Canale L.C.F., Mesquita R.A., Totten G.E. Failure Analysis of Heat Treated Steel Components
Подождите немного. Документ загружается.


Steel Failures due to Tempering and
Isothermal Heat Treatment
Jan Vatavuk, Universidade Presbiteriana Mackenzie
L.C.F. Canale, Universidade de Sa˜o Paulo USP
FAILURE of swords made by early metal-
smiths was a complex phenomenon for blade-
smiths. The repeated working, heating, and
cooling could cause embrittlement, with sword
failure occurring in the most critical moments of
a battle. Likewise, some of the earliest cannons
would break apart after the first shots following
manufacture. These problems occurred in the
ferrous alloy application until the benefit of
tempering became recognized. In the middle of
the 18th century, the tempering process (and/or
stress relief) received attention as a fundamen-
tally important process in the heat treatment of
the ferrous components of tools. Some ironsmith
tools were treated by the so-called process of
water annealing, whereby steel was tempered in
the range of 300 to 600
C. The slow cooling
was substituted by water cooling.
At the beginning of the 20th century, Krupp
developed a great number of patents based on
water cooling after tempering of chromium-
nickel steel. This phenomenon received atten-
tion after the start of WWI, when large amounts
of steel were used by the armament industry. In
1917, the term tempering embrittlement was
introduced by Dickenson, having been pub-
lished in papers by Brarley, Hatfield, Philpot,
and Grenet. Some investigators, such as Greves
and his collaborators, began a set of experi-
mental methods using notched bars to determine
the susceptibility of tempering embrittlement.
A relationship between the energy absorbed
after water cooling and annealing was termed
the steel susceptibility ratio. At that time, all
the experiments were performed at room tem-
perature, because no one anticipated that tem-
perature may also have an effect on the results.
The effect of test temperature received atten-
tion in the beginning of 1944, when Jolivet
and Vidal introduced experiments at different
temperatures, resulting in a revision of all for-
mer data.
A very important technological mark was the
development of the beneficial molybdenum
effect on the embrittlement reduction phenom-
enon, through work by Greaves and Jones
(Ref 1). For some time, embrittlement due to the
tempering process has been shown to be an
important failure related to heat treatment. In
this chapter, the causes and cases associated with
problems originated by tempering are reviewed.
However, to provide background on this phe-
nomenon, a brief description of the martensite
reactions and the steel heat treatment of tem-
pering is given to review the different stages of
microstructural transformation.
Martensite
Before describing the solid-state reactions
resulting from the tempering process in the fer-
rous matrix, it is important to define the mar-
tensitic structure as a function of the alloying
elements, especially for the carbon effect. Fer-
rous martensite is composed of a body-centered
tetragonal crystallographic structure, with lat-
tice parameters (c and a) related to the carbon
contents of its chemical composition, as shown
in the expression (Ref 2):
c=a=1+0:0467 · (wt% C)
The lattice ratio for the tetragonal structure is
approximately 1.0467, with 1 wt% C in solid
solution. As shown in Fig. 1, hardness varies
with carbon content, and that effect is strongly
related to the distortions caused by the carbon
atom in the body-centered tetragonal structure.
Martensite is extremely hard (maximum of 800
to 900 HV) and brittle.
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:32PM Plate # 0 pg 285
Failure Analysis of Heat Treated Steel Components
L.C.F. Canale, R.A. Mesquita, and G.E. Totten, editors, p 285-309
DOI: 10.1361/faht2008p285
Copyright © 2008 ASM International®
All rights reserved.
www.asminternational.org
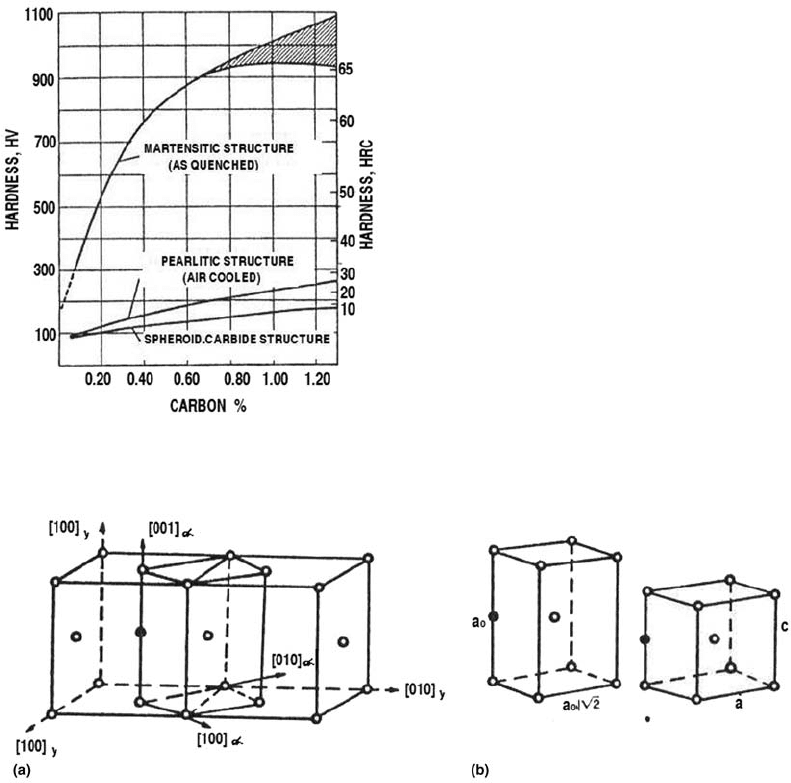
The transformation of martensite from aus-
tenite is a nonequilibrium (athermal) process
that occurs during rapid cooling from the aus-
tenite phase. Unlike the phase transformation
from atomic diffusion at equilibrium tempera-
tures, the martensitic transformation occurs
when many atoms together undergo a shear
displacement. This rapid shear displacement of
atoms results in a rapid change in crystal struc-
ture during cooling. Thus, the martensitic
transformation is referred to as a diffusionless
process, because the transformation involves a
nonequilibrium (athermal) crystal change from a
shear transformation during rapid cooling from
austenite.
