Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

physiological parameters allow some distinction from
other food-relevant lactobacilli, it is not possible to
use a phenotypical basis to discriminate sufficiently
among L. acidophilus, L. johnsonii, L. gasseri, L.
crispatus, and L. amylovorus. All five species are
usually assigned to the L. acidophilus cluster. A dis-
tinction of these species can be facilitated by applying
genotypical techniques and methods based on DNA
homology, the molar amounts of guanine plus cyto-
sine in the DNA, or by the analysis of certain cell wall
components.
Physiological Actions of
Lactobacillus
acidophilus
0005 Because of the properties described above and its
pronounced bile salt resistance, L. acidophilus is
well adapted to the environmental conditions of the
gastrointestinal tract. Proteins in the cell wall may be
important in attaching the bacterium to the mucosal
cells of the intestine. With strain-dependent vari-
ations, L. acidophilus contributes to the inhibition
of the multiplication of pathogenic and putrefactive
bacteria in the intestine due to the production of
organic acid and trace amounts of H
2
O
2
. Further-
more, strain-specific inhibitory substances can be
excreted by certain strains. In this context, numerous
antagonistic peptides (bacteriocins) have been isol-
ated from certain strains of L. acidophilus. For
example, some of them were described as lactocidin,
acidophilin, acidolin, lactosin B, and lactacin B
and possess some ‘antibiotic’ potential against
salmonellae, staphylococci, Escherichia coli, and
clostridia, and partly also against other species of
lactic acid bacteria. Because of their beneficial L.
acidophilus-related properties, products containing
this bacterium have been used in the treatment of
gastrointestinal disorders and to reestablish the func-
tion of the intestine after treatment with antibiotics.
Other features of these products are the provision of
b-galactosidase to humans having an enzymatic defi-
ciency for lactose digestion or, particularly when used
in conjunction with fructooligosaccharides (oligo-
fructose), the reduction of fecal enzymes (glucuroni-
dase, nitroreductase, azoreductase) which obviously
play some role in some stages of precancerogenesis.
Since L. acidophilus produces equimolar amounts of
l(þ) and d() lactic acid, products fermented with
this bacterium offer the advantage of a reduced d()
lactate content, compared to classical yogurt. How-
ever, the acidification potential of this bacterium is
often low and varies considerably among strains.
Products with
Lactobacillus acidophilus
0006At present, a broad variety of products containing
L. acidophilus is on the market. This bacterium
has been incorporated into fermented as well as
nonfermented milks of different levels of dry matter
and fat (Figure 2). Cows’ milk is the main substrate
which is processed using the same basal technology as
applied for the manufacture of yogurt or other cul-
tured dairy products. Hence, continuous production
lines with conventional or aseptic filling systems are
used. Some of the products also contain added fruits
and flavoring agents.
0007Fermented dairy products containing L. acidoph-
ilus as a single bacterial culture are primarily of local
importance in Russia, Eastern European countries,
and Scandinavia. In contrast, in other European
regions, L. acidophilus is usually used in combination
with other microorganisms (e.g., Bifidobacterium
spp., Streptococcus thermophilus, L. delbrueckii
subsp. bulgaricus, L. casei). Milk cultured with such
multicomponent starter cultures (including L. acido-
philus) is produced in increasing numbers and
varieties and consumed frequently by many people.
Among these dairy products, a distinction can be
made between the so-called ‘mild’ yogurt products
(yogurt-related fermented milks, with or without
fruits) which are based on a fermentation with vari-
ous thermophilic bacteria (many of them are assigned
to the area of probiotics), and so-called ‘Acidophilus
milk’ products which are usually fermented by means
of mesophilic lactic acid bacteria (e.g., strains of
Lactococcus lactis or Leuconostoc cremoris or
combinations of both), in addition to L. acidophilus.
