Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


lipids are sequestered away from the water to the
interior of the bilayer in the most thermodynamically
favorable arrangement. Because of such chemical
characteristics, membrane phospholipid molecules
spontanously form bilayers in aqueous environments
and form sealed compartments.
0004 Cellular membranes are organized in mosaic-like
structures which are dynamic and mobile. The terms
‘fluidity’ and ‘motility’ are used to describe properties
of membrane and membrane components, respect-
ively. Fluidy reflects the viscosity within the mem-
brane that depends on both the composition and
temperature within the membrane. The change in vis-
cosity or in phase transition (from liquid to gel or
crystalline state) is determined by the chain length
and saturation of the hydrocarbon chains. A shorter
chain length reduces the tendency of the hydrocarbon
tails to interact with one another and cis double bonds
produce kinks in the hydrocarbon chains that make
them more difficult to pack together, making mem-
branes more fluid at lower temperatures. Phospho-
lipid mobility is determined by intramolecular and
intermolecular motion. Intramolecular motion in-
cludes three main types: (1) rotation or vibration
about each C–C bond within the fatty acyl chain (seg-
mental motion); (2) rotation of the entire molecule
about the long axis perpendicular to the plane of the
bilayer; (3) a pendulum-like motion of the fatty acyl
chains. Intermolecular motion refers to lateral diffu-
sion of complete phospholipid molecules and occurs
by interchange of one lipid molecule for another.
These time motions have a different time scale, i.e.,
segmental motion is very fast, whereas lateral diffu-
sion is slower. Flip-flop or migration of phospholipid
molecules from one monolayer to another is a com-
paratively less common type of intermolecular
motion. These movements are possible because of
the presence of a special class of endoplasmic reticu-
lum (ER) membrane-bound enzyme, called phospho-
lipid exchange proteins or phospholipid translocators,
which catalyze the rapid flip-flop of specific phospho-
lipids from the inner to the outer monolayer of plasma
membrane or from one organelle membrane to an-
other, e.g., from microsomal vesicles to mitochondria.
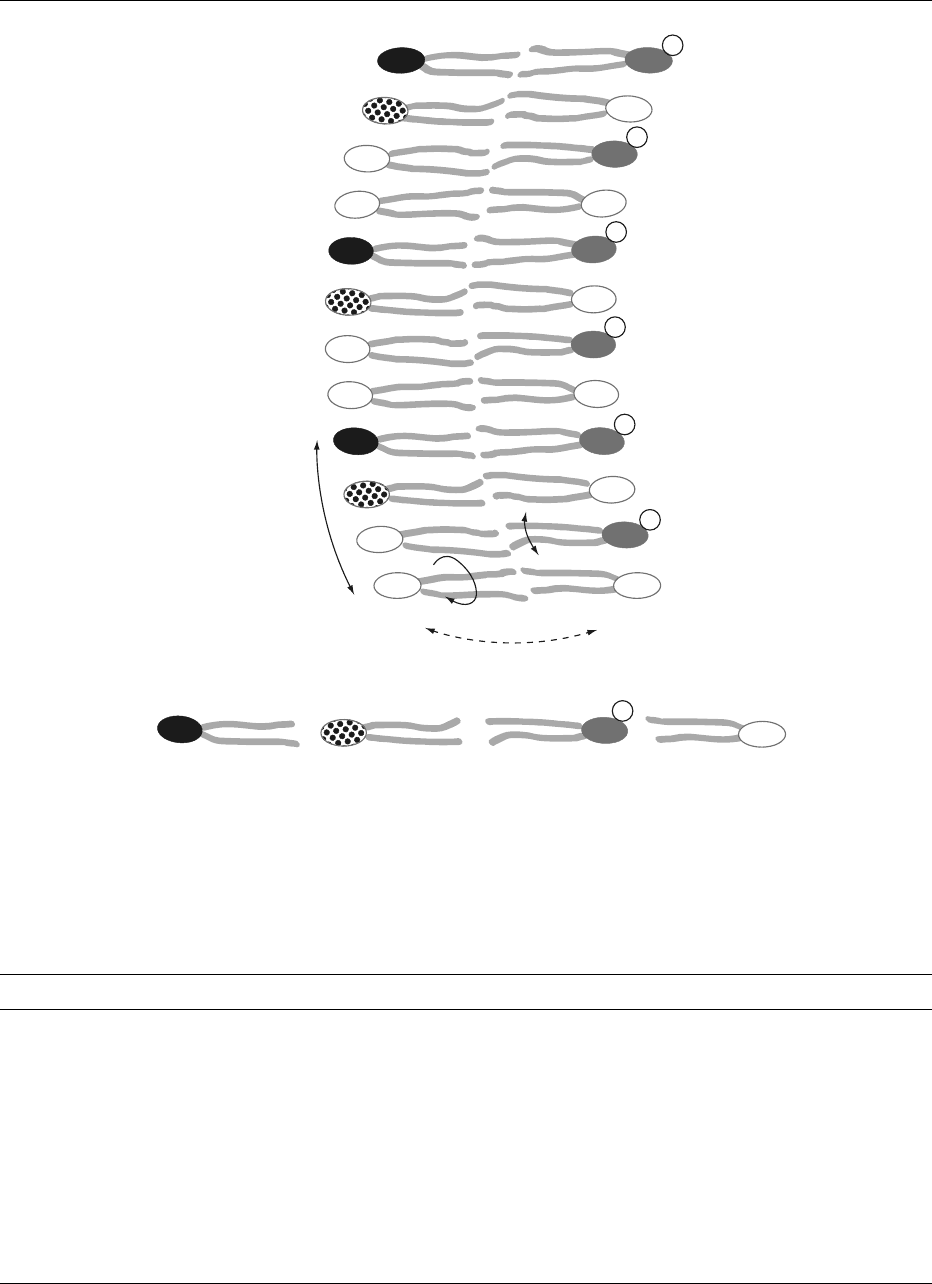
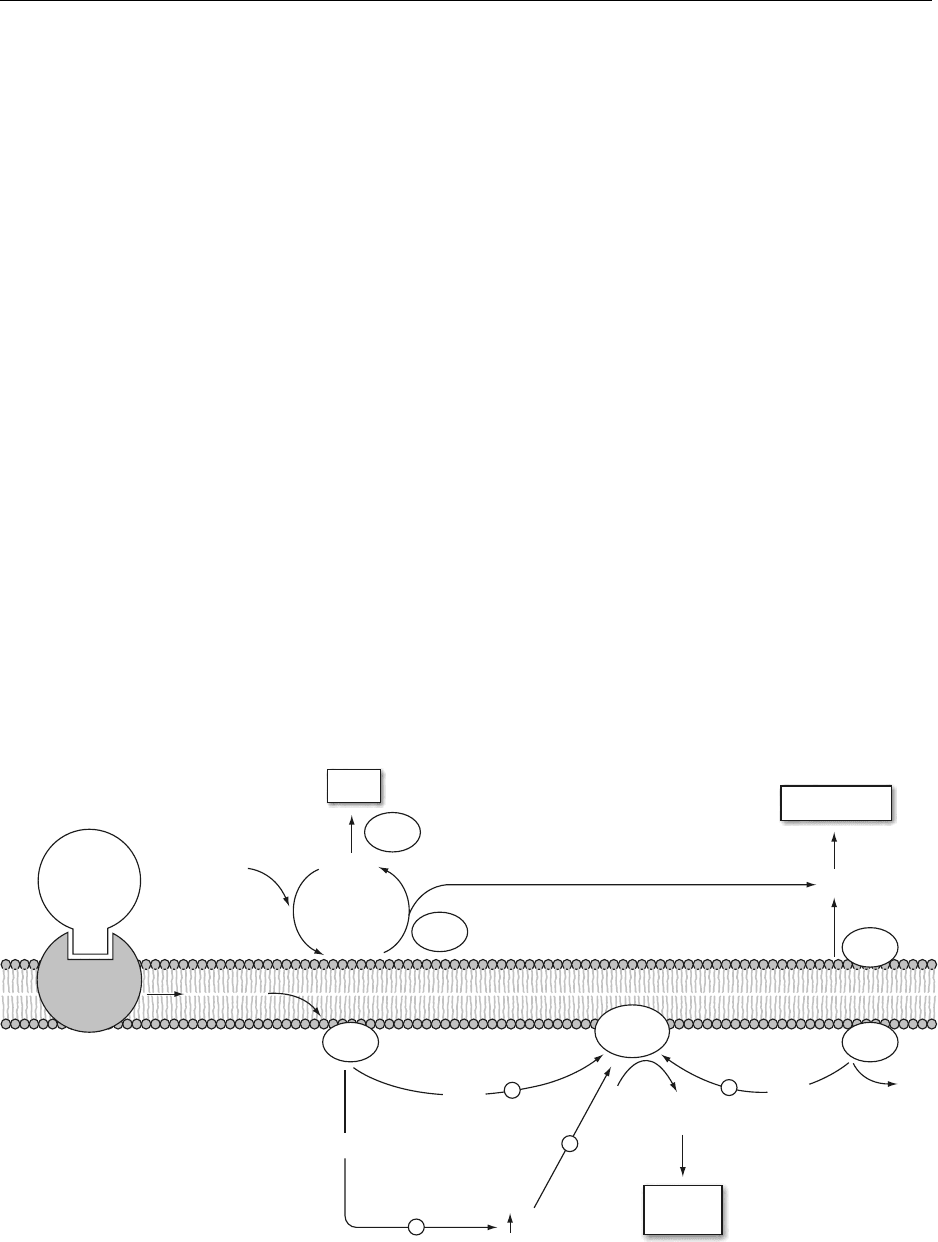
The phospholipid movements in biological mem-
branes are presented schematically in Figure 2.
0005 Phospholipid fraction of cellular membranes varies
considerably in total amount and in the composition