Martensite is a nonequilibrium structure and
thus does not appear on the iron-carbon equili-
brium phase diagram. The face-centered cubic
lattice shearing of austenite (Bain’s deforma-
tion) in a martensitic transformation is illu-
strated in Fig. 2. The deformation is large and
rapid over many atoms in the lattice, and the
change in the polycrystalline system is accom-
modated by lattice deformation. This can occur
by slide, mechanical twinning, or even a mixture
of both mechanisms according to the steel che-
mical composition. The crystal change results in
an expansion of the polycrystalline system.
The effect of temperature on martensite for-
mation is directly related to the transformation
temperatures of martensite start (M
s
) and mar-
tensite finish (M
f
). Carbon is the alloy element
that has a higher influence on M
s
temperature,
which is mainly responsible for the martensite
morphology of steels. There are several
empirical formulas to calculate M
s
temperature.
Some examples are reported as follows (Ref
2–4):
M
s
=539 432 (%C) 30:4 (%Mn) 17:7 (%Ni)
12:1 (%Cr) 7:5 (%Mo)
For medium-carbon alloy steels (Ref 4):
M
s
=520 320 (%C) 50 (%Mn) 30 (%Cr)
20 ½%(Ni+Mo)5 ½%(Cu+Si)
In Ref 3:
M
s
=561 474 (%C) 33 (%Mn) 17 (%Ni)
17 (%Cr) 21 (%Mo) (Ref 3)
The higher the transformation temperature,
the higher the probability of the plastic defor-
mation mechanism occuring by dislocation
slide, although the low temperatures provoke a
Fig. 1
Hardness of martensite as a function of carbon content.
Source: Ref 3
Fig. 2
(a) Body-centered tetragonal cell in austenite. (b) Body-centered tetragonal cell before (left) and after (right) the lattice
deformation from austenite to martensite. Source: Ref 2, 3
286 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:32PM Plate # 0 pg 286
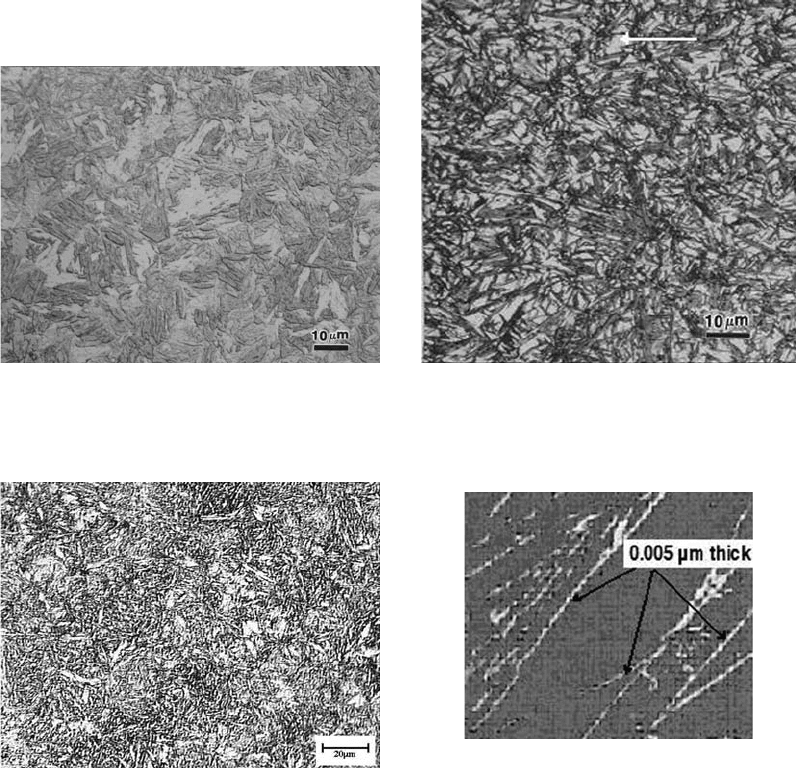
plastic deformation by mechanical twinning.