A general flow diagram for the production of such
an Acidophilus milk fermented under mesophilic
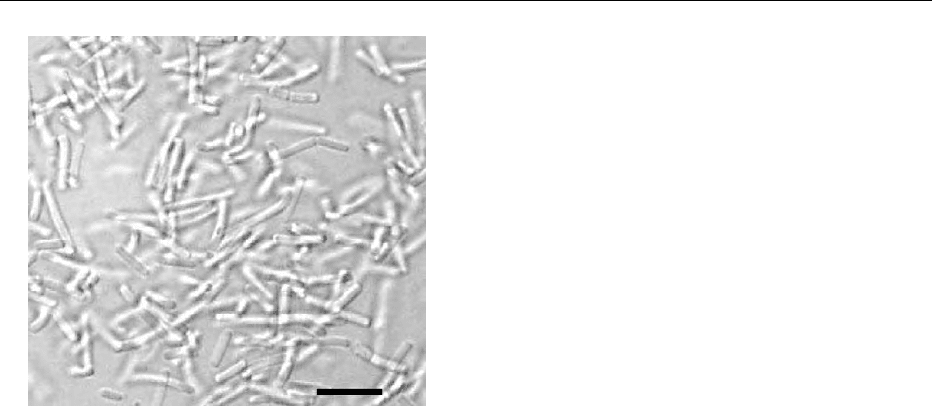
10µm
fig0001 Figure 1 Microphotograph of a Lactobacillus acidophilus culture
(deep-frozen culture concentrate cultured in MRS broth; for
detailssee Table 1).
4
ACIDOPHILUS
MILK
conditions is presented in Figure 3. Deep-frozen cul-
ture concentrates or freeze-dried bacteria or, very
rarely, liquid cultures are inoculated into the milk
base. The fermentation is usually performed over-
night for 15–20 h. Stirred products, with a liquid
character, are usually made, but set-style fermented
Acidophilus milk products with increased levels of
solid-nonfat are also available.
0008 Other categories of products include specially fer-
mented drinks (e.g., L. acidophilus plus yeasts, with
or without other lactic acid bacteria, resembling kefir
and named acidophilin), texturized products with a
reduced water content, which are offered in a pasty
form or cut in cubes, or powdered milk which has
been fermented with L. acidophilus before drying.
Many of these product types have a local significance
as dietary adjuncts. In Russia, these products even play
some role as therapeutic agents and have been well
recognized with regard to their medical relevance.
0009 Nonfermented milk containing L. acidophilus is
also offered by some dairies. Such products are usu-
ally produced from standardized milk which is sup-
plemented with a culture concentrate (deep-frozen
pellets or lyophylisate) of L. acidophilus under cooled
conditions, followed by stirring before filling into
cartons or beakers. Some of these products are also
fortified with fat-soluble vitamins (A, D, E), water-
soluble vitamins (thiamin), and trace elements (iron).
While a pronounced metabolic activity of the L. acid-
ophilus strains is desired for all those products which
are produced by fermentation, storage-resistant but
not fast-growing cultures (strains) are needed for the
manufacture of ‘sweet’ (nonfermented) Acidophilus
milk in order not to alter the sensory properties
during storage.
0010The sensory characteristics of nonfermented
‘sweet’ Acidophilus milk are comparable with
regular milk; those of fermented Acidophilus milk
(mesophilic varieties) are similar to those of regular
cultured or sour milks which are manufactured using
a butter flavor-producing mesophilic culture, since
almost no acetaldehyde, which is typical for yogurt,
but some diacetyl-based butter aroma is generated
during fermentation caused by citrate-fermenting
mesophilic lactic acid bacteria. Since L. acidophilus
possesses alcohol dehydrogenase activity, which is
capable of reducing acetaldehyde, only low levels of
this compound are found in the corresponding prod-
ucts. Thus, yogurt-related dairy products (thermo-
philic varieties) containing L. acidophilus often
exhibit a milder and less acidic taste than classical
yogurt, i.e., that manufactured by a cofermention of
S. thermophilus and L. delbrueckii subsp. bulgaricus.
Sensorically, this classical yogurt is dominated by
acetaldehyde which introduces some kind of astrin-
gent characteristic and typical sharpness. Moreover,
many classical yogurt cultures, in particular owing to
the Lactobacillus component of the culture, exhibit a
continued acidification activity even under cooled
conditions on the shelves of retail shops. Besides the
sensory changes, this ‘overacidification’ can also lead
to textural problems (syneresis, whey separation).
