Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Structure of the Major Phospholipids
0005 The chemical backbone of the major phospholipids is a
diacylglycerol molecule with the third carbon attached
to a phosphate molecule. Choline, ethanolamine,
serine, and inositol can be attached to the phosphate
group to change the physical and functional proper-
ties, leading to the formation of phosphatidylcholine,
phosphatidylethanolamine, phosphatidylserine, and
phosphatidylinositol, respectively. The groups at-
tached to positions 1 and 2 (a or b) are C
14
–C
18
fatty
acids with double bonds associated with the lecithin
source. The second carbon of the glycerol molecule,
the b-position, usually contains linoleic acid.
Physical Properties
0006 There are two major physical classes of lecithins: (1)
fluid, and (2) waxy solids.
0007 The fluid lecithins can have viscosities from 5000
to 100 000 cP, depending on processing conditions
and diluents. The low-viscosity products are made
through the addition of fatty acids and vegetable oil,
depending on the function and stability required. Di-
valent metal ions like calcium can be added during
drying to decrease viscosity. The moisture content can
also make a difference. Water levels above 1% will
increase the viscosity, eventually to a plastic state.
0008Deoiled lecithins are waxy solids that can be
ground to various particle sizes. They are stable,
free-flowing granules or powders.
Functional Properties
0009Lecithins are multifunctional agents. They can be
used for many purposes in a food system, as shown
in Table 2. The most popular functionalities are
discussed below.
0010Antidusting agents Lecithins reduce static electri-
city by wetting dusty particles. They can be used
alone or in conjunction with vegetable oils. Oils can
be selected for the degree of shelf-life required.
0011Crystal formation modifier Lecithins retard nucle-
ation in fats and even monoglycerides, reducing
graininess in texture.
0012Emulsifiers Lecithins are most often used as ampho-
teric emulsifiers. They promote stable formation of
oil-in-water and water-in-oil emulsions by reducing
tbl0001 Table 1 Chemical composition of soya bean lecithin
Granularlecithin Typicalliquidlecithin
Phosphatides (acetone-insolubles) (%) 95 (minimum) 60 (minimum)
Soya bean oil (%) 2–3 39
Moisture (%) 1 0.7
Fat (g per 100 g product) 90 93
Monounsaturated (oleic acid) (%) 9.2 17.9
Polyunsaturated (linoleic, linolenic acids) (%) 65.9 60.7
Saturated (palmitic, stearic acids) (%) 24.9 20.3
Carbohydrates (g per 100 g of product) 8 5
Approximate composition (100 -g sample)
Fatty acid content (g) 50 66
Fatty acid content (relative composition) (%)
Linoleic 58.9 54.0
Linolenic 7.0 6.7
Oleic 9.2 17.9
Palmitic 20.3 15.6
Stearic 4.6 4.7
Other fatty acids 0.0 1.1
Total 100.0 100.0
Primary acetone insolubles (g)
Phosphatidylcholine 23 15
Phosphatidylethanolamine 20 12
Phosphatidylinositol 14 9
Elemental analysis (mg)
Calcium 65 40
Iron 2 1
Magnesium 90 60
Phosphorus 3000 2000
Potassium 800 440
Sodium 30 10
Reproduced from Central Soya, Chemurgy Division (1989) The Lecithin Book. Fort Wayne: Central Soya.
PHOSPHOLIPIDS/Properties and Occurrence 4515

the interfacial surface tension between immiscible
liquids. (See Emulsifiers: Organic Emulsifiers.)
0013 Mixing and blending aids Lecithins decrease time
and increase efficiency of mixing of unlike ingredients
such as sugar and shortening by providing lubricity as
well as viscosity reduction at the contact surfaces of
the incompatible solids.
0014 Release agents Lecithins provide easy release from
metallic surfaces by attaching to the metal surface
during hot or cold cooking. They assist in the clean-
ing of hot surfaces where proteins or batters are
applied. They also reduce sticking between frozen
food products.
0015 Separating agents Lecithins prevent the adhesion of
products that normally stick when in contact, like
cheese slices and caramel confectionery.
0016Viscosity modifiers Lecithins reduce viscosity by
coating particles to reduce particle–matrix friction
such as in chocolates.
0017Wetting agents Lecithins provide complete wetting
of fatty or hydrophilic powders in aqueous systems.
The fatty acids are attracted to the fatty portion and
the hydrophilic portions of the molecules actively
imbibe water and control the hydration of the
powder.
Manufacture
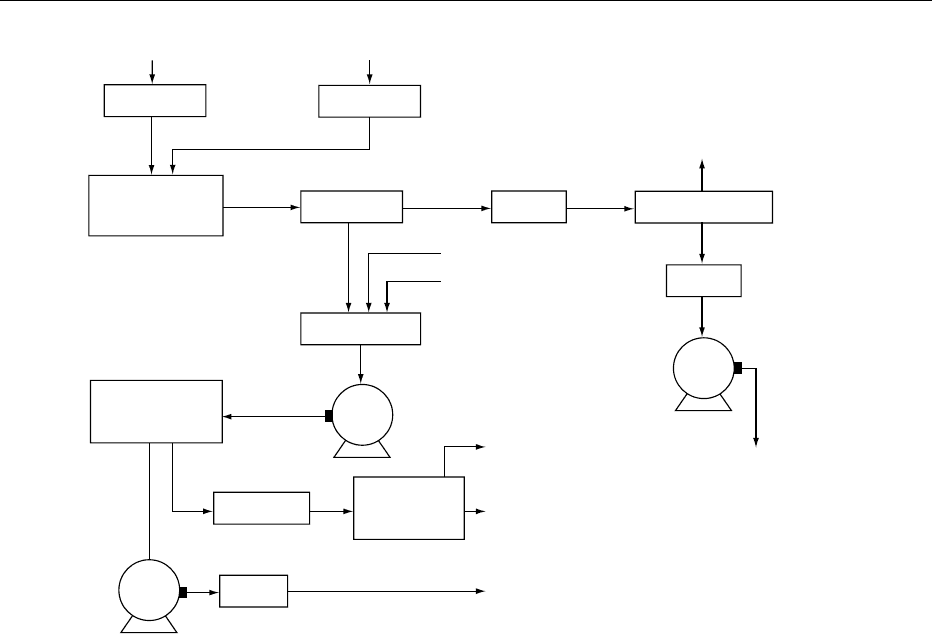
0018The majority of phospholipids are solvent-extracted
from their source. Usually, nonpolar hydrocarbons,
like hexane, are used. Soya beans, for example, are
cleaned, cracked, and dehulled before flaking and
extraction. The hexane is removed from the solvent
micelle and the crude soya bean oil is cooled for
further refining. Figure 1 shows a flow diagram for
the degumming of crude soya bean oil and the pro-
duction of lecithin. Approximately 2–3% water is
added to the crude oil and agitated for at least
30 min. The phosphatides hydrate, swell, and are
separated by centrifugation. The wet gums with
50% moisture are dried through a thin-film drier.