This way, it is possible to establish a martensite
morphology with respect to the alloy content,
giving special attention to carbon.
Figures 3 to 5 show the morphological aspects
of martensite as a function of carbon content for
steels through optic microscopy. In the case of
lath martensite, the deformation mechanism of
the lattice is dislocation slip. This kind of mar-
tensite is also known as slipped martensite.
Morphologically, this martensite presents lath
packages, which are separated by low-angle
boundaries (Ref 5). In the past, it was thought
that the transformation units happened as lath
packages, although recently it became clear that
each lath is independently formed, and the evi-
dence shows that an austenite film exists, which
can be seen in Fig. 6.
Measurements of dislocation density found
in martensite are on the order of 0.3 to
0.9 · 10
13
cm/cm
3
of the crystal. This disloca-
tion density is higher than the maximum that can
be obtained by elevating the percentage of cold
plastic deformation. Some observations, made
by transmission electron microscope, show a
very small cellular structure (approximately 0.2
to 0.3 mm) inside the lath (Ref 5).
Figure 4 shows the martensite with a high
carbon content, observed with an optical
microscope. With a high carbon content, the
microstructure has twinned martensite or plate
martensite. With higher carbon contents, higher
volume of retained austenite (Fig. 5) occurs,
because a higher carbon content lowers the M
s
Fig. 3
Lath (low-carbon) martensite in SAE 8620 alloy steel
(Fe, 0.2% C, 0.8% Mn, 0.55% Ni, 0.5% Cr, 0.2% Mo)
after heat treatment (954
C, or 1750
F, for 1 h, water quench)
Fig. 4 High-carbon martensite (0.8% C). Etched with nital
Fig. 5
Microstructure of quenched 1.3% C steel. Dark nee-
dles of plate martensite and white areas of retained
austenite (white arrow)
Fig. 6
Illustration of the austenite film surrounding martensite
laths in a Fe-10Cr-0.2C steel. Source: Adapted from
Ref 2
Steel Failures due to Tempering and Isothermal Heat Treatment / 287
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:33PM Plate # 0 pg 287
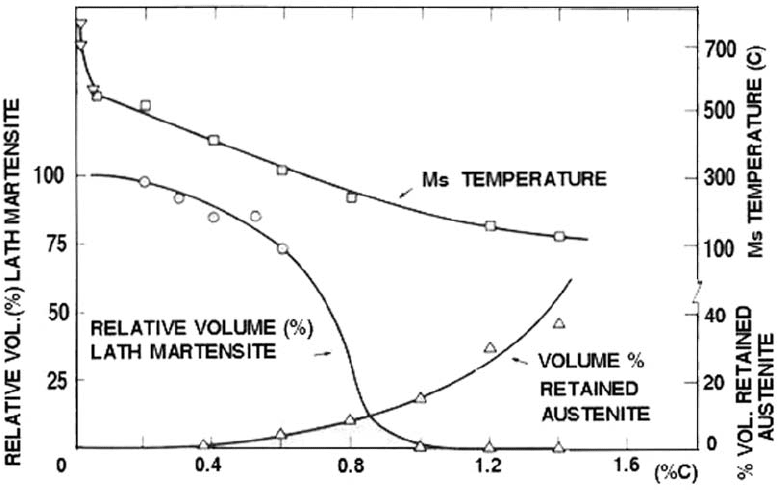
temperature. Twinning density can be seen by
transmission electron microscopy, because
density is high even though twins are very nar-
row (on the order of 10 A
˚
).
The percentage of slip martensite and twinned
martensite in carbon steel and tool steel can be
experimentally determined, as shown in Fig. 7.
As the carbon content increases, the amount of
lath martensite decreases. The untransformed
austenite increases by the M
s
temperature
(martensite start temperature) reduction. The
increase in the retained austenite volume frac-
tion can reduce the as-quenched hardness
mainly in the higher content range.
Martensitic transformation causes an increase
in volume and size variations, which contributes
to the residual tension stresses that develop in
the surface after the heat treatment of quenching,
when transformation takes place in all of the
sample cross sections, and transformation be-
tween surfaces and nucleus occurs anachroni-
cally. The volume variation measured during the
transformation from austenite to martensite in a
1% C steel is approximately 4% (the transfor-
mation to pearlite results in a 2.4% expansion)
(Ref 2), decreasing as far as the carbon is added
in the matrix. This occurs because of the dif-
ferent carbon effect in the austenite related to the
martensite. In the first, the deformation has a
volumetric character, while in the second, it is
more directional (Ref 7). This behavior can be
seen in Fig. 8.
It can be seen in Fig. 8 that the difference
in the specific volume between austenite and
martensite is approximately 15% from very low
carbon content to very high carbon content
(2% C). It is also interesting to observe that for
low carbon, the change of volume from an as-
annealed condition to an as-hardened condition
is practically nil. On the other hand, increasing
carbon content raised that difference.