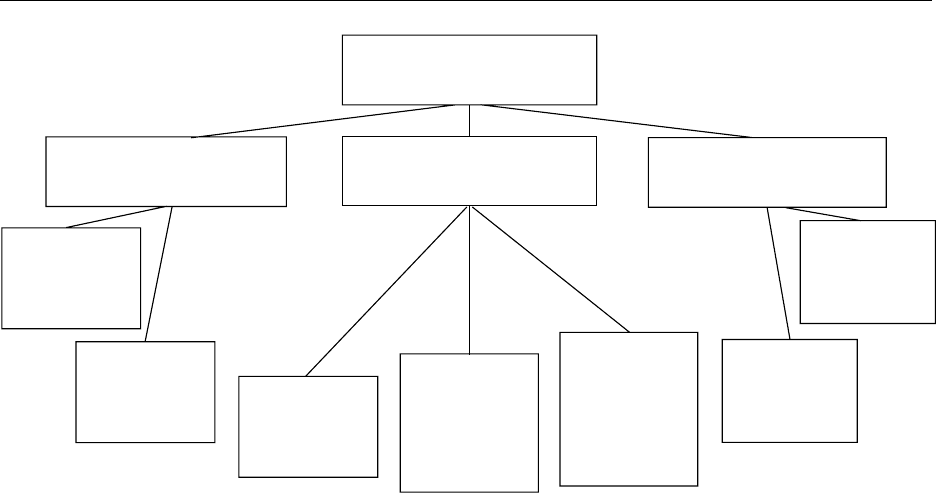
Acidophilus products
Single-culture products
Other products
'Sweet'
Acidophilus milk
Fermented
Acidophilus milk
(liquid, dried)
Acidophilus milk
cofermented with
mesophilic lactic
acid bacteria
Fermented milk
manufactured with
L. acidophilus and
other thermophilic
lactic acid bacteria
and/or
bifidobacteria
Fermented milk
manufactured with
L. acidophilus and
yeasts,
facultatively plus
meso- or
thermophilic lactic
acid bacteria
Fermented
Acidophilus paste
or cubes
(enriched with
sugar), texturized
Soymilk-based
Acidophilus milk
Multiple-culture products
fig0002 Figure 2 Survey of the diversity of food products containing Lactobacillus acidophilus.
ACIDOPHILUS
MILK 5
Obviously because of these effects, preferences of
consumers for the milder yogurts with L. acidophilus
have been observed in many countries.
0011A completely different group of Acidophilus prod-
ucts are the pharmaceutical preparations containing
L. acidophilus. Capsules, suppositories, and soluble
powders packaged in sachets are enriched with bac-
terial lyophylisates and used for therapeutic purposes.
They are marketed in pharmacies and health stores.
Bacterial Viable Count and Bacterial
Stability of Acidophilus milk
0012Although milk is a substrate containing almost a
universal array of nutrients, it does not fully meet
the growth requirements of L. acidophilus. For this
purpose, additives and growth promoters consisting
of a mixture of natural compounds which support
and enhance bacterial growth are recommended for
supplementation of the fermentation milk by most
culture suppliers. Usually, they are added to the milk
base in small amounts, before inoculation. In add-
ition, the use of multicomponent cultures offers the
advantage of inducing synergistic effects among
the bacterial microflora which may also positively
influence the propagation rate and the stability of
the bacteria.
0013According to legal aspects and to consumer expect-
ations, products labeled as Acidophilus milk or as
containing L. acidophilus necessarily have to contain
a significant number of these microorganisms. In this
context, a group of experts of the International Dairy
Federation has recommended that L. acidophilus
shall be detected in such products at a level of at
least 1 million CFU ml
1
or g, at their sell-by dates.