of the fatty acids. This diversity occurs across species,
among tissues, and even among organelles in the same
cell (Table 1).
0006 The functional role of differences in fatty acid com-
position of cell membranes and the fatty acid asym-
metry between phospholipids from the outer and
the inner membrane have an important functional
significance. In human red blood cell membranes,
most of the PtdSer, which is negatively charged, is
located in the inner monolayer. This position is im-
portant for the activity of the enzyme protein kinase
C which requires the negative charge of phosphati-
dylserine (Figure 2). Similarly, specific inositol phos-
pholipids are concentrated in the cytoplasmatic half
of the plasma membrane where it can be reached by
specific enzymes and the product of the phosphatidy-
linositol molecules serves as intracellular signals.
Biosynthesis of Phospholipids
0007A more direct approach about the biosynthetic path-
ways of phospholipids, known as the Kennedy
pathway, became possible with the utilization of
radioactive and stable isotopes, which permitted ad-
vances in the understanding of phospholipid active
synthesis and breakdown in the cells and their role as
a metabolic pool for cellular signals. These techniques
are now combined with genetic manipulation for
the study of the pathways involved in phospholipid
metabolism.
0008The ER of eukaryotic cells is the predominant site
of phospholipid and sphingolipid assembly, but there
are phospholipid biosynthetic enzymes localized in
the mitochondria and microsomes. The yeast Sac-
charomyces cerevisiae has become the model of
choice for the study of the regulation of phospholipid
biosynthesis, owing to the possibility of a biochem-
ical, molecular, and genetic approach. The model
system of S. cerevisiae has allowed advances in the
study of intracellular transport and assembly of lipids
into membranes, analysis of the genes, and gene prod-
ucts required for lipid metabolism and definition of
the role of lipids in signal transduction. Many of the
enzymes were isolated and purified and metabolic
pathways were targeted by genetic manipulation of
specific genes which were impossible in other eukary-
otic organisms. In addition, the pathways of phos-
pholipid metabolism in S. cerevisiae are, with few
exceptions, similar to those found in higher eukary-
otes. They also display the intracellular compartmen-
talization and subcellular membranes typical of
eukaryotes which are important for the study of lo-
calization of the phospholipid biosynthesis enzymes.