These observations are important during the
component process design. As mentioned ear-
lier, the greater the carbon content, the greater
the embrittlement of the martensite plates
(Ref 8). However, since retained austenite also
increases with carbon content, this fraction of
retained austenite will bring some toughness to
the matrix as well as reduce the volume varia-
tion as shown in the curve “A-FC” in Fig. 8.
This fact results in a lower load to the cold sur-
face because of the incomplete transformation
of the nucleus. It is important to remember
that the nucleus presents a low yield strength
when retained austenite has a low mechani-
cal resistance, decreasing the possibility of
Fig. 7
Effect of carbon content on the lath martensite volume, retained austenite volume fraction, and Ms temperature. Source:
Adapted from Ref 6
288 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:33PM Plate # 0 pg 288
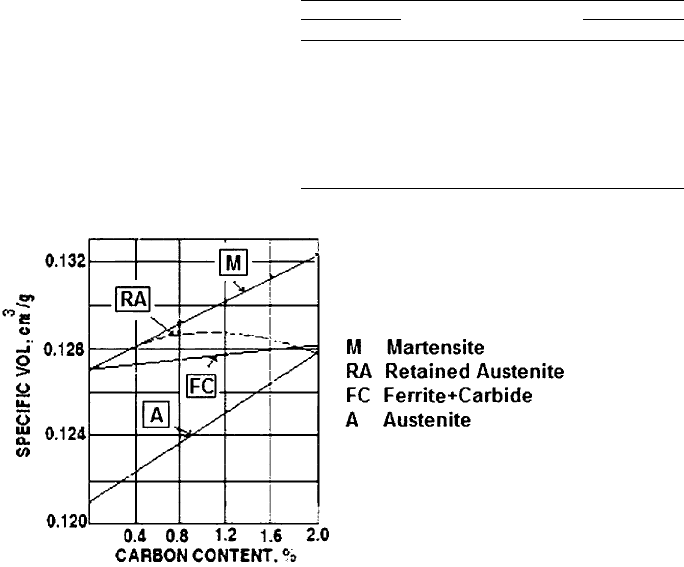
developing tension stress in the surface of the
component.
It can be seen in Fig. 8 that for 2% C, theo-
retically it could be possible to obtain a complete
transformation to martensite, but, this is not
the case. However, it is interesting to observe
that the retained austenite line matches the aus-
tenite line for very high carbon content. This is
the case for Hadfield steels (Ref 9), which have a
high manganese content (approximately 12%)
that guarantees an austenitic microstructure,
even though the carbon amount is approximately
1.2%. In this situation, it is possible to quench
large components with complex geometry
without the risk of developing cracks, even
while increasing quenchant severity.
The ability to form martensite is described
in terms of hardenability, which is related to
the presence of other alloy elements besides
carbon. For example, molybdenum and manga-
nese increase hardenability, while cobalt lowers
the hardenability of steel. A higher hardenability
allows martensite formation with a slower
cooling rate. This is beneficial for reducing
the tensile residual stresses in the component
surface.
Tempering
Tempering is historically associated with the
heat treatment of martensite in steels. The
resultant microstructure is called tempered
martensite. The main purpose for tempering is to
develop a usable combination of hardness and
toughness. The microstructure and mechanical
properties can be changed when the component
is held isothermally at a temperature where
austenite cannot form.
It is important to emphasize that tempered
martensite usually does not contain martensite.
Instead, it is a structure of fine carbide particles
in ferrite, which has formed from martensite
during the tempering. This structure has a lower
hardness than the martensite, but by proper
choice of temperature and time used, the struc-
ture developed will be fine to give the desired
hardness. Table 1 lists the colors associated with
the tempering heats, and Table 2 illustrates the
times required to reach furnace temperature
during tempering (Ref 10).