The important points here are careful drying tem-
peratures and cooling of the product below 20
C.
Lightening the color of the product can be achieved
with hydrogen peroxide in the gum stage. The hydro-
gen peroxide is removed through drying.
Modification
0019The chemistry and functionality can be altered by
simple chemical additions with acids and bases as
well as hydrogen peroxide and acetic anhydride.
These modifications increase the dispersion and hy-
dration properties of the lecithin. Enzyme modifica-
tion is also possible with lipases and phospholipases.
These changes markedly affect the functionality in
emulsification.
Composition of Lecithins
0020The composition of lecithins and their phospholipids
will vary depending on their source: vegetable,
animal, or bacterial. There are minor differences
within a class but major differences between the
sources. Vegetable lecithins are high in phosphatidyl-
choline, phosphatidylethanolamine, phosphatidyl-
inositol, and phosphatidic acid, but very low in
phosphatidylserine and contain no sphingomyelin.
Animal lecithins are high in phosphatidylcholine,
phosphatidylethanolamine, phosphatidylserine and
tbl0002 Table 2 Functionality of lecithins
Adhesion aid
Antibleed agent (as in fat bloom)
Anticorrosive
Antidusting agent
Antioxidant
Antispatter agent
Biodegradable additive
Biologically active agent
Catalyst
Color intensifier
Conditioning agent
Coupling agent
Dispersing agent, mixing aid
Emollient, softening agent
Emulsifier or surfactant
Flocculant
Grinding aid
Lubricant
Liposomal encapsulating agent
Machining aid
Modifier
Moisturizer
Nutritional supplement, vitamin source
Penetrating agent
Plasticizer
Promoter
Release agent, antisticking agent
Spreading agent
Stabilizer
Strengthening agent
Suspending agent
Synergist
Viscosity modifier
Water repellent
Wetting agent
Reproduced from Schmidt JC and Orthoefer FT (1985) In Szuhaj BF and
List GR (eds) Lecithins, p. 187. Champaign: American Oil Chemists’
Society, with permission.
4516 PHOSPHOLIPIDS/Properties and Occurrence
sphingomyelin but contain no phosphatidylinositol.
Microbes have phospholipids similar to the plant
kingdom with high levels of phosphatidylethanol-
amine and phosphatidylcholine and phosphatidyl-
serine or sphingomyelin.
Specifications of Lecithins
0021 Lecithins may be qualified in several ways, chem-
ically, physically, and functionally, but there are also
specifications used to assess quality and purity
(Table 3). These include acetone-insolubles (AI),
acid value (AV), hexane-insoluble matter (HI), perox-
ide value (PV), moisture, color, free fatty acids (FFA),
divalent metals (DVM), iodine value (IV), and phos-
phorus. Most of these analytical methods are found in
the American Oil Chemists’ Society (AOCS) Official
Methods and Recommended Practices, Section J.
Acetone-Insolubles
0022 Phospholipids are nearly insoluble in cold acetone.
This quantitative method should measure the active
ingredients in lecithin. Depending on the type of
lecithin, the range is 35–98%.
Acid Value
0023The phosphorus group in lecithins have a titratable
acidity that is measured with this volumetric method.
FFAs are also measured in this test and should not be
confused with phospholipid acidity. The AV range is
20–36 mg of potassium hydroxide per gram.
Hexane-Insolubles
0024In the processing of soya bean lecithin, particulate
matter finds its way through some processes and
gives the product a hazy appearance. The HI can
be determined by dissolving the product in hexane,
centrifuging and gravimetrically measuring the
insolubles. The HI range for soya bean lecithins is
0.05–0.3%.
Peroxide Value
0025The PV is a measure of oxidation in fats and oils. In
lecithin, however, the PV usually measures the
residual hydrogen peroxide from processing. The
range in unbleached products is 0–10 mmol kg
1
and in bleached products is 10–75 mmol kg
1
.
Filtered, warm, crude oil
Flowmeter
Pipeline dwell
agitator
Centrifuge
Oil
Heater
Vacuum
Vacuum
Condensate
Fluidity agent
Bleaching agent
Gums
Pump
Condenser
Condensate
receiver
Mixing tank
Dry lecithin
Cooler
Pump
To packaging
Agitated-film
evaporator
Vacuum drier
Cooler
Pump
To storage
Degummed
dry
soya bean oil
Flowmeter
Water
fig0001 Figure 1 Flowsheet for degumming and crude lecithin production. Reproduced from List GR (1989) In Szuhaj BF (ed.) Lecithins:
Sources, Manufacture and Uses, p. 149. Champaign: American Oil Chemists’ Society, with permission.