0009Figure 3 shows the biosynthetic pathways in
S. cerevisiae. The important steps are outlined, to-
gether with the enzymes responsible for specific reac-
tions. The whole biosynthetic process is highly
interconnected to the sorting and delivery of plasma
and subcellular membrane. Synthesis of long-chain
fatty acids is achieved by the coordinated action
of acetyl-coenzyme A (acetyl-CoA) carboxylase, fatty
acid synthase and acyl-CoA synthetases (FAAs),
PHOSPHOLIPIDS/Physiology 4525
Lateral
diffusion
Flip-flop
PtdCho PtdEtn PtdSer PtdIns
Rotation
Flexion
Extracellular space Cytosol
−
−
−
−
−
−
−
fig0002 Figure 2 Schematic representation of a biological membrane section, showing the possible phospholipid movements within each
phospholipid molecule, between molecules and between the inner and outer phospholipid monolayer. The asymmetry of phospholipid
species is also presented, with the predominance of phosphatidylcholine (PtdCho) and phosphatidylethanolamine (PtdEtn) facing the
outer half and phosphatidylserine (PtdSer) with a negative charge and phosphatidylinositol (PtdIns) facing the inner half of the
membrane.
tbl0001 Table 1 Phospholipid composition of cell membranes
Membrane PtdCho PtdEth PtdSer PtdIns PtdGro diPtdGro PtdOH
Rat liver
Endoplasmic reticulum 58 17 4 9
Plasma membrane 56 15 10 2
Mitochondrial (inner) <3 3 25 1 6 2 18
Mitochondrial (outer) <5 5 23 2 13 3 3
Nuclear 55 20 3 7
Rat brain
Myelin 11 14 7
Erythrocytes
Rat 31 15 7 2
Human 23 20 8 3
Sheep 1 23 8 1
Escherichia coli plasma membrane 80 15 5
PtdCho, phosphatidylcholine; PtdEth, phosphatidylethanolamine; PtdSer, phosphatidylserine; PtdIns, phosphatidylinositol; PtdGro,
phosphatidylglycerol; diPtdGro, diphosphatidylglycerol (cardiolipin), PtdOH, phosphatidic acid.
Adapted with permission from Jain MK and Wagner RC (1980) Introduction to Biological Membranes, p. 36. New York: John Wiley.
4526 PHOSPHOLIPIDS/Physiology
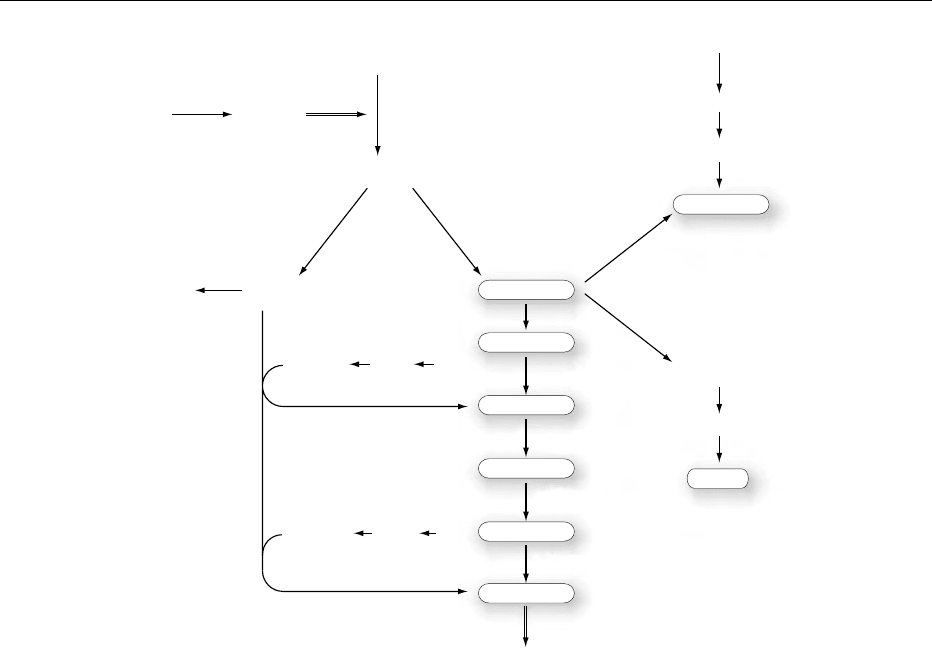
-elongase(s) and -desaturase(s). The fatty acids are
esterified to sn-1 and sn-2 to glycerol-3-phosphate by
the enzyme glycerol-3-phosphate acyltransferase
(GAT) (Figure 3). Cytidine diphosphate-diacylgly-
cerol (CDP-DAG) synthase (CDS) converts phospha-
tidic acid to CDP-diacylglycerol (CDP-DAG), the
major intermediate in phospholipid synthesis. The
availability of CDP-DAG at specific cellular sites will
direct the synthesis of different phospholipids, such as
cardiolipin (CL), which occurs exclusively in mito-
chondria and phosphatidylinositol (PtdIns) and PtdSer
to distinct subfractions of the ER or the Golgi appar-
atus. There is extensive transfer of intermediates and
cross-compartment integration for the synthesis of
phospholipids. For example, the enzyme phosphati-
dylserine synthase (CHO1) is localized in the ER,
while the steps of conversion of PtdSer to PtdEtn take
place in the inner mitochondrial membrane. The
PtdEtn synthesized in the mitochondria must again
migrate to the ER to insure synthesis of PtdCho, the
most abundant phospholipid of S. cerevisiae.
0010In the absence of exogenous choline, PtdCho is
synthesized by the de novo pathway by a three-step
methylation of PtdEtn, which is catalyzed by the
ER methytransferases. In the case of mutants with
defects in phosphatidylserine decarboxylase (PSD)
and methyltransferases, choline and ethanolamine
must be provided in the medium to enter phospho-
lipid biosynthesis via the salvage pathway (Figure 3).