Effect on Mechanical Properties
As noted, martensitic structures are too brittle
for most practical applications. However, it
is possible to enhance the structure tough-
ness through tempering. The toughness usually
comes at the expense of a decrease in yield
Fig. 8 Specific volume (DV/V) of carbon steels relative to room temperature. Source: Adapted from Ref 7
Table 1 Colors of tempering heats
Temperature(a) Temperature(b)
°C ° F Color of oxides °C °F
188 370 Faint yellow 238 460
199 390 Light straw 265 510
210 410 Dark straw 293 560
221 430 Brown 321 610
232 450 Purple 337 640
254 490 Dark blue 349 660
265 510 Light blue 376 710
(a) Temperature held for 1 h. (b) Temperature held for 8 min
Steel Failures due to Tempering and Isothermal Heat Treatment / 289
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:33PM Plate # 0 pg 289
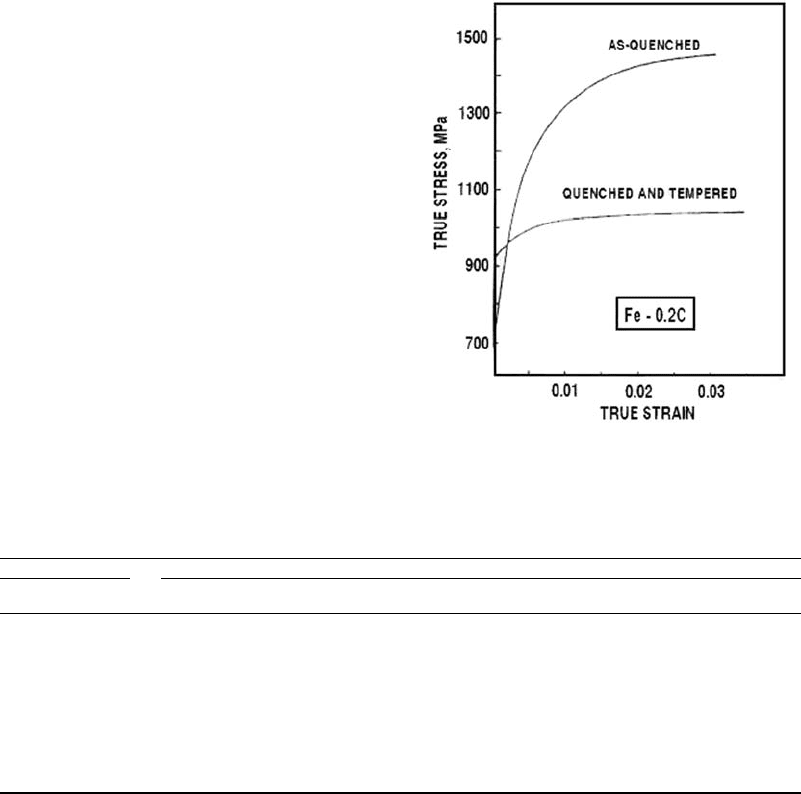
strength and hardness, as illustrated in Fig. 9
and 10. Figure 11 shows other modifications
of mechanical properties that occur when an oil-
quenched AISI 4340 steel is tempered at tem-
peratures above 200
C.
The interrelationship between tempering
temperature, steel chemistry, and hardness can
be estimated by the equation (Ref 13):
HB=2:84H
h
+75(%C) 0:78(%Si)
+14:24(%Mn)+14:77(%Cr)+128:22(%Mo)
54:0(%V) 0:55T
t
+435:66
where HB is the Brinell hardness after hardening
and tempering, H
h
is the Rockwel (HRC) hard-
ness after hardening, and T
t
is the tempering
temperature in
C. This equation was developed
for the following conditions:
H
h
= 20 to 65 HRC and T
t
= 500 to 600
C
C = 0.20 to 0.54%, Si = 0.17 to 1.40%,
Mn = 0.50 to 1.90%, and Cr = 0.03 to
1.20%
An average relation between the hardness
after hardening (H
h
) and the hardness after
hardening and tempering (H
t
) can be found
through:
H
h
=(T
t
=167 1:2)H
t
17 ½HRC
where H
t
is the hardness after hardening and
tempering (HRC), and T
t
is the tempering tem-
perature (
C). This equation is valid for
490
C5T
t
5610
C and for a tempering time
of 1 h.
The tempering temperature for a specified
hardness after hardening and tempering is also
possible to calculate when chemical composi-
tion and the degree of hardening are known
(Ref 13):
T
t
=647 [S(60
ffiffiffiffi
C
p
+20)=H
t
70:9]
1=4
73:45 SH
t
+(5377561S)(%C)+505S(%V)+219S(%Mo)
+75S(%Cr)+66S(%Si)751 [C
]
where H
t
is the hardness after hardening and
tempering (HRC), S is the degree of hardening,
Sj1.0, and the alloying elements are given in
weight percent. This formula is valid for a
tempering time of 2 h.
Tempering Reactions
Tempering is a process in which the micro-
structure approaches equilibrium under the
influence of thermal activation. It follows that
the tendency to temper depends on how far
the starting microstructure deviates from
Table 2 Approximate heating times for tempering
Per inch of diameter or thickness, with furnace maintained steadily at T
max
, and steel having dark or scaled surface
Temperature Heating time, min
°C °F
Cubes or
spheres(a)
Squares or
cylinders(a)
Average
flats(a)
Cubes or
spheres(b)
Squares or
cylinders(b)
Average
flats(b)
121 250 30 55 80 15 20 30
149 300 30 50 75 15 20 30
177 350 30 50 70 15 20 30
204 400 25 45 65 15 20 30
260 500 25 40 60 15 20 30
316 600 25 40 55 15 20 30
371 700 20 35 50 15 20 30
427 800 20 30 45 15 20 30
482 900 20 30 40 15 20 30
(a) In hot air oven, without circulation. (b) In circulation air furnace or oil bath (can be used only in lower temperatures)
Fig. 9
Effect of tempering on the true stress in a carbon steel.
