well casing is vented directly to the
atmosphere, after the steam has dissipated in the ambient air, a residual blue aerosol
forms. This aerosol consists of volatile hydrocarbons which, at steam temperature,
were predominantly gas phase compounds but whch, after cooling down in the
ambient air, become light oil aerosols. This oil is of value to the oil producer as
product and is considered a pollutant by the regulatory agencies because it can
contribute to photochemical smog formation. For this reason, oil producers must
collect the vent gas streams from each of the oil wells, cool these gas streams
sufficiently to condense the oils from them, and separate the oil and water which
forms. Both air- and water-cooled heat exchangers have been used for this purpose.
The steam
is
simply condensed back to water, the oil simultaneously condenses and
floats
on
the water, and the mixture can be taken to an oil-water separator.
Occasionally, an emulsion will form and the emulsion may have to again be heated
slightly to produce proper oil-water separation.
In a number of locations, reduced sulfur compounds can be present in the casing
vent gas. Gaseous sulfides may be encountered simultaneously with steam emissions
from steamflood operations or they will sometimes be produced from sour gas
vented from the hydrocarbon subsurface formation. The reduced sulfur compounds
typically take the form
of
hydrogen sulfide (H2S) or mercaptans (organic sulfides).
In relatively low concentrations, these sulfides can be scrubbed from the gas stream
using simple caustic soda scrubbers. The reactions between sodium hydroxide and
hydrogen sulfide produce sodium sulfide.
As
long as the liquid waste from the
scrubbing system remains alkaline, the sodium sulfide remains relatively stable.
However, if this waste liquid is contacted with any acidic liquid, causing the pH of
the mixture to drop below 7.0, hydrogen sulfide emissions will be reemitted
to
the

451
TABLE
11-111
Removal processes for hydrogen sulfide
Name Reaction
Girbotol
Phenolate
'
Phosphate
Sodium carbonate
Seaboard
Lime
Iron oxide
Caustic soda
(vacuum)
Ironite sponge
2 RNH, +H,S
%
(RNH3),S
NaOC,H,
+H2S
%
NaHS+C,H,OH
K,PO,
+H,S%
KHS+K,HPO,
Na,CO, +H,S
@
NaHCO, +NaHS
Na,CO,
+
H,S
fi
NaHCO,
+
NaHS
Ca(OH), +H,S
+
CaS+2 H,O
FeO+H,S
+
FeS+H,O
2 NaOH+H,S
+
Na,S+2 H20
Fe,04+4H,S+3FeS+4H,0+S
FeS
+
S
+
FeS,
Fe,04
+6
H,S
+
3
FeS,
+4
H,0+2
H,
'
Regenerated by steaming.
Regenerated by vacuum steaming.
Regenerated by
air
blowing.
atmosphere.
To
prevent this from occurring, it
is
quite common to use a strong
oxidant in the scrubbing solution to oxidize the collected sulfide to sulfate. Once the
sulfate has been formed, it remains stable in aqueous solution and is not affected by
contact with acidic liquids. The most common oxidant for this application
is
sodium
hypochlorite (NaOC1) (also see Table 11-111).
When hydrogen sulfide concentrations exceed about
50
ppm (vol), it
is
common
to
use an amine solution with both monoethanolamine or diethanolamine present to
absorb the hydrogen sulfide from the gas stream, take the sulfide-rich amine
solution and steam strip the sulfide from it in a concentrated gas stream, and then
cool and reuse the amine solution for absorption again.
This
is done in a closed loop
system where the waste gas enters and exits the absorber and a concentrated gas
stream of hydrogen sulfide is produced out
of
the steam stripper. This concentrated
hydrogen sulfide gas is then typically flared to burn the reduced sulfur compounds
to sulfur dioxide. In some instances, a small sulfur dioxide scrubber must then be
used to absorb the sulfur dioxide from the reduced sulfur compound incinerators. In
California, there are an increasing number
of
oil-fired steam generators whch are
being used to burn sulfide-rich gases from amine absorption systems to simulta-
neously produce heat and to eliminate the sulfide gas problem. Exhaust gases from
these steam generators must then be scrubbed for sulfur dioxide emissions.
Produced water treatment and disposal
Where steam or water flood techniques are used for oil production, the produced
oil is mixed with significant quantities of produced water. In many steam flood