This route also insures recycling of PtdEtn and
FFA
Gro-3-P
PtdOH
INO1
Cho-CDP
Etn-CDP
Salvage pathway
Cho-P
Etn-P
Acyl-CoA
FFA1−4
PAP
DAGTAG
GAT
CDP-DAG
Ins
PtdGro-P
PtdGro
CLS
CL
Ins-1-P
Glc-6-P
Ptd Ins
PtdSer
Ptd Etn
Ptd MMEtn
Ptd DMEtn
Ptd Cho
Bulk membrane
PSD1
PSD2
CDS1
CHO1
CHO2
OPI3
OPI3
PGP
PIS1
Etn
Cho
fig0003 Figure 3 Phospholipid biosynthetic pathways in Saccharomyces cerevisiae. FFA, free fatty acid; Acyl-CoA, acyl coenzyme A; Gro-3-P,
glycerol-3-phosphate; PtdOH, phosphatidic acid; DAG, diacylglycerol; TAG, triacylglycerol; CDP-DAG, cytidine diphosphate-diacylgly-
cerol; PtdSer, phosphatidylserine; PtdEtn, phosphatidylethanolamine; PtdMMEtn, phosphatidylmonomethylethanolamine; PtdDMEtn,
phosphatidyldimethylethanolamine; PtdCho, phosphatidylcholine Cho, choline; Cho-P, choline phosphate; Cho-CDP, cytidine diphos-
phate-choline. Etn, ethanolamine; Etn-P, ethanolamine phosphate, Etn-CDP, cytidine diphosphate-ethanolamine. Glc-6-P, glucose-6-
phosphate; Ins-1-P, inositol 1-phosphate; Ins, inositol; Ptd Ins, phosphatidylinositol. PtdGro-P, phosphatidylglycerol-phosphate;
PtdGro, phosphatidylglycerol; CL, cardiolipin. Enzymes abbreviations (italic): FFA 1^4, acyl-CoA synthetases 1–4; GAT, glycerol-3-
phosphate acyltransferase; PA P , phosphatidate phosphatase; CDSI, CDP-diacylglycerol synthase; CHO1, phosphatidylserine synthase;
PSD1,2, phosphatidylserine decarboxylase 1,2; CHO2, phosphatidylethanolamine N-methyltransferase; OPI3, phosphatidyl-N-methyl-
transferase; PISI, phosphatidylinositol synthase; INO1, inositol-1-phosphate synthase; PGP, phosphatidylglycerophosphate phosphat-
ase; CLS, cardiolipin synthase.
PHOSPHOLIPIDS/Physiology 4527
PtdCho degradation products and control of local
levels of the potent second messenger diacylglycerol.
Degradation of Phospholipids
0011 Undoubtedly, the most important function of phos-
pholipids in the cell membrane relates to their
breakdown by the action of phospholipases. The
phospholipases are enzymes that hydrolyze phospho-
lipids on water–lipid interfaces and are distinguished
from other esterases by their relatively low activity in
soluble monomeric phospholipids below their critical
micellar concentrations (CMC), but become fully
active in aggregated phospholipid structures above
their CMC, such as in micelle, bilayers, or hexagonal
structures.
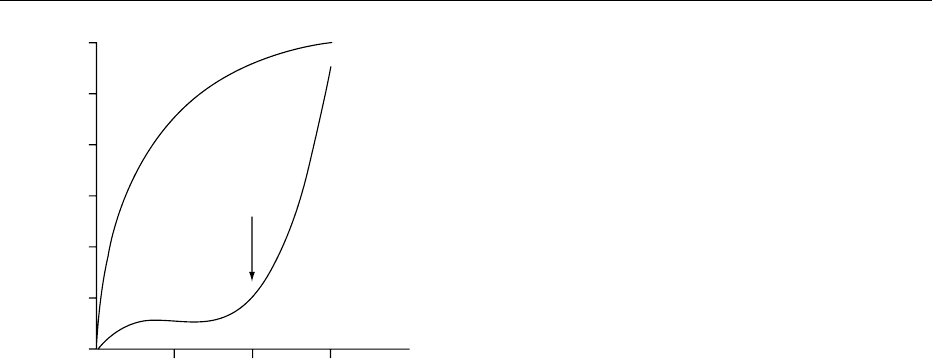
0012 As shown in Figure 4, whereas esterases show clas-
sical Michaelis–Menten kinetics, the phospholipases
may reach more than 1000-fold increase in activity as
the substrate phospholipid concentration reaches the
CMC. Important factors responsible for this in-
creased rate of hydrolysis include high local substrate
concentration, amphipatic substrate orientation at
the interface, enhanced diffusion of products from
the enzyme to lipid or aqueous moieties, and con-
formational change of enzyme upon binding to the
interface.
0013 Phospholipases play a central role in the activation
of various events related to phospholipid degrad-
ation. In general: (1) many phospholipases are digest-
ive enzymes found in high concentrations in venoms,
bacterial secretions, and digestive fluids of higher
animals; (2) many phospholipases have a regulatory
function with their products being cellular medi-
ators such as diacylglycerols, inositol trisphosphate,
platelet-activating factor, and the eicosanoids. The
actions of lipid mediators, known collectively as
second messengers, are highly sensitive and under
rigid control by a variety of anabolic and catabolic
enzymes. Many pathological states, as in inflamma-
tion, allergic reactions, and hypertensive states, are
related to activation of the cascade of events involv-
ing phospholipid degradation and the intracellular
second messenger pathway.
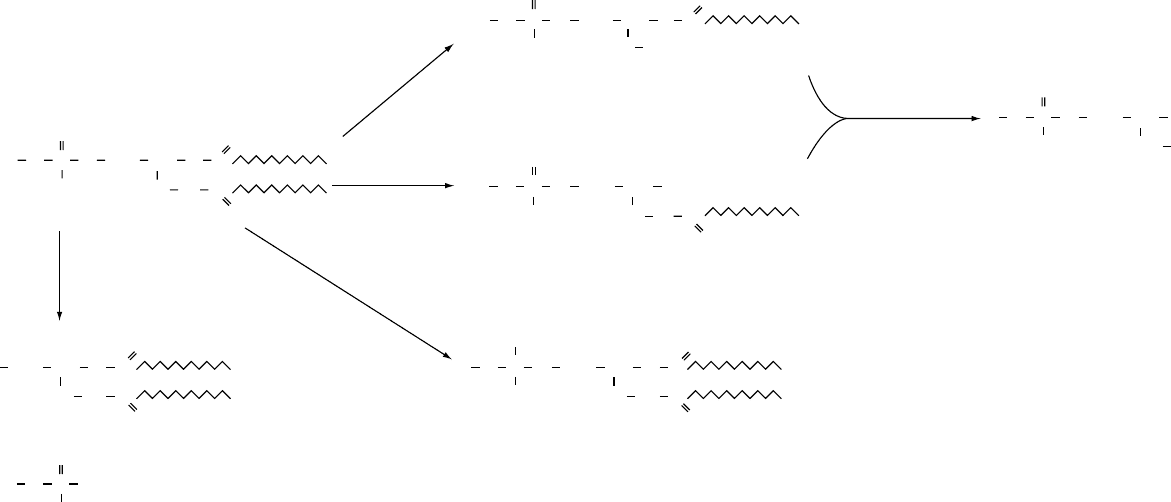
0014Phospholipases are classified as type A
1
,A
2
,B,C,
or D, according to where they act on the substrate
phospholipid (Figure 5).