PHOSPHOLIPIDS/Properties and Occurrence 4517
Moisture
0026 The water content of lecithins is quite low, at
0.1–1.0%. It can be measured by oven drying but is
more accurately determined by the Karl–Fischer
method. This water content is so low that there is
no measurable water activity for microbial growth.
Moisture contents above 1% will change the viscosity
from a fluid to a plastic state. (See Water Activity:
Principles and Measurement.)
Viscosity
0027 The Brookfield viscosity at 25
C will have a range of
150–20 000 cP. Diluents and divalent metals can alter
the viscosity to usable levels.
Free Fatty Acids
0028 This method measures the true fatty acid levels in
lecithins which range from 1 to 5 mg of potassium
hydroxide per gram. Fatty acids are added as additives
to adjust the viscosity. (See Fatty Acids: Analysis.)
Iodine Value
0029 The IV is a traditional method for qualifying lecithin
sources. The more unsaturated fatty acids are found
in soya bean lecithins, which have a range of 95–110
in natural fluid lecithins, to 80–90 in deoiled lecithin.
Phosphorus
0030This wet chemical method is an indirect way of meas-
uring the phospholipid content. The typical level of
phosphorus is 2.0% in fluid lecithin and 3.0% in
deoiled products. The AOCS method Ca 12–55 has
an approximation for converting percent phosphorus
to the phosphatides in soya bean oil. The equivalent
phosphatides content is equal to percent phosphorus
30.
Uses of Lecithin
0031There are many uses of phospholipids in the food
industry. As seen from the functional properties,
there are multiple functions for lecithins. The
following is a listing of the major areas of use:
.
0032margarines – emulsifier, stabilizer, and antispatter;
.
0033confectionery and snack foods – crystallization
control, viscosity control, antisticking;
.
0034instant foods – wetting and dispersing agent, emul-
sifier;
.
0035commercial bakery products – crystallization con-
trol, emulsifier, wetting agent, release agent;
.
0036cheese products – emulsifier, release agent;
.
0037meat and poultry processing – browning agent,
phosphate dispersant;
.
0038dairy and imitation products – emulsifier, wetting
and dispersing agent, antispattering and release
agent;
.
0039packaging aid – release agent, sealant;
.
0040processing equipment – internal or external release
agent, lubricant.
Refer to individual foods
Applications of lecithins in foods are clearly sup-
ported by the Food Chemicals Codex, the European
E322 regulations, and they are considered as gener-
ally regarded as safe substances by the US Food and
Drugs Administration.
Storage and Handling
0041Lecithins are very stable products. They are shipped
in drums or in bulk containers. They can be stored at
ambient temperatures for up to 2 years without loss
of activity or becoming rancid or spoiling. The water
activity is so low that no microbial growth can occur.
The products may be heated to 25
C for easier appli-
cation. (See Water Activity: Effect on Food Stability.)
See also: Eggs: Structure and Composition; Emulsifiers:
Organic Emulsifiers; Fatty Acids: Properties; Analysis;
Soy (Soya) Beans: Properties and Analysis; Spices and
Flavoring (Flavouring) Crops: Tubers and Roots;
Triglycerides: Structures and Properties; Water Activity:
Principles and Measurement; Effect on Food Stability
tbl0003 Table 3 Specifications range for commercial lecithins
Analysis Typicalrange
Acetone-insolubles (%) 35–98
Acid value (mg KOH g
1
) 20–36
Hexane-insolubles (%) 0.05–0.3
Peroxide value (mmol kg
1
)
Unbleached 0–10
Bleached 10–75
Moisture (%) 0.1–1.0
Viscosity (Brookfield, 25
C) (cP) 150–20 000
Free fatty acids (mg KOH (g
1
) 1–5
Iodine value (cg I g
1
)
Natural 95–110
Oil-free 80–90
Fattyacid composition (%)
Soya bean oil Natural Oil-free
C
16:0
10.3 15.6 20.3
C
18:0
4.4 4.7 4.6
Total saturates 14.7 20.3 24.9
C
18:1
24.5 17.9 9.2
C
18:2
53.8 54.0 58.9
C
18:3
7.0 6.7 7.0
Total unsaturates 85.3 78.6 75.1
Unsaturated: saturated ratio 5.8:1 3.9:1 3.0:1
Reproduced from Central Soya, Chemurgy Division (1990), with
permission.
4518 PHOSPHOLIPIDS/Properties and Occurrence

Further Reading
Burner D (ed.) (1991) Official Methods and Recommended
Practices. Champaign: American Oil Chemists Society.
Central Soya, Chemurgy Division (1990) The Lecithin
Book. Fort Wayne: Central Soya.
Charalambous G and Doxastakis G (1989) Food Emulsi-
fiers: Chemistry, Technology, Functional Properties and
Applications. New York: Elsevier.
Hanin I and Pepeu G (1990) Phospholipids: Biochemical,
Pharmaceutical, and Analytical Considerations. New
York: Plenum Press.
Szuhaj BF (ed.) (1989) Lecithins: Sources, Manufacture and
Uses. Champaign: American Oil Chemists’ Society.
Szuhaj BF and List GL (eds) (1985) Lecithins. Champaign:
American Oil Chemists’ Society.
Determination
B F Szuhaj, Central Soya Company Inc., Fort Wayne,
IN, USA
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001 Phospholipids are a well-known class of lipids that
have been thoroughly analyzed over the past three
centuries. Their complete analysis was facilitated by
the great advances in separation science and qualita-
tive procedures that have occurred in the last 50
years. This article will cover the analysis of phospho-
lipids from their structure and composition, extraction
techniques, qualitative and quantitative assays, and
industrial methodology. (See Fats: Classification.)