0014Recently, studies performed in several countries
have shown that many commercially available prod-
ucts can meet this limit, but with a considerable
number of products a decrease in the L. acidophilus
counts has been observed during a storage period of
approximately 3–5 weeks. Due to the fact that the
expression of beneficial effects is based on a high
number of active bacteria, a high viable count and
pronounced bacterial stability have become import-
ant goals in product development and optimization.
0015Viable counts of L. acidophilus-containing dairy
products are usually enumerated by culture methods
based on plate count techniques with media designed
for culturing lactic acid bacteria (e.g., MRS, Rogosa
agar, TGV agar; for details see Table 1). To enhance
the discriminatory power of these media (this is of
particular relevance in the examination of products
which contain a mixed microflora), media are modi-
fied by slight acidification and/or by supplementation
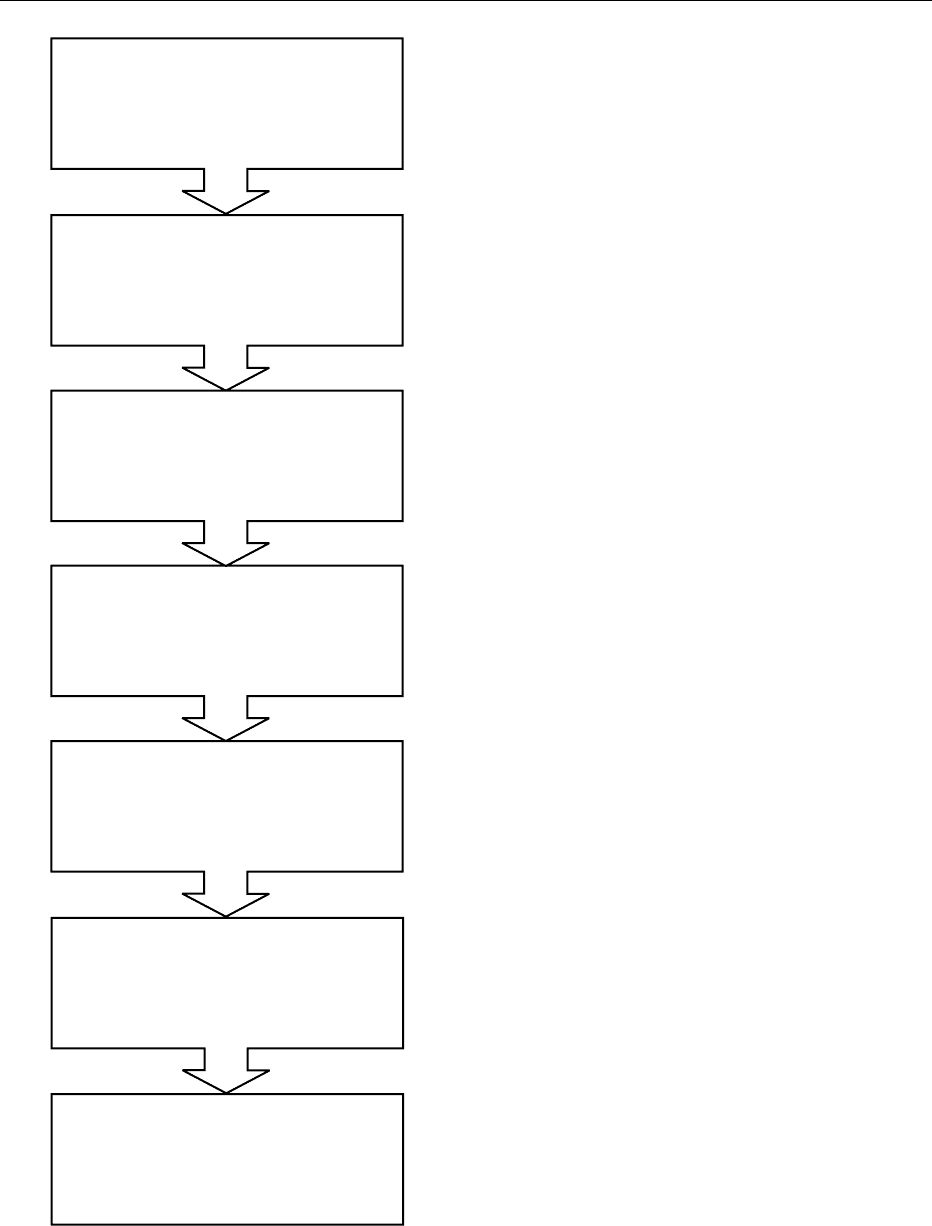
Process milk with variable dry matter
and fat contents
Dual-step homogenization
with 15 000-20 000 kPa at 65-70 8C
Heat treatment for 5-10 min
at 90-95
8C
Cooling to fermentation temperature
(varies from 22 to 30
8C)
Inoculation with L. acidophilus and a
mesophilic starter culture
Fermentation period at
defined temperature (varies from 22 to 30
8C)
Stirring, cooling to 10-12
8C
and filling into packaging units
(beakers, cartons, etc.)