0015The phospholipases A are acyl hydrolases, which
means they remove one acyl group, yielding one fatty
acid and lysophosphatide. Phospholipase A
1
and
phospholipase A
2
remove fatty acids at positions
sn-1 and sn-2, respectively. Phospholipase A
1
is widely
distributed in nature and in mammals the major
sources are found in the pancreas and the brain. Intra-
cellularly, phospholipase A
1
is dominant in the ER.
The best known examples of phospholipase A
2
are
snake venom and the pancreatic enzyme that acts
mostly on PtdCho and PtdEdn. It is the major phos-
pholipase of mitochondria. Phospholipase B hydro-
lyzes both acyl groups (sn-1 and sn-2) and also has
high lysophospholipase activity. Phospholipase C acts
on the glycerophosphate bond while phospholipase D
catalyzes the removal of the polar head group. In
mammalian cells, phospholipase C is an important
initiator of the PtdIns cycle. Phospholipase D is the
major phospholipase in many plant tissues and acts
specifically on intact phospholipids to give phospha-
tidic acid and an alcohol. In plants, it seems to be
involved in cell turnover and energy utilization. Phos-
pholipase D is also found in bacterial and mammalian
sources, where it is involved in replacement of the
polar head groups of membrane phospholipids.
Phospholipids as Precursors of Cellular
Signal Transduction
0016In the cell membrane, phospholipids store important
precursors of second messengers. Transduction of sig-
nals from hormones, across a membrane, involves the
coordinated actions of receptor, membrane proteins,
and phospholipids that either stimulate or inhibit the
synthesis of a second messenger. A number of pro-
cesses inside the cell are controlled by the level of
second messengers.
0017Among the best described second messenger
systems is the G-protein and phosphatidylinositide
Substrate concentration
(arbitrar
y
)
CMC
Activity (arbitrary units)
Esterase
6
5
4
3
2
1
0
123
fig0004 Figure 4 Esterase exhibits Michaelis–Menden kinetics,
whereas phospholipase needs critical micellar concentration
(CMC) of the substrate for full activity.
4528 PHOSPHOLIPIDS/Physiology
Lysophospholipase
X
C
D
O
O
C
OC
O
O
−
O
+
OP
OH CH
OH
CH
2
H
2
C
XO
O
OC
OH + RCOOH
+
RCOOH + RCOOH
+ RCOOH
+ XOH
A
2
, B
A
1
, B
O
PO CH
CH
2
H
2
C
XO
O
OH
O
O
PO CH
CH
2
H
2
C
HO
O
O
O
C
OC
O
O
PO CH
CH
2
H
2
C
XO
O
OH
OH
O
PO CH
CH
2
CH
2
O
C
O
XO
O
O
O
C
OC
O
O
PO CH
CH
2
H
2
C
Figure 5 The action of phospholipases A
1
,A
2
, B, C, and D and lysophospholipase in the hydrolysis of phospholipids. X represents choline, serine, ethanolamine, etc.
fig0005
system. This system is distinctive in that one stimulus
activates membrane reactions that generate two
second messengers. The first experimental observa-
tions noted that administration of neurotransmitter
acetylcholine in pigeon pancreas led to a rapid turn-
over of PtdIns fraction of membrane phospholipids
and release of the digestive enzyme amylase. Similar
observations were made in other systems by hor-
mones, neurotransmitters, or growth factors. Despite
intensive efforts, the understanding of these mechan-
isms had not progressed until the early 1980s. It is
now recognized that the initial events of inositol
phospholipid metabolism occur within 20–30 s of
binding of the agonist to the receptor.
0018 Today, the role of specific inositol phospholipids
in the phosphoinositide family, namely, phosphati-
dylinositol 4,5-bisphosphate (PI(P
2
)), as a mem-
brane-associated storage form of two second
messengers, is quite clearly understood.
0019 Figure 6 shows a simplified scheme of the events
involved. When an agonist binds to a receptor, mem-
brane G-protein is stimulated to bind guanosine
triphosphate. The activated G-protein then acts on
the membrane-bound enzyme, phospholipase C,
which in turn cleaves PI(P
2
) to yield two products,
sn-1,2-diacylglycerol (DAG) and inositol 1,4,5-
trisphosphate (IP
3
). Both of these products are second
messengers. The function of IP
3
is to stimulate the
release of calcium ion from intracellular stores,
largely from the ER. The increased calcium concen-
tration originates a cascade of events in the intracel-
lular metabolism, including the activation of the
membrane-bound protein kinase C (C from calcium).
This enzyme requires calcium and PtdSer for its activ-
ity. The specific role of the second messenger DAG is
to increase the affinity of the protein kinase C for
calcium ions. Activated protein kinases will then
phosphorylate target proteins inside the cell, which
will then be activated and proceed within the cascade.
Since many metabolic events are controlled by cal-
cium fluxes and by phosphorylation of specific pro-
teins, the phosphoinositol system shows great
ingenuity as a control mechanism. Some of the
known target proteins are the insulin receptor, b-
adrenergic receptor, and glucose transporter. It is
now quite clear that PtdIns is not the only source of
DAG which can activate protein kinase C. PtdCho
and PtdEtn also seem to serve this role. PtdCho is also
a major source of arachidonic acid for the biosyn-
thesis of eicosanoids.
0020Another well-described example of a regulatory
function involving phospholipids in membrane is the
generation of another second messenger, known as
arachidonate cascade. As described earlier, when an
Cytosol
Extracellular space
Receptor
Stimulus
AcylCoA
G-protein
PLA
2
PLA
1
FA
Cellular
response
Active
protein
Eicosanoids
AA
LPI (P
2
)
PLA
2
PLC
PKC
PLC
PI (P
2
)
Ptd Ser
PtdCho
Ca
2+
CP
IP
3
+
DAG
+
+
+
DAG
fig0006 Figure 6 The role of phospholipids in signal transduction. PLA
1
, PLA
2
, PLC, phospholipases A
1
,A
2
, C; PI(P
2
), phosphatidylinositol 4,5
bisphosphate; IP
3
, inositol 1,4,5-trisphosphate; AcylCoA, acyl coenzyme A; DAG, diacylglycerol; FA, fatty acids; AA, archidonic acid;
CP, choline phosphate; PKC, protein kinase C; Ptd Ser, phosphatidylserine; PtdCho, phosphatidylcholine; LPI, lysophosphatidylino-
sitol.