Structure of Phospholipids
0002 There are at least a dozen compounds that fall into
the class of phospholipids. They have a basic struc-
ture of a diacylglycerol backbone with a phosphate
ester on the a or third carbon of the glycerol mol-
ecule. Usually another compound is attached that
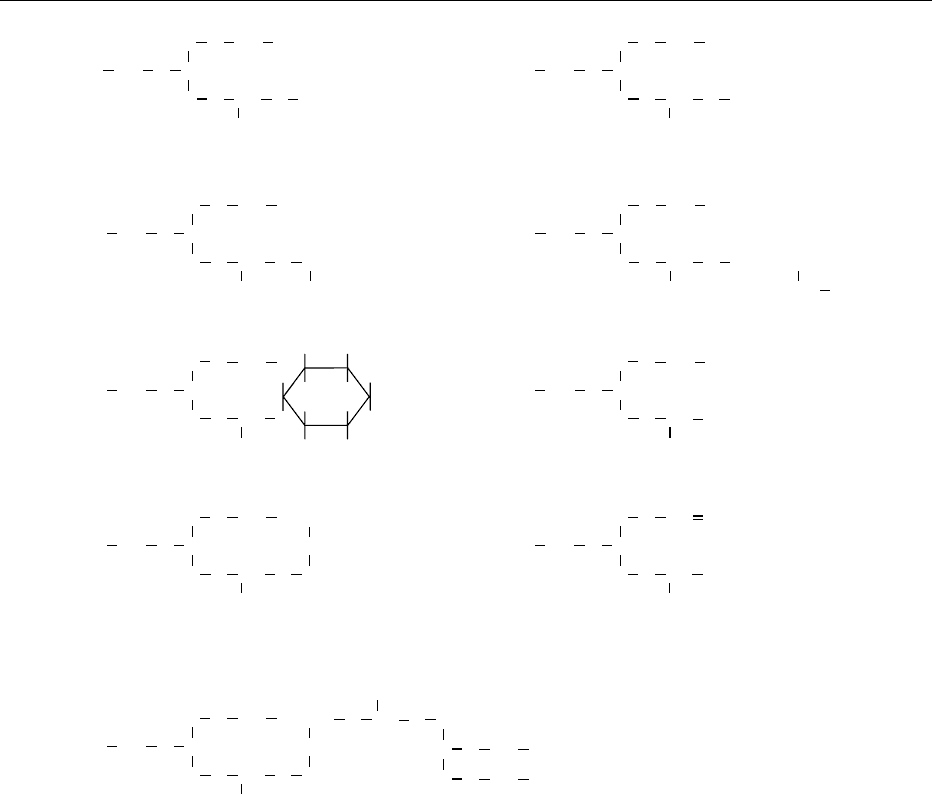
characterizes the phospholipid. Figure 1 shows
examples of the structures of the major phospho-
lipids. These phospholipids (and their common ab-
breviations) are:
.
0003 phosphatidylcholine (PC, PtdCho)
.
0004 phosphatidylethanolamine (PE, PtdEth)
.
0005 phosphatidylserine (PS, PtdSer)
.
0006 N-acylphosphatidylethanolamine (NAPE, N-acyl-
Ptd-Eth)
.
0007 phosphatidylinositol (PI, PtdIns)
.
0008phosphatidic acid (PA, PtdA)
.
0009phosphatidylglycerol (PG, PtdGro)
.
0010plasmologen (PM)
.
0011diphosphatidylglycerol (DPG, diPtdGro)
.
0012lysophosphatidylcholine (LPC, lysoPtdCho)
.
0013lysophosphatidylethanolamine (LPE, lysoPtdEth)
The proper nomenclature for phospholipids has been
defined by the 1976 revised recommendations of the
International Union of Pure and Applied Chemistry
(IUPAC) and the International Union of Biochemis-
try (IUB) Committee on Biochemical Nomenclature.
For example, the term ‘lecithin’ is permitted for
phosphati-dylcholine but the systematic name is
1,2-diacyl-sn -glycero-3-phosphorylcholine. The gen-
eric name of 3-sn-phosphatidylcholine could be used.
The abbreviation PtdCho is also allowed. This article
will use the common names listed above since
the literature has thousands of references with this
terminology.
Composition of Phospholipids
0014The composition of phospholipids depends on the
source of the phospholipids. Those from animal,
plant, and microbial sources will have different com-
positions, depending on the nature of the tissue from
which the lipids are extracted – for example, brain,
liver, or blood. In plants, it will vary on whether they
are from soya beans, corn, cotton, rapeseed, or sun-
flower. In microbial sources it depends upon the
organism.
0015The phospholipid classes are similar within a
species, but differ primarily in fatty acid acyl compos-
ition around the 1 and 2 positions on the glycerol
backbone.
Fatty Acids
0016The fatty acid chain length is commonly from C
4
to
C
26
, with different degrees of unsaturation from one
to six double bonds, which may be at different loca-
tions on the acyl group. However, there is a pattern
that is relevant to the present discussion. (See Fatty
Acids: Properties.)
0017In animals the primary fatty acids range from C
12:0
(lauric acid) to C
24:0
(tetracosanoic acid). Again,
depending on the species and tissue extracted, the
fatty acids can be variable.
0018In the plant kingdom, the primary diacyl groups on
the phospholipids will range from C
12:0
to C
18:3
, i.e.,
lauric to linolenic acid. There are usually no C
20
fatty
acids and higher, as in the animal kingdom. The degree
of unsaturation depends on the origin of the crop, i.e.,
from temperate or tropical regions. Also, the climate
within the zone can make a seasonal difference.
PHOSPHOLIPIDS/Determination 4519
0019 Microbial phospholipid acyl groups are more simi-
lar to those found in the plant kingdom than they are
to those from the animal kingdom. The predominant
acyl fatty acids range from C
16:0
to C
18:3
. There are
more odd-numbered carbon fatty acids in micro-
organisms than elsewhere.
0020 A further factor that makes phospholipid compos-
ition more complex is the ability to have different
fatty acids on the 1 and 2 positions of the molecule.