fig0003 Figure 3 General production steps of the manufacture of
fermented Acidophilus milk using a combined fermentation with
Lactobacillus acidophilus and mesophilic lactic acid starter culture.
Data compiled after various manufacturers’ recommendations.
6
ACIDOPHILUS
MILK

with antibiotics (e.g., vancomycin at different levels)
or with other selective agents (cellobiose, conjugates
with chromogenic indicator dyes, esculin, etc.). In
many cases, the parallel use of different media select-
ive for each of the bacterial components is necessary
to allow the reliable microbiological monitoring of
these Acidophilus products. Moreover, microscopical
verification of isolates harvested from the different
media usually completes their routine assessment.
Although a number of media and methodologies
have been described in the literature, no official
standard method is available yet.
See also: Fermented Milks: Types of Fermented Milks;
Functional Foods; Lactic Acid Bacteria; Probiotics;
Yogurt: The Product and its Manufacture; Yogurt-based
Products; Dietary Importance
Further Reading
Fonde
´
n R, Mogensen G, Tanaka R and Salminen S (2000)
Effect of culture-containing dairy products on intestinal
microflora, human nutrition and health – current know-
ledge and future perspectives. In: IDF Bulletin, no. 352,
pp. 5–30, Brussels: International Dairy Federation.
Hammes WP (1995) The genus Lactobacillus. In: Wood JB
and Holzapfel WH (eds) The Genera of Lactic Acid
Bacteria, pp. 19–54. London: Blackie Academic & Pro-
fessional.
Kanbe M (1992) Uses of intestinal lactic acid bacteria and
health. In: Nakazawa Y amd Hosono A, (eds) Functions
of Fermented Milk. Challenges for the Health Sciences,
pp. 289–304. London: Elsevier Applied Science.
Kneifel W and Pacher B (1993) An X-Glu based agar
medium for the selective enumeration of Lactobacillus
acidophilus in yogurt-related milk products. Inter-
national Dairy Journal 3: 277–291.
Lee YK, Nomoto K, Salminen S and Gorbach SL (1999)
Handbook of Probiotics. New York: John Wiley.
Mital BK and Garg SK (1992) Acidophilus milk products:
manufacture and therapeutics. Food Reviews Inter-
national 8: 347–389.
Tamime AY and Robinson RK (1999) Yoghurt Science and
Technology. Cambridge: CRC, Woodhead Publishing.
ACIDS
Contents
Properties and Determination
Natural Acids and Acidulants
Properties and Determination
J D Dziezak, Dziezak & Associates, Ltd., Hoffman
Estates, IL, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 In very general terms, an acid is a compound that
contains or produces hydrogen ions in aqueous solu-
tions, has a sour taste, and turns blue litmus paper
red. A more comprehensive definition, given by the
US chemist G.N. Lewis, states that acids are sub-
stances that can accept an electron pair or pairs, and
bases are substances that can donate an electron pair
or pairs. This definition, applicable to both non-
aqueous and aqueous systems, requires that an acid
be either a positive ion or a molecule with one or
more electron-deficient sites with respect to a corres-
ponding base.