4530 PHOSPHOLIPIDS/Physiology

agonist stimulates a membrane receptor, a series of
events occurs which may lead to the release of 20
carbon polyunsaturated fatty acids from membrane
phospholipids, most commonly arachidonic acid
(Figure 6). These are tissue-specific stimulants by
hormones such as bradykinin or epinephrine, or
proteases such as thrombins, to name but a few.
Pathological release can occur if membranes are per-
turbed; for example, the bee sting may stimulate the
release of arachidonate from local cell membrane and
cause inflammation. This release involves the action
of a specific phospholipase A
2
on PtdCho or PtdEdn,
yielding arachidonate, and the action of a phospho-
lipase C on PtdIns, yielding a diacylgycerol, which in
turn undergoes cleavage to give free arachidonate,
which is then converted into a specific eicosanoid in
the cell. Eicosanoids, such as prostaglandins, leuko-
trienes, and thromboxanes are compounds with
potent physiological properties which are formed
from 20-carbon unsaturated fatty acids into one of a
series of eicosanoids according to the enzyme present
in the cell and the unsaturated fatty acid released
from the membrane. Long-chain polyunsaturated
fatty acids of the n-3 series, especially from marine
origin, when present in the diet will be incorporated
into the cell membrane and may replace arachidonic
acid in the signal pathway. All eicosanoids are metab-
olized very rapidly. We still know relatively little
about the subsequent effects of eicosanoids at a mo-
lecular level, though recent evidence points to their
function in the communication pathway with nuclear
receptors.
0021 Figure 6 presents events as a coordinated response
involving membrane phospholipids and their degrad-
ation products. This figure exemplifies the events
associated with arachidonic acid release (top) and
the mobilization of calcium ions and protein kinase
C (bottom). The action of lipid mediators is highly
sensitive and under rigid control and when this regu-
lation is not maintained, a number of pathological
states, such as those mentioned above, may result.
0022Current knowledge on the mechanisms involved in
intracellular signaling is rapidly advancing and it
underscores the importance of the physiological,
pathological, as well as the pharmacological prop-
erties of this diverse and ubiquitous group of com-
pounds, known as phospholipids. The nutritional
significance of phospholipid physiology may be sum-
marized by the fact that foods are the main sources of
essential elements of this system.
See also: Choline: Properties and Determination;
Essential Oils: Properties and Uses; Fatty Acids:
Properties; Metabolism; Gamma-linolenic Acid; Fish:
Dietary Importance of Fish and Shellfish; Fish Oils:
Dietary Importance; Fats: Classification; Occurrence;
Prostaglandins and Leukotrienes; Vegetable Oils:
Dietary Importance
Further Reading
Carman GM and Henry AS (1989) Phospholipid bio-
synthesis in yeast. Annual Review of Biochemistry 58:
635–669.
Eyster KM (1998) Introduction to signal transduction – a
primer for untangling the web of intracellular messen-
gers. Biochemistry and Pharmacology 55: 1927–1938.
Kohlwein SD, Daum G, Schneiter R and Paltauf F (1996)
Phospholipids: synthesis, sorting, subcellular traffic – the
yeast approach. Trends in Cell Biology 6: 260–266.
Mead JF, Alfin-Slater RB, Howton DR and Popja
´
k G (1986)
Lipids: Chemistry, Biochemistry and Nutrition. New
York: Plenum.
Sim E (1982) Membrane Biochemistry. Outline Studies in
Biology. London: Chapman and Hall.
Vance DE (1991) Phospholipid metabolism and cell signal-
ling in eucaryotes. In: Vance DE and Vance J (eds)
Biochemistry of Lipids, Lipoproteins and Membranes –
New Comprehensive Biochemistry, vol. 20, pp. 205–
239. Netherlands: Elsevier.
Waite M (1991) Phospholipases. In: Vance DE and Vance J
(eds) Biochemistry of Lipids, Lipoproteins and Mem-
branes – New Comprehensive Biochemistry, vol. 20,
pp. 269–295. Netherlands: Elsevier.
PHOSPHOLIPIDS/Physiology 4531

PHOSPHORUS
Contents
Properties and Determination
Physiology
Properties and Determination
A N Garg, Indian Institute of Technology, Roorkee,
U.A., India
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The extensive and varied chemistry of phosphorus
transcends the traditional boundaries of inorganic
chemistry because of its vital role in the biochemistry
of all living organisms as a constituent of adenosine
triphosphate (ATP) and phosphoproteins. It was first
isolated from urine by Hennig Brandt in 1669 as a
white waxy substance. The spontaneous chemilumin-
escent reaction of white phosphorus with moist air
was the first observed property and was also the
origin of its name (Greek: ‘phos,’‘light’; ‘pherein,’
‘bearing’). It was also the ancient name for the planet
Venus, when it appeared before sunrise.