Most research has shown that polyunsaturated fatty
acids are usually in the 2 position.
Extraction Techniques
0021 One of the most important factors in phospholipid
analysis is the initial extraction procedure. If the
analysis is on a finished commercial lecithin there is
no problem, but if the analysis is from tissue samples
or food samples the extraction technique will be crit-
ical in obtaining meaningful results.
Tissue Samples
0022For many years the Folch extraction of tissue hom-
ogenates with chloroform/methanol 2:1 (v/v) has
been the method of choice by most researchers.
Some found that the use of chloroform/methanol 1:1
(v/v) was preferable and some have used a biphasic
system of butanol/methanol with dilute hydrochloric
acid. Some have used hexane/2-propanol 3:2 (v/v).
Which solvent system used depends largely on the
required accuracy, but in most cases chloroform/
methanol 2:1 (v/v) is the best solvent to try initially.
H
2
CO
++
+
+
CO
CH
H
2
CO
Phosphatidylcholine
Phosphatidylserine
Phosphatidylinositol
Phosphatidylglycerol
Diphosphatidylglycerol
N-Acylphosphatidylethanolamine
Phosphatidic acid (PA)
Plasmalogen
X = Choline or ethanolamine
Phosphatidylethanolamine
PO
O
−
O
CH
2
CH
2
N (CH
3
)
3
OCOR
2
R
1
H
2
COCO
CH
H
2
COPO
O
−
O
CH
2
CHNH
3
OCOR
2
R
1
H
2
COCO
CH
H
2
COPO
O
−
COO
−
O
CH
2
CHNH
3
OCOR
2
R
1
H
2
COCO
CH
OH
OH
OH
OH
HO
H
2
CO O PO
O
−
O
OCOR
2
R
1
H
2
COCO
CH
H
2
COPO
O
−
O
HCOH
H
2
COH
CH
2
OCOR
2
R
1
H
2
COCO
CH
H
2
COPO
O
−
O
HCOH
CH
2
OCOR
2
R
1
O
−
H
2
COPO
O
CH
2
H
2
C O CO R
4
HC O CO R
3
H
2
COCH
CH
H
2
COPO
O
−
OX
OCOR
2
CHR
1
H
2
COCO
CH
H
2
COPO
O
−
O
−
OCOR
2
R
1
H
2
COCO
CH
H
2
COPO
O
−
CO R
3
O
CH
2
CH
2
NH
OCOR
2
R
1
fig0001 Figure 1 Structures of the major phospholipids. Reproduced from Scholfield CR (1985) In: Szuhaj BF and List GR (eds)Lecithins, p.3.
Champaign: American Oil Chemists’ Society, with permission.
4520 PHOSPHOLIPIDS/Determination
Food Samples
0023 Since food samples may have lecithin or phospholipid
added rather than being incorporated into the tissues
of the food matrix, the dried sample can be ground
and extracted with petroleum ether. If the lipids are
bound in the product through processing, chloro-
form/methanol 2:1 (v/v) can be used. Because of
environmental considerations di- or trichloroethane
should be used in place of chloroform.
0024 Drying of high-moisture products is required, but
not by oven drying or air drying, as oxidation of the
fatty acids can occur. Freeze drying of the product is
preferred.
Qualitative Analysis
0025 There are several ways to detect the presence of phos-
pholipids. Traditional instrumental methods using
ultraviolet and infrared are not used as often since
thin-layer chromatography (TLC) can give a qualita-
tive and semiquantitative result in one assay. Also, the
use of conformational chemical sprays on the TLC
plates can further identify the products.
Thin-Layer Chromatography
0026 There are several types of silica gel plates available for
TLC. Silica gel G and H are the most useful. Phos-
pholipids may be separated on a 20 20 cm plate in
one or two directions. A polar solvent and a nonpolar
solvent system are used. The polar system is chloro-
form/methanol/water (65:25:4, v/v/v) and the nonpo-
lar solvent is petroleum ether/diethyl ether/acetic acid
(90:10:1, v/v/v). See the American Oil Chemists’
Society (AOCS) recommended practice Ja 7-86
for alternative methodology. (See Chromatography:
Thin-layer Chromatography.)
0027 These TLC plates are air- or oven-dried after separ-
ation of 20–50 mg of sample and are sprayed with
10% sulfuric acid and heated to char the phospho-
lipids. Alternatively, they may be sprayed with a
phosphorus spray containing molybdenum blue.
Phospholipids stain a deep blue on heating the TLC
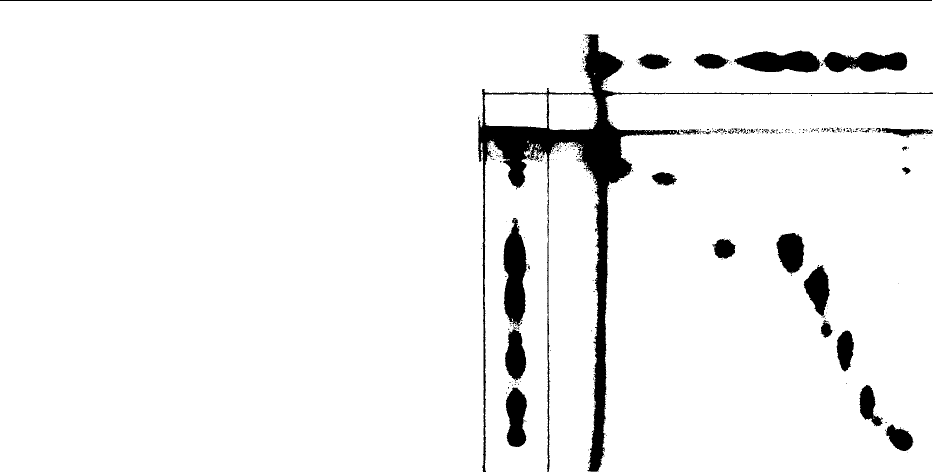
plate. An example is shown in Figure 2.