0002The definition most widely used to describe acid–
base reactions in dilute solution is one that was pro-
posed independently by two scientists in 1923 – the
Danish chemist J.N. Br
Ønsted and the US chemist
T.M. Lowry. The Br
Ønsted–Lowry theory defines an
acid as a proton donor, that is, any substance (charged
or uncharged) that can release a hydrogen ion or
proton. A base is defined as a proton acceptor
or any substance that can accept a hydrogen ion or
proton.
tbl0001 Table 1 Media used for culturing Lactobacillus acidophilus
Media References
MRS agar Lactobacillus agar according to De Man JD,
Rogosa M and Sharpe ME (1960) Journal of
Applied Bacteriology 23: 130–135.
Rogosa agar Lactobacillus selective agar according to
Rogosa M Mitchell and JA, Wiseman RF (1951)
Journal of Bacteriology 62: 132–133.
TGV agar Agar medium according to Galesloot T,
Hassing F and Stadhouders J (1961)
Netherlands Milk and Dairy Journal 15: 127–150.
ACIDS/Properties and Determination 7

tbl0001 Table 1 Structure, ionization constant, pK
a
, and key physical and chemical properties of acidulants
a
Acid Structure Ionization constant(s) pK
a
Physical form Melting
point (
C)
Solubility
(gper100 mlof water)
Hygroscopicity Taste characteristics
Acetic acid
CH
3
COOH
1.76 10
5
at 25
C 4.76 Clear, colorless
liquid
8.5 Soluble na Tart and sour
Adipic acid
COOH
COOH
CH
2
CH
2
CH
2
CH
2
K
1
¼ 3.71 10
5
4.43 Crystalline
powder
152 1.9 g at 20
C Low level of
hygroscopicity
Smooth lingering tartness;
complements grape flavors
K
2
¼ 3.87 10
6
at 25
C
5.41 83 g at 90
C
Citric acid
COOH
COOH
COOH
CH
2
HO C
CH
2
K
1
¼ 7.10 10
4
3.14 Crystalline
powder
Moderately
hygroscopic
Tart; delivers a ‘burst’
of flavor
K
2
¼ 1.68 10
5
4.77
K
3
¼ 6.4 10
7
at 25
C
6.39
Anhydrous 153 181 g at 25
C
Hydrous 135–153 208 g at 25
C
Fumaric acid
COOH
COOH
CH
HC
K
1
¼ 9.30 10
4
3.03 White granules
or crystalline
powder
286 0.5 g at 20