Source: Adapted from Ref 11
290 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:33PM Plate # 0 pg 290
equilibrium. Martensite microstructure is the
farthest, followed by bainite, ferrite, and
cementite (Ref 14).
When the martensitic structure is metastable,
there is a natural tendency to transform it to a
structure with more stability, and those mod-
ifications are accelerated by increasing the
temperature during the tempering.
The modification that occurs during temper-
ing is complex, and the transformations that take
place during the treatment conditions necessary
to produce the best mechanical properties com-
bination are a result of accumulated knowledge,
not just from the academic point of view but also
the practical aspect of observation. Most of the
time, the structures developed during isothermal
heat treatments are influenced by the low content
of other elements besides iron and carbon.
Tempering stages
Solid-state reactions follow a sequence of
precipitation that is related to variables such as:
Diffusivity of the involved element
Surface energy of interfaces produced by the
reactions
Crystallographic adjustment (coherence
stresses) between the precipitated phases
and the ferrous matrix
Thermodynamic stability of reactions
During tempering, the martensitic structure is
submitted to a sequence of reactions, often
superimposed and defined as temper stages (Ref
2, 3, 4, 5, 12).
Stage 1. In high-carbon steels, the pre-
cipitation of excess carbon begins with the for-
mation of a transition carbide, such as e (Fe
2.4
C).
The e-carbide can grow at temperatures as low
as 50
C. Martensite is said to be supersaturated
with carbon when the concentration exceeds its
equilibrium solubility with respect to another
phase. However, the equilibrium solubility
depends on the phase. The solubility will be
larger when the martensite is in equilibrium with
a metastable phase such as e-carbide. Approxi-
mately 0.25 wt% C is said to remain in solution
after the precipitation of e-carbide is completed.
Although most textbooks will begin a discussion
of tempering with this first stage of tempering,
involving the redistribution of carbon and pre-
cipitation of transition carbides, cementite can
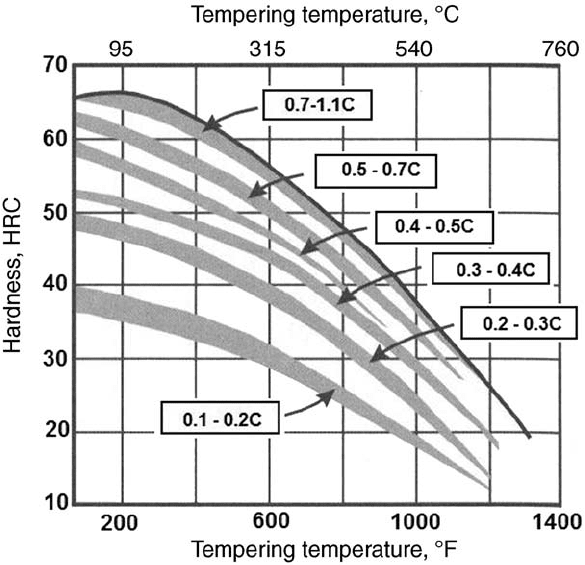
Fig. 10 Effect of carbon content on the hardness of tempered plain steels. Source: Ref 10
Steel Failures due to Tempering and Isothermal Heat Treatment / 291
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:33PM Plate # 0 pg 291
precipitate directly (Ref 14). This is the case for
the lath martensite structure, where the disloca-
tion density can be as high as 10
12
to 10
13
/cm
2
(Ref 15). Trapped carbon atoms will not pre-
cipitate as transition carbides, but cementite is
more stable than trapped carbon. This stage
begins at room temperature and extends to
250
C. A fine adjustment between the e-
carbides and the ferrous structure is attributed to
the precipitation-hardening effect of martensite
in high-carbon steels tempered between 50 and
100
C.
Stage 2. Tempering at higher temperatures,
in the range of 200 to 300
C, for 1 h induces the
retained austenite to decompose into a mixture
of cementite and ferrite. When the austenite is
present as a film, the cementite also precipitates
as a continuous array of particles that have the
appearance of a film (Ref 3, 5, 12, 14). The
martensite of the steels with less than 0.5% C
content has a retained austenite amount lower
than 2%, reaching 6% for 0.8% C. There are
some indications that austenite decomposes,
turning into ferrite and cementite, but presently a
consensus does not exist about whether this
structure can be correlated to lower bainite,
typically from the isothermal decomposition of
austenite, in the temperature range of 230 to
300
C.