0028 Nondestructive visualization techniques can be
used if the phospholipids are to be determined or the
fatty acid composition is to be run. Ultraviolet light
and 2
0
,7
0
-dichlorofluorescein easily detects lipids on
TLC. On prep plates, the bands are scraped off and
extracted with chloroform/methanol (1:1, v/v) and
the fatty acids converted to methyl esters using
boron trifluoride and then determined using gas–
liquid chromatography (GLC).
Quantitative Analysis of Phospholipids
Column Chromatography
0029Column chromatography precedes TLC in the separ-
ation of phospholipids. The techniques are slow and
require good skill with column preparation, flow
rates, and solvent removal. Commercial lecithins
can be separated by dissolving the crude mixture in
petroleum ether and passing it through a deactivated
silica gel column with petroleum ether. The phospho-
lipids are adsorbed and do not pass through the
column, whilst triglycerides and sterol esters are
eluted. The phospholipids are subsequently quanti-
fied by TLC and wet phosphorus analysis.
High-Performance Liquid Chromatography (HPLC)
0030Newer technologies have found that HPLC can sep-
arate and quantify phospholipids more quickly and
accurately. Separation is carried out on several types
of columns, including silica gel and an amino group
bonded to the silica surface (mBondapak-NH
2
). The
columns are eluted with chloroform/methanol gradi-
ents, acetonitrile/methanol/85% phosphoric acid, or
acetonitrile/methanol/ water. The eluent is measured
at 205 nm or detected with flame ionization. Figure 3
Acylated
lecithin
TG
SG
PA
PC
Pl
Ori
g
in
NAPE
PE
LysoPE
LysoPC
fig0002Figure 2 Thin-layer chromatogram of soya bean lecithin in two
dimensions: triglycerides (TG), sterol glucosides (SG), phospha-
tidic acid (PA), N-acylphosphatidylethanolamine (NAPE), phos-
phatidylethanolamine (PE), lysophosphatidylethanolamine (Lyso
PE), phosphatidylcholine (PC), phosphatidylinositol (PI), lyso-
phosphatidylcholine (LysoPC). Silica gel plate; first dimension
chloroform/methanol/acetic acid/water, 85:15:15:3 (v/v/v/v);
second dimension chloroform/acetone/methanol/acetic acid/
water (10:4:2:2:1) (v/v/v/v/v). Courtesy of J. Yaste, Central Soya,
Food Research, Fort Wayne, Indiana, USA.
PHOSPHOLIPIDS/Determination 4521
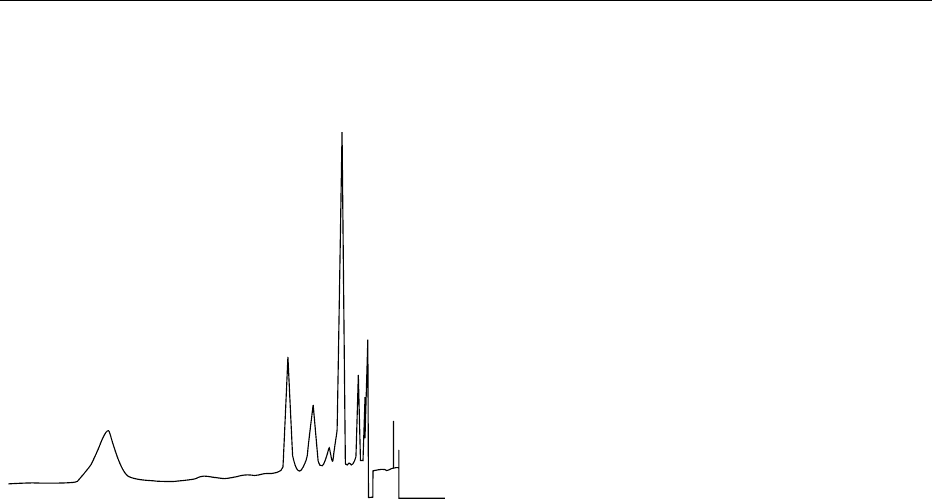
shows an HPLC separation of commercial lecithin,
using ultraviolet detection. The mass detector,
an evaporative analyzer, has also been successfully
used for the HPLC determination of phospholipids.
(See Chromatography: High-performance Liquid
Chromatography.)
Densitometry
0031 Densitometric scanning has been used as an indirect
method for determining phospholipid content on TLC
plates. While the method has some promise, a problem
is the quanitative charring of the phospholipid spots.
Each phospholipid has a different charring density and
this depends on fatty acid composition. Only with
proper standards can this method be useful.
Thincography
0032 Thin-rod TLC combines TLC with quantification by
flame ionization detection. Rods are used rather than
plates but controversy still exists over the suitability
of the technique for routine lipid analyses.
Phosphorus Analysis on Phospholipids
0033 Phosphorus analysis is an indirect method for
the quantification of phospholipids because the
qualitative composition of the sample must be
known, if accurate values are to be obtained. With
pure phosphatides this will work well, but most sep-
aration techniques give mixed phospholipids. The
preferred method for phosphorus in lecithins is the
AOCS method Ja 5-55. This determines the total
phosphorus content of the sample. For commercial
lecithins a multiple factor of 30 is used to convert
total phosphorus values to acetone-insoluble value.
0034There are various methods to determine phos-
phorus through molybdenum blue and molybdova-
nadophosphate yellow. To improve reproducibility
many factors need to be evaluated. This includes the
digestion method, chromogenesis, and sensitivity.
The AOCS method is the most straightforward and
should be used especially in the food area.