452
operations, the oil is emulsified with the water. Most regulatory agencies prohibit
disposal of the water, even after oil removal, to natural streams and rivers adjacent
to the oil fields, unless total suspended solids are reduced to extremely low levels
and dissolved solids are also minimal. Most oil producers try to reuse the produced
water, as a result. If the water is emulsified with oil, it is first taken through a free
water knockout system and then to a heater-treater. After it leaves the heater-treater,
it is commonly taken to an oil-water separator. The water becomes relatively oil-free
at this point and is then taken through water softeners
to
reduce hardness and to
make the water reuseable in steamflood applications.
The produced water frequently contains high concentrations
of
sodium chloride.
There is no recognized technique for chloride removal other than reverse osmosis
and/or distillation. Neither of these techniques is feasible for the large volumes
of
water which must be processed in the oilfields. Therefore, it is often necessary to
dispose of some of this produced water to prevent the sodium chloride concentra-
tion from building to an intolerable level. Disposal can take two forms. It can be
injected into a subsurface formation which does not communicate with potable
water. It can also be taken to a liquid waste disposal site which has an impermeable
liner which prevents the chloride containing produced water from communicating
with any potable water source. Water is often in relatively short supply in the
oilfields. Some producers are giving serious consideration to casing their wells with
high nickel alloys and providing non-metallic surface piping to allow use of these
higher chloride liquids without corrosive effect on both surface and subsurface
equipment, for reinjection into the wells.
Summary
I
In oilfield surface operations, pollution control becomes a serious consideration
when utilizing steamflood techniques for secondary and tertiary recovery. The three
most highly regulated emissions have been sulfur dioxide, nitrogen oxides, and
particulate matter. Techniques are available for reducing these emissions to regu-
lation levels in the oilfields. In these same oilfields, produced water presents a
significant challenge to the oil companies to comply with water pollution control
requirements.
For sulfur dioxide removal, liquid scrubbing systems are used. Nitrogen oxides
are controlled using catalytic techniques, ammonia injection or burner modifica-
tions. Particulate matter is commonly collected in the same scrubber which absorbs
sulfur dioxide, but secondary particulate removal devices may also be used, includ-
ing bag houses and electrostatic precipitators. Hydrocarbon emissions are produced
at the wellhead in steamflood operations. These are controlled by condensing the
steam, cooling the gas stream, and condensing the organics out of the gas stream for
recovery. Finally, sulfides and mercaptans may be produced along with crude oil
By
J.D.
Brady
453
and may be treated by simple alkaline scrubbing solution absorption or by more
complex amine absorption and steam stripping techniques. Equipment is available
to the oilfield operator to accomplish all of this necessary pollution control in
surf ace operations.
SAMPLE PROBLEMS
Sample problem
I
1-1
In the flotation equipment presented below
(Fig.
11-12) gas is intimately mixed
(a) Determine:
(1)
The weight of pollutant handled per day,
if
the flotation unit processes 12,000
(2) The horsepower necessary to drive the pump at an overall pump and motor
(b) Draw a diagram
of
a system to maintain the proper liquid level in the tank.
(c) List all the other methods that are commonly used in pollution control.
with polluted wastewater
in
order to remove the sludge prior to disposal.
bbl/day of water containing 90 ppm of sludge.
efficiency of 70%.
Solution
:
(a-1)
Volume
of
pollutant
=
90
X
Weight of pollutant
=
350
X
1.08
=
378 lb/day.
X
12,000
=
1.08
bbl/day. Specific weight of
water
=
350 Ib/bbl. Thus:
(a-2)
Total dynamic head (TDH)
=
900
-
8
=
892 psig.
Total dynamic head
=
892
X
2.31
=
2061 ft.
Flow rate
=
12,000
:
34.3
=
35 gal/min (GPM).
Hydraulic horsepower
=
(TDH
X
GPM
X
sp. gr.)
:
3960
=
(2061
X
350
X
1)
:
3960
Brake horsepower
=
hydraulic horsepower
:
efficiency
=
182
:
0.70
=
260 HP.
Assuming sp. gr.
=
1,
=
182 HP.
SLUDGE
,Jxqdgr~
(AIR)
8
psig
900
psig
POLLUTED WATER
12.
000
bbllday
Fig.
11-12.
Schematic diagram
of
a
flotation equipment-Sample Problem
11-1

454
Fig.
11-13, Schematic diagram
of
a
system
for
maintaining the proper
liquid
level
in
the tank-Sample
Problem 11-1.
A
liquid level controller (LLC) is installed on the outside
of
the tank within the
required level range
of
operation. The controller is normally supplied with a 20-psig
air supply. Through proper linkage, the float (within the LLC) will transmit
3-5
psig output air signal to the liquid level control valve (LLCV) diaphragm, which will
throttle the opening
of
the valve and thereby maintain the required liquid level, as
shown
below (Fig. 11-13). (c)
(1)
Gravity settling tanks, (2) primary skimming tanks, (3) lagooning,
(4)
clari-
fiers,
(5)
centrifuging,
(6)
filtration,
(7)
sedimentation tanks, and
(8)
chemical
treatment tanks.
Sample problem
I
1-2
It is required to design a chemical injection system for a water treatment plant.
Field data
(1) 20,000 bbl/day
of
water
to
be treated through
a
dissolved air flotation cell.
(2) Field testing indicates a requirement
of
20 ppm
of
a
6%
solution mix
of
a dry
(3)
A
second chemical requirement downstream of the flocculant clay injection, is
clay flocculant, 1/2 lb/gal of water
mix.
5
ppm of a liquid polymer.
Determine:
(1) How many lb
of
dry clay per day will be required.
(2) How many gallons per day of the liquid polymer will be required.
REFERENCES
Brady,
J.D.
and Legatski, L.K., 1978. Improved process for separating
sulfur
oxides from gas streams.
U.S.
Patent
No.
3,989,797.
Brady,
J.D.,
1979. Emission control for oil fired steam generators.
In:
Struthers-Andersen Seminar on
New Developments in Oilfeld Steam Generators.
Rep. 79-900101. Bakersfield, Calif., Nov. 1979, 47 pp.
Nemerow,
N.L.,
1963.
Theories and Practices
of
Industrial Waste Treatment.
Addison-Wesley, Reading,
Reilly,
P.B.,
1972. Wastewater treatment for removal of suspended solids.
Plant Eng.,
May 18: 88-91.
557 pp.