C Nonhygroscopic Tart; has an affinity for
grape flavors
K
2
¼ 3.62 10
5
at 18
C
4.44 9.8 g at 100
C
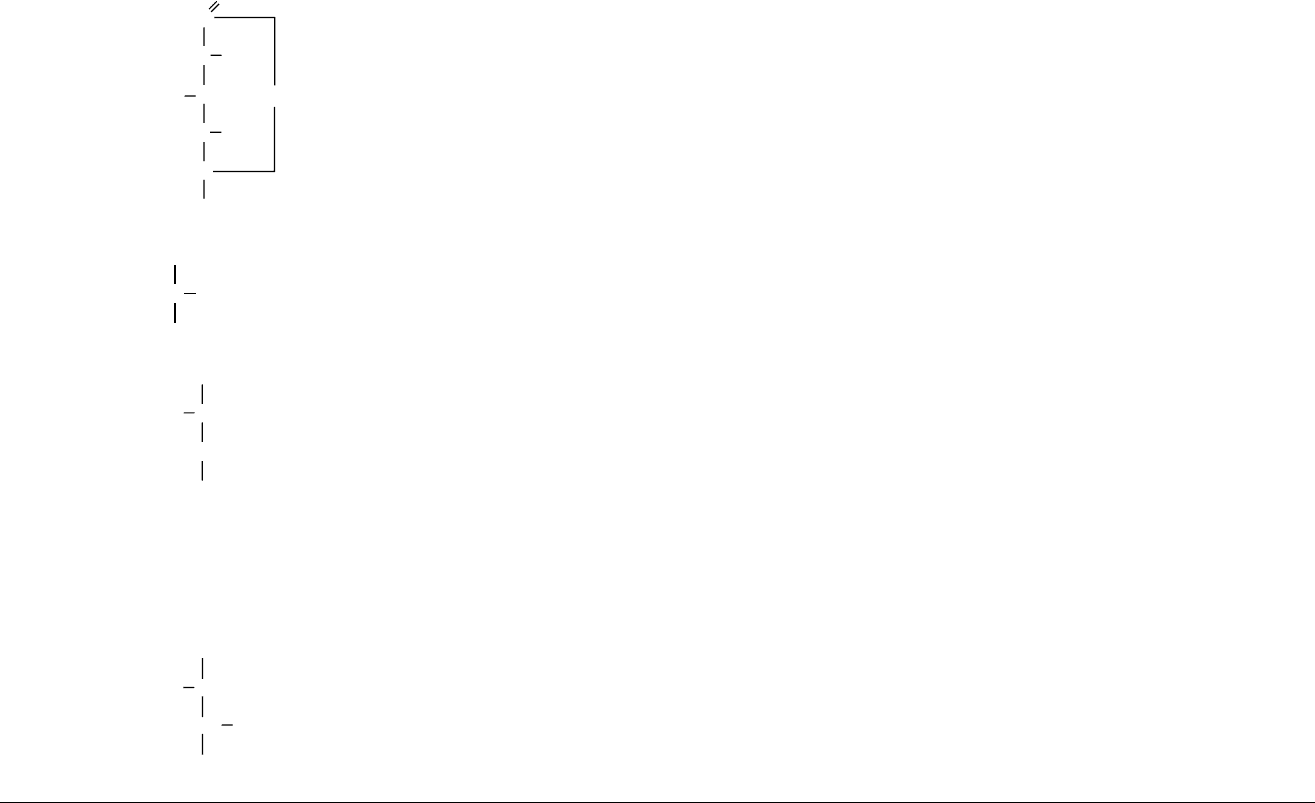
Glucono-d-lactone
C
O
HC OH
O
HO CH
HC
HC
OH
CH
2
OH
1.99 10
4
(for gluconic acid)
3.7 White crystalline
powder
153 59 g at 25
C Nonhygroscopic Neutral taste with acidic
aftertaste, when hydrolyzed
Lactic acid
HC OH
CH
3
COOH
1.37 10
4
at 25
C 3.86 Liquid; also
available in
dry form
16.8 Very soluble na Acrid
Malic acid
COOH
COOH
HO CH
CH
2
K
1
¼ 3.9 10
4
3.40 Crystalline
powder
132 62 g at 25
C Nonhygroscopic Smooth tartness
K
2
¼ 7.8 10
6
at 25
C
5.11
Phosphoric acid K
1
¼ 7.52 10
3
2.12 Liquid Very soluble
in hot water
na Acrid
K
2
¼ 6.23 10
8
7.21
K
3
¼ 2.2 10
13
12.67
K
1
and K
2
at 25
C;
K
3
at 18
C
Tartaric acid
COOH
HO CH
HC OH
COOH
K
1
¼ 1.04 10
3
2.98 Crystalline
powder
168–170 147 g at 25
C Nonhygroscopic Extremely tart; augments
fruit flavors, especially
grape and lime
K
2
¼ 4.55 10
5
at 25
C
4.34
na, not applicable.
a
Adapted with permission from Food Technology, Acidulants: Ingredients that do more than meet the acid test. 44(1): 76–83. Institute of Food Technologists, Chicago, Illinois, USA.

0003 This article discusses the physicochemical proper-
ties of acids and describes several methods for their
analysis.
Strong Versus Weak Acids
000 4 The strength of a BrØnsted–Lowry acid depends on
how easily it releases a proton or protons. In strong
acids, owing to their weaker internal hydrogen
bonds, the protons are loosely held. As a result, in
aqueous solutions, almost all of the acid reacts with
water, leaving only a few unionized acid molecules in
the equilibrium mixture. The reaction takes place
according to eqn (1):
HA þ H
2
O Ð H
3
O
þ
þ A
ð1Þ
In this equation, HA represents the undissociated
acid, H
3
O
þ
the hydronium ion formed when a proton
combines with one molecule of water, and A
the
conjugate base of HA.