Industrial Methods of Analysis
0035Phospholipids are characterized by a different set of
assays than the determination of compounds used for
academic or biochemical use. Most commercially
available phospholipids come from soya beans and
from eggs, and the methods outlined below can be
used to qualify or categorize the products.
Acetone-Insolubles (AI)
0036This method determines the content of phospho-
lipids in commercial lecithins. The method employs
AOCS method Ja 4-46. The AI is an approximation of
the active ingredients in formulations. Cold lecithin-
saturated acetone must be used in this test.
Acid Value (AV)
0037This method determines the phosphatide and fatty
acid content of commercial phospholipids. The
method utilized is AOCS method Ja 6-55. Phospha-
tide acidity is often confused with fatty acid addition
to commercial lecithin. It is a combination of organic
acids and phosphoric acid.
Peroxide Value (PV)
0038This is a measurement of oxidative state of commer-
cial phospholipids. It measures the milliequivalents of
peroxide per kilogram of sample which oxidize po-
tassium iodide. It also measures the residual peroxide
used in process stabilization and bleaching. AOCS
method Ja 8-87 is used.
Free Fatty Acids (FFA)
0039This method utilizes AOCS method Ca 5a-40. When
run on the acetone-soluble portion of the AI method,
it gives the added fatty acids.
PC (RT 23.68)
PA ( RT 6.65)
PI ( RT 8.75)
PE (RT 4.35)
Deoiled lecithin 83.2 mg 50 ml
−1
hexane
fig0003 Figure 3 High-performance liquid chromatography of deoiled
soya bean lecithin. PC, phosphatidylcholine; Pl, phosphatidylino-
sitol; PA, phosphatidic acid; PE, phosphatidylethanolamine.
Column, m Porasil 10 m 3.98 300 mm; mobile phase, hexane/2-
propanol/acetate (8:8:1, v/v/v), buffer pH 4.2; detection, ultraviolet
(206 nm); injection, 10 ml; flow rate, 1 ml min
1
. Retenton time (RT)
in minutes. Courtesy of P. Balazs, Central Soya, Food Research,
Fort Wayne, Indiana, USA.
4522 PHOSPHOLIPIDS/Determination

Phosphorus Content
0040 The determination of total phosphorus is an indirect
method for quantifying phospholipids. This method
(AOCS method Ja 5-55) quantifies phospholipids
through a molybdate reaction to a chromophore
quantitated by phenolphthalein titration.
Gas Chromatography (GC)
0041 This method is commonly used to measure the fatty
acid composition and does not quantify the phospho-
lipids themselves (AOCS method Ce 1-62).
High-performance Liquid Chromatography
0042 This is gradually replacing the older techniques for
qualifying and quantifying particular phospholipids
in commercial mixtures. A uniform technique is being
addressed by the AOCS.
0043 Phospholipid analyses have come a long way since
their study by Theuticum, c. 1800. Each analyst must
choose the best method depending on constraints of
accuracy and time.
See also: Chromatography: Thin-layer Chromatography;
High-performance Liquid Chromatography; Fatty Acids:
Properties; Fats: Classification
Further Reading
Burner D (ed.) (1991) Official Methods and Recommended
Practices. Champaign: American Oil Chemists’ Society.
Central Soya, Chemurgy Division (1990) The Lecithin
Book. Fort Wayne: Central Soya.
Charalambous G and Doxastakis G (1989) Food Emulsi-
fiers: Chemistry, Technology, Functional Properties and
Applications. New York: Elsevier.
Hanin I and Pepeu G (1990) Phospholipids: Biochemical,
Pharmaceutical, and Analytical Considerations. New
York: Plenum Press.
Szuhaj BF (1989) Lecithins: Sources, Manufacture and
Uses, Champaign: American Oil Chemists’ Society.
Szuhaj BF and List GL (1985) Lecithins. Champaign:
American Oil Chemists’ Society.
Physiology
T H M Da Costa and M K Ito, Universidade de
Brası
´
lia, Brası
´
lia, DF, Brazil
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 Phospholipids are ubiquitous molecules that, due to
their chemical properties, have important structural
as well as metabolic roles in the cell. The first phos-
pholipid to be discovered was phosphatidylcholine.
It was originally named lecithin after the Greek
lekithos, which means egg yolk. The concept of phos-
pholipids as mere structural and metabolic inert
molecules associated with methological difficulties
delayed the interest in these compounds until the
1950s, when elucidation on the biosynthetic pathway
of phospholipids began to be published. Today, the
metabolic and physiological roles of phospholipids
are an active and exciting area of research. In this
article we discuss the physical and biochemical prop-
erties of phospholipids in cellular and subcellular
membranes, the biosynthetic and hydrolytic path-
ways, and the role of phospholipids in signal trans-
duction.