Stage 3. Tempering at even higher tem-
peratures leads to a coarsening of the cementite
particles, with those located at the plate bound-
aries growing at the expense of the intraplate
particles. This precipitation is responsible for
the embrittlement phenomenon observed at the
temperature of 250 to 400
C. It can be avoided
by adding silicon, which is an insoluble element
in cementite. This allows cementite formation at
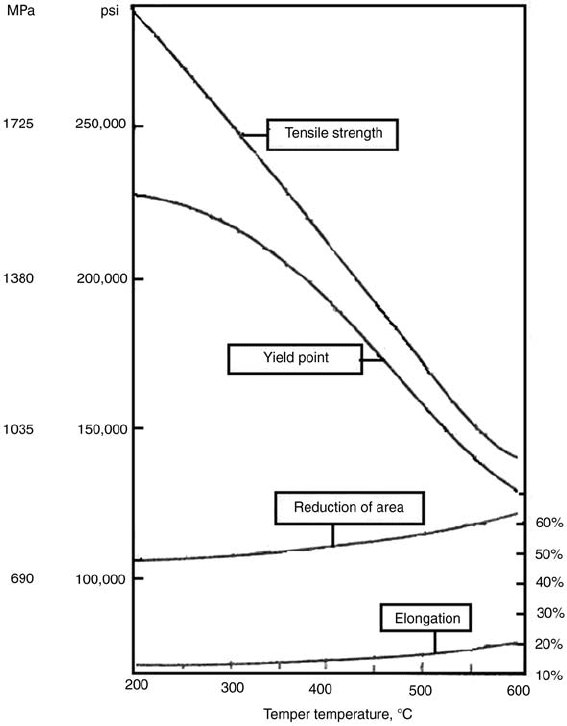
Fig. 11 Changes in the mechanical properties of AISI 4340 steel with tempering temperature. Source: Ref 12
292 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:33PM Plate # 0 pg 292
temperatures where silicon diffusion occurs,
thus slowing the process.
Silicon is a substitutional alloy element that
accumulates in the ferrous matrix adjacent to the
Fe
3
C carbide in the growth process. It increases
the carbon activity in this region, decreasing the
carbon flow to the growing particle and conse-
quently reducing its growth velocity (Ref 5, 16,
17). This silicon effect resulted in the develop-
ment of alloy 300M, which substitutes for 4340
steel in those applications where it is necessary
to use tempering temperatures that cause
embrittlement of tempered martensite, which is
soon defined. This reaction begins to occur at
temperatures on the order of 100
C. Cementite
can also be observed during quenching when the
M
s
temperature is elevated, as is the case of steel
with a low carbon content, mainly in the mar-
tensite formed just below the M
s
temperature.
This phenomenon is known as self-tempering
(Ref 5).
Stage 4. In carbon steels, stage 3 marks the
end of the tempering process. Spheroidization of
Fe
3
C occurs as cementite coalesces. This phe-
nomenon is sometimes called stage 4 of tem-
pering (Ref 2). The lath boundary maintains
stability up to approximately 600
C. Intense
rearrangement occurs between the lath and its
low-angle boundaries above 600
C. This
recovery process is replaced by recrystallization
and coarsening (Fig. 12) at temperatures be-
tween 600 and 700
C (Fig. 13).
Effect of Te mperature and Alloying. The
effect of the tempering temperature on steels
with increasing carbon contents can be inferred
from Fig. 13. During tempering, the continuous
decomposition of martensite to ferrite and car-
bides changes the state of stress because of
continuous dimensional changes. At low tem-
peratures (first stage), a volume contraction
takes place as a consequence of e-carbide pre-
cipitation. In the second stage, with the trans-
formation of retained austenite (approximately
300
C), the volume is increased. In stage 3, the
progressive decomposition of martensite leads
to a volume decrease.
It is important to observe that the austeniti-
zation temperature, which determines the
amount of carbon dissolved and the amount of
retained austenite, has a strong influence on the
expected volume changes (Ref 2, 4, 12, 13).
Table 3 shows the changes in length for various
steels as a function of tempering temperature.
Alloyed steels can also have another stage
with the precipitation of alloy carbides, includ-
ing M
2
C (molybdenum), M
7
C
3
,M
6
C, M
23
C
6
(chromium rich), V
4
C
3
, TiC, and so on, where
the “M” refers to a combination of metal atoms.
However, all of these carbides require long-
range diffusion of substitutional atoms. They
can only precipitate when the combination of
time and temperature is sufficient to allow this
diffusion. The alloy carbides grow at the
expense of the less stable cementite. If the con-
centration of strong carbide-forming elements,
such as molybdenum, chromium, titanium,
vanadium, and niobium, is large, then all of the
carbon can be accommodated in the alloy car-
bide, thereby completely eliminating the
cementite.
Figure 14 illustrates the effect of alloying
elements on hardness as a function of tempering
temperature in carbon steels (Ref 5). Increases in
hardness with additions of titanium, vanadium,
molybdenum, and chromium are related to the
alloy carbide precipitation. This phenomenon is
common for tool steels and can affect their
toughness, as illustrated in Fig. 15.