Chemical Properties
0002 Phosphorus is a typical nonmetal placed in group
VA of the periodic table. The element phosphorus
(P) has an atomic number of 15 and atomic
weight of 30.97 with electrons distributed as
1s
2
2s
2
2p
6
3s
2
3p
x
1
3p
y
1
3p
z
1
and atomic energy levels
as shown in Figure 1. Thus, three unpaired electrons,
together with the availability of low-lying vacant 3d
orbitals, account for the predominant oxidation
states III and V in phosphorus chemistry. The most
important biological form is the pentavalent oxygen
compound phosphate PO
3
4
. Phosphorus exists in
three main allotropic forms; white, red, and black,
each of these being polymorphic. There are at least 11
known modifications, some amorphous, others of
some indefinite identity, and all but three of unknown
structure. Of these, white phosphorus is soft, waxy,
most reactive, and thermodynamically least stable. It
has a melting point of 44.1
C, a boiling point of
80
C and a specific gravity of 1.82 g cm
3
. Red and
black forms are heavier with specific gravities of
2.2 and 2.69 g cm
3
, respectively. White phosphorus
reacts with moist air and gives out light. It ignites
spontaneously in air at about 35
C and is therefore
stored in water to prevent combustion. It is soluble in
organic solvents such as CS
2
and benzene. If white
phosphorus is heated to about 250
C, or a lower
temperature in the presence of sunlight, then red
phosphorus is formed. It is a polymeric solid with a
melting point of 280.5
C and sublimes at 430
C. It
is much less reactive than white phosphorus and does
not phosphoresce in air. Unlike white phosphorus,
red phosphorus need not be stored under water. Its
structure is extremely complex, involving a crisscross
packing of infinite tubular chains of P atoms. When
white phosphorus is heated under high pressure, a
highly polymerized form called black phosphorus is
obtained. It is also obtained by heating at 220–370
C
for 8 days in the presence of mercury as a catalyst and
with a seed of black P. This is thermodynamically the
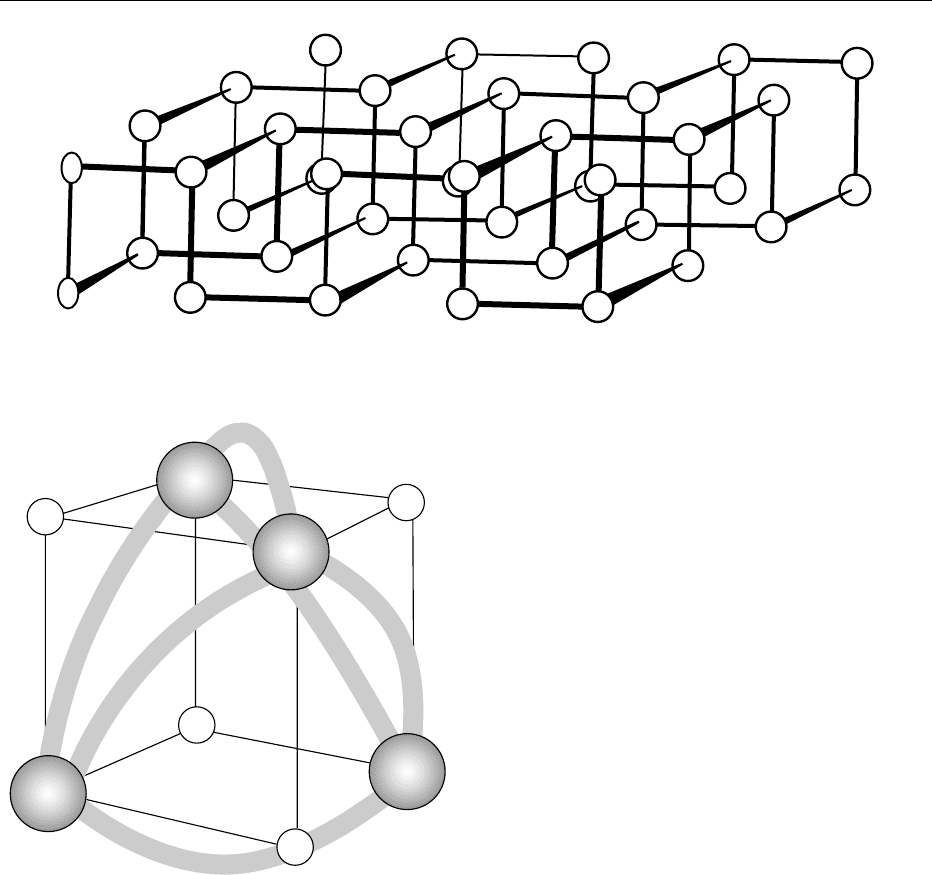
most stable allotrope. It is inert and has a double layer
structure with P atoms being bound to three neigh-
bors, as shown in Figure 2. The entire structure con-
sists of a stacking of these double layers with the
closest P–P distances within each layer being 2.17–
2.20 A
˚
and the shortest P–P distance between layers
at 3.87 A
˚
. Therefore, the crystals are flaky like graph-
ite. All forms of phosphorus melt to give the same
liquid, which consists of symmetric P
4
molecules with
phosphorus atoms occupying corners of a regular
tetrahedron. Each atom is directly bonded to the
other three atoms P–P ¼ 2.21 + 0.02 A
˚
. The same
molecular form exists in the gas phase at >800
C and
low pressure. The bonding orbitals in P
4
have only
2% of 3s and 3d character. It is most likely that pure
3d
3p
3s
7.5 eV
9eV
~2 eV
fig0001Figure 1 Atomic energy levels in phosphorus.
4532 PHOSPHORUS/Properties and Determination
3p orbitals are involved, even though the bonds in P
4
are bent with ffP–P–P ¼ 60
and a strain energy of
96 kJ mol
1
, which accounts for its high reactivity.
Electronic spectral studies suggest a resonance struc-
ture with strong p bonds. The regions of high electron
density in the P
4
molecule are shown in Figure 3. The
moment of inertia about any of its three major axes
is 2.5 10
40
gcm
2
. Thus, the P
4
molecule might be
expected to have donor ability.
0003 The only naturally occurring isotope,
31
P, has a
nuclear spin I ¼ ½h/2p and a large magnetic moment
(1.13 NM) but no quadrupole moment. It is suitable
for nuclear magnetic resonance (NMR) spectroscopy.