Functional Role of Membrane
Phospholipids
0002Phospholipids are a major component of cellular
membrane and play a pivotal role in the communi-
cation between extra- and intracellular space. Phos-
pholipids represent a large class of compounds that
have fatty acyl chains esterified to glycerol and a
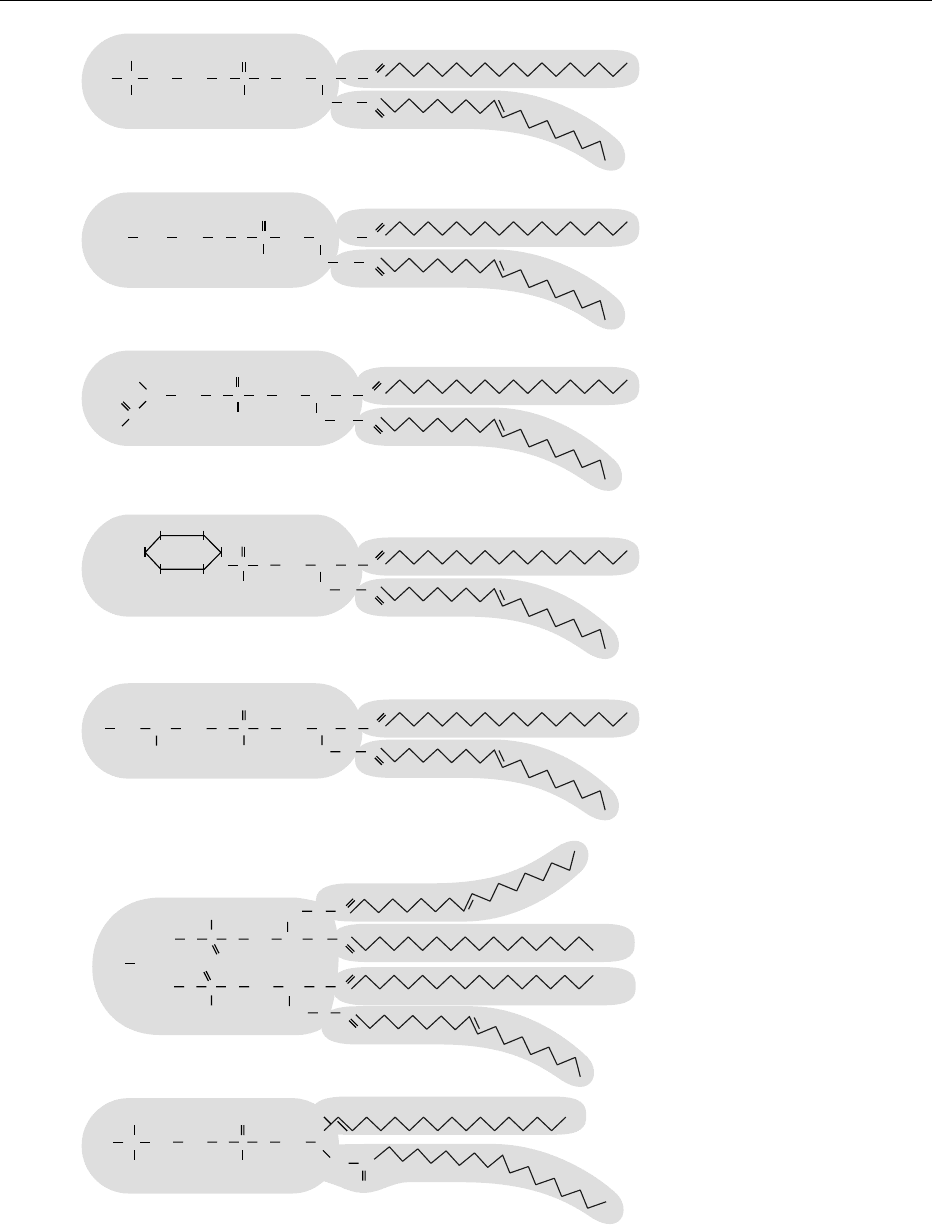
charged or zwitterionic head group (Figure 1). The
fatty acyl chains usually have an even number of
carbon atoms from 12 to 26, with 80% being 16–20
carbon atoms long. They may have up to six double
bonds, commonly present as a cis isomer in one of the
fatty acids that creates a kink in the chain. Differences
in the length and saturation of the fatty acid tails are
important for their influence in the ability of phos-
pholipid molecules to pack against one another,
and for this reason they affect the fluidity of the
membrane.
0003In animal cells phosphatidylcholine (PtdCho) is
the major phospholipid, whereas in bacteria phos-
phatidylethanolamine (PtdEtn) is the predominant
species. Sphingomyelin is found in most animal cell
membranes and belongs to a different group of phos-
pholipids that fits into the overall pattern of phospho-
lipid structure (Figure 1). In many mammalian cells,
four major phospholipids predominate in the plasma
membrane: PtdCho, phosphatidylserine (PtdSer),
PtdEtn, and sphingomyelin. Only PtdSer carries a
net negative charge, while the other three are electric-
ally neutral at physiological pH with one positive and
one negative charge. Sphingomyelin and PtdCho
carry a molecule of choline in their head group
(Figure 1). The phospholipid bilayer of biological
membranes confers the amphipathic feature with
hydrophobic and hydrophilic domains. The polar
head groups of phospholipids face the aqueous exter-
ior of the membrane while hydrophobic regions of the
PHOSPHOLIPIDS/Physiology 4523
H
3
C
+
N
CH
2
CH
2
OPOCH
2
CH O C
H
2
C
OC
CH
3
O
O
O
CH
3
O
−
Phosphatidylcholine
(PtdCho or lecithin)
CH
3
+
NCH
2
CH
2
OPO CH
2
CH
CH
3
O
OH
NH C
O
CH
3
O
−
Sphingomyelin
H
3
N
+
CH
2
CH
2
OPOCH
2
CH O C
H
2
CO C
O
O
O
O
−
Phosphatidylethanolamine
(PtdEtn)
CH CH
2
OP OCH
2
CH O C
NH
3
H
2
COC
O
O
C
O
O
O
−
O
−
Phosphatidylserine
(PtdSer)
Diphosphatidylglycerol
(cardiolipin)
CH
2
OP OCH
2
CH O C
CH
2
OPO CH
2
CH C C
HO CH
H
2
COCO
−
O
−
O
O
O
O
O
O
CH
2
OC
HO CH
2
CH CH
2
OPOCH
2
CH
OC
H
2
C
OC
O
O
O
O
−
OH
Phosphatidylglycerol
(PtdGro)
OPOCH
2
CH O C
H
2
COC
O
O
O
O
−
Phosphatidylinositol
(PtdIns)
OH
OH
OH OH
HO
+
fig0001 Figure 1 Chemical structure of phospholipids. The head group with electron charge is presented as the oval part of the molecule and
the tails are the fatty acyl chains. The presence of a double bond creates a kink in the chain.
4524 PHOSPHOLIPIDS/Physiology