Embrittlement
Hardness decreases with increasing temper-
ing temperature (Fig. 10, 11). Consequently,
yield strength and tensile strength decrease as
well. On the other hand, elongation and ductility
increase. In this general context, a failure related
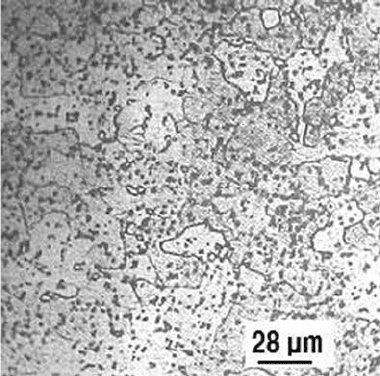
Fig. 12
Fe-0.17C alloy quenched in water from 900
C and
tempered at 650
C for 5 h. Microstructure shows
ferrite grains and spheroidized Fe
3
C
Steel Failures due to Tempering and Isothermal Heat Treatment / 293
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:34PM Plate # 0 pg 293
to tempering may be attributed to an incorrect
choice of temperature (and/or time of temper-
ing), resulting in an incorrect hardness or low
toughness.
However, most failures are related to
embrittlement phenomena. Quenched and tem-
pered steels are susceptible to a number of dif-
ferent types of embrittlement. Some of them
are due to structural modifications during tem-
pering, as previously described. However,
there are some due to the interaction of the
environment with the quenched and tempered
microstructures, such as hydrogen embrittle-
ment and liquid metal embrittlement. Examples
of the first type of embrittlement are tempered
martensite embrittlement and temper embrittle-
ment, which are described as follows.
Tempered Martensite Embrittlement. It is
well known that tempered martensite embrit-
tlement (TME) is related to tempered martensite
of specimens tempered between 250 and
370
C, as shown in Fig. 16. The impact
toughness after tempering at this temperature
range is lower than that obtained on tempering
at temperatures below 250
C. This type of
brittleness is inherent to some extent in all steels,
including carbon grades. For that reason, med-
ium-temperature tempering is, as a rule, not
employed in practice, although it can ensure a
high yield limit. According to Krauss (Ref 12),
TME may or may not be associated with
impurity atom segregation to prior-austenitic
grain boundaries, but the most common factor,
at least for medium-carbon steels, is the phe-
nomenon that takes place due to decomposition
of retained austenite to cementite in the interlath
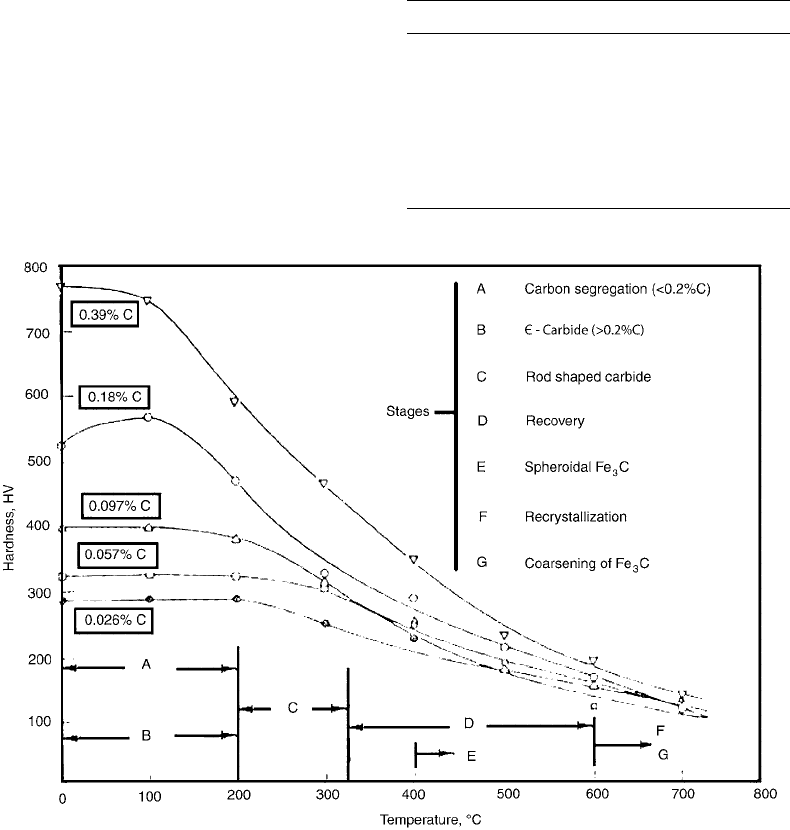
Fig. 13 Hardness as a function of tempering temperature for plain carbon steels. Source: Ref 6
Table 3 Length variations related to
metallurgical reactions as a function of
tempering temperature ranges
Stage
Temperature
range, °C Metallurgical reactions
Expansion (E) or
contraction (C)
1 0–200 Precipitation of e-carbide;
loss of tetragonality
C
2 200–300 Decomposition of retained
austenite
E
3 230–350 e-carbides decompose to
cementite
C
4 350–700 Precipitation of alloy
carbides; grain
coarsening
E
Source: Ref 18
294 / Failure Analysis of Heat Treated Steel Components
Name ///sr-nova/Dclabs_wip/Failure_Analysis/5113_285-309.pdf/Chap_09/ 18/8/2008 3:34PM Plate # 0 pg 294