In a 10-kG field
31
P resonates at 17.24 MHz. Phos-
phorus forms compounds with all elements except
tin, bismuth, and the inert gases. It also reacts readily
with heated aqueous solutions to give a variety of
products. The bonding and stereochemistry of the
phosphorus atom are varied, essentially due to
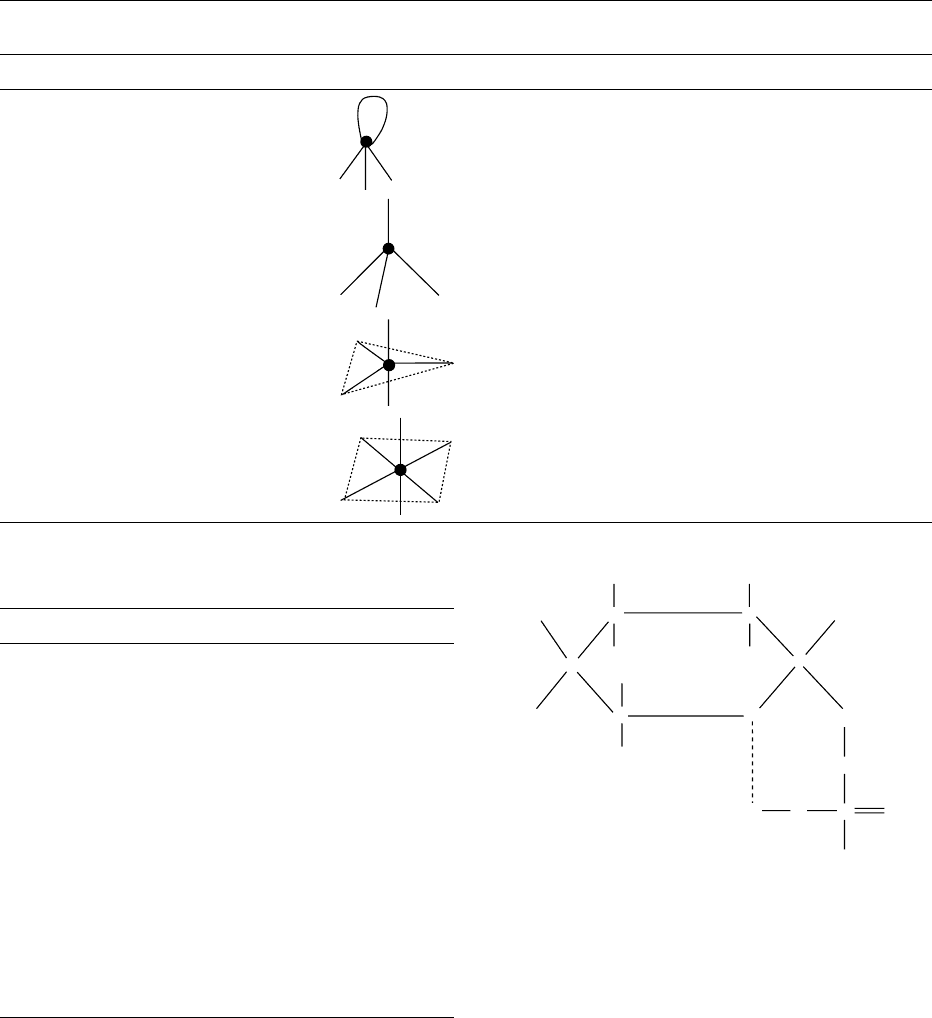
empty d orbitals. It is known in at least 14 coordin-
ation geometries with coordination numbers up to 9,
though the most frequently met coordination numbers
are 3, 4, 5, and 6. Some typical geometries along with
orbitals used in the formation of bonds are given in
Table 1. Phosphorus has a significant tendency to
catenation, forming a series of cyclic compounds
(RP)
n
where n ¼ 3–6, as well as some R
2
PPR
2
type
of compounds. (See Spectroscopy: Nuclear Magnetic
Resonance.)
Forms in Foods
0004Plants need phosphate for healthy growth, especially
for the development of roots, flowers, fruits, and
seeds, though the requirement is less compared with
that for nitrogen and potassium. All foods contain
phosphorus in the form of the phosphate anion
ðPO
3
4
Þ, and is consumed by living organisms as
such. Very few natural compounds contain phos-
phorus in any other form. A summary of the types
of biologically important phosphorus compounds is
presented in Table 2. It is picked up from the soil,
where it is present as organic and inorganic phos-
phates (soluble as well as insoluble) in nucleopro-
teins, nucleic acids, and the coenzymes nicotinamide
adenine dinucleotide (NAD) 2-phosphate, ATP, and
other high-energy phosphates. Organic phosphates
include sugar phosphates such as glucose 6-phosphate
(Figure 4), phospholipids, and pigments. Phosphate
in the form of nucleotides serves as a source of a high-
energy bond and performs an important function in
conserving and providing bursts of metabolic energy.
fig0002 Figure 2 Arrangement of P atoms in the double layers in crystalline black phosphorus.
fig0003 Figure 3 Three-dimensional distribution of valence shell elec-
tron density in the P
4
molecule. From Hart RR, Robin MB and
Kuebler NA (1965) Journal of Chemical Physics 42: 3631–3638, with
permission.
PHOSPHORUS/Properties and Determination 4533
0005 Most of the phosphorus in foods is in the form of
organic phosphates that are digested in the intestines
to form inorganic phosphates of sodium, calcium,
and potassium. A special regulatory mechanism, the
parathyroid hormone (PTH)–vitamin D–thyro calci-
tonin axis is involved in the control of both calcium
and phosphorus balance. This hormonal axis controls
the absorption rate in the gut, the excretion rate in the
kidney, and the storage capacity of bones. Therefore,
phosphorus is involved in a multitude of process in
the entire life cycle, serving both a structural and
catalytic function.
0006In egg yolk and fish, it occurs in the form of phos-
pholipids, phosphatidylcholine, and phosphatidyl-
ethanolamine. In certain foods such as cereal grains
and proteins from vegetable sources, 50–80% of the
phosphorus occurs in the form of phytin, which is
usually the calcium/magnesium salt of phytic acid
(the hexaphosphate ester of inositol). Natural starch,
particularly of potato, contains phosphoric acid as
an ester (Figure 5). (See Phospholipids: Properties
and Occurrence; Phytic Acid: Properties and Deter-
mination; Starch: Structure, Properties, and Deter-
mination.)
0007The phosphate cycle in water is controlled by the
biocycle. The inorganic part of the cycle consists of
HPO
2
4
in solution equilibrium with H
2
PO
4
, ðPO
3
4
Þ,
and H
3
PO
4
. Planktonic algae can absorb inorganic
phosphates. Some algae also have a method of
tbl0001 Table 1 Types of phosphorus compounds and their molecular structure
Numbers of bonds Hybridization Geometry Shape Examples
3sp
3
(with one lone pair) Pyramidal PH
3
4sp
3
Tetrahedral PH
4
þ
, POCl
3
, PO(OH)
3
(d
p
–p
p
bonding in
compounds with PO)
5sp
3
d Trigonal bipyramid PCl
5
,PF
5
,Ph
5
P
6sp
3
d
2
Octahedral PF
6
tbl0002 Table 2 Type of biologically important phosphorus compounds
and their functions
Type Example Function
Nucleic acids DNA Storage of genetic
information
RNA Transcription of DNA and
protein synthesis
Phospholipid Phosphatidyl
choline
Structural components of
membranes
Bone salt Hydroxyapatite Bone structure and function
Phosphoproteins Casein Nutrient storage
Glutaminase NH
3
production; acid–base
regulation
Stathmin Cell proliferation and
differentiation
Sugar phosphate Glucose-6-P Glycolysis
Nucleotides ATP Energy transformation,
molecular activation
cAMP Second messenger
Uridine di-P
glucose
Glycogen synthesis
CH
2
O
P
H
O
O
−
O
O
C
C
C
C
C
H
H
OH
OH H
H
OH
H
HO
fig0004Figure 4
4534 PHOSPHORUS/Properties and Determination