0005 Unlike strong acids, weak acids exist largely in the
undissociated state when mixed with water, since
only a small percentage of their molecules interact
with water and dissociate. Most acids found in
foods, including acetic, adipic, citric, fumaric, malic,
phosphoric and tartaric acids, and glucono-d-lactone,
are classified as weak or medium strong acids.
Physicochemical Properties
0006 Physicochemical properties, including the ionization
constant, pH, the apparent dissociation constant
(pK
a
) and buffering capacity, are discussed below
and are listed in Table 1.
Ionization Constant
0007 The tendency for an acid or acid group to dissociate is
defined by its ionization constant, also denoted as
pK
a
. The ionization constant, given at a specified
temperature, is expressed as:
K
a
¼
½H
3
O
þ
½A
½HA
,
ð2Þ
where the brackets designate the concentration in
moles per liter. The ionization constant is a measure
of acid strength: the higher the K
a
value, the greater
the number of hydrogen ions liberated per mole of
acid in solution and the stronger the acid.
0008 Acids with more than one transferable hydrogen
ion per molecule are termed ‘polyprotic’ acids.
Monoprotic or monobasic acids are those that can
liberate one hydrogen ion, such as acetic acid and
lactic acid. Those containing two transferable
hydrogen ions are called diprotic or dibasic acids
and include, for example, adipic acid and fumaric
acid. Acids such as citric acid and phosphoric acid,
which have three transferable hydrogens, are called
triprotic or tribasic acids. Ionization of polyprotic
acids occurs in a stepwise manner with the transfer
of one hydrogen ion at a time. Each step is character-
ized by a different ionization constant.
pH
0009Measurement of acidity is an important aspect of
ascertaining the safety and quality of foods. Such
measurements are given in terms of pH, which is
defined as the negative logarithm of the hydronium
ion concentration (strictly, activity):
pH ¼ log
10
1
½H
3
O
þ
¼log
10
½H
3
O
þ
:
ð3Þ
0010The lower the pH value, the higher the hydrogen
ion concentration associated with it. A pH value of
less than 7 indicates a hydrogen ion concentration
greater than 10
7
M and an acidic solution; a pH
value of more than 7 indicates a hydrogen ion concen-
tration of less than 10
7
M and a basic solution.
When the hydronium and hydroxide ions are equal
in concentration, the solution is described as neutral.
(See pH – Principles and Measurement.)
0011It is also important to note that, because the pH
scale is logarithmic, a difference of one pH unit rep-
resents a 10-fold difference in hydrogen ion concen-
tration.
p
K
a
0012The term pK
a
is defined as the negative logarithm of
the dissociation constant:
pK
a
¼ log
10
1
K
a
¼log
10
K
a
: ð 4Þ
0013The pK
a
corresponds to the pH value at the mid-
point of a titration curve developed when one equiva-
lent of weak acid is titrated with base, and the pH
resulting from each incremental addition of base is
plotted against the equivalents of hydroxide ions
added.
0014The pH of a system is at the pK
a
when the concen-
trations of acid (HA) and conjugate base (A
) are
equal. At the pK
a
and, to a lesser extent, in the area
extending to within one pH unit on either side of the
pK
a
, the system resists changes in pH resulting from
addition of small increments of acid or base. In other
words, at the pK
a
, acids and their salts function as
buffers.
0015The number of pK
a
s that an acid has depends on
the number of hydrogen ions it can liberate. Mono-
protic acids have a single pK
a
, whereas di- and tri-
protic acid have two and three pK
a
s, respectively.
10 ACIDS/Properties and Determination

001 6 Strong acids have low pK
a
values, and strong bases
have high pK
a
values.
Buffering Capacity
0017 A solution of a weak acid (or a weak base) and its
corresponding salt is called a buffer solution. In these
systems, the hydronium ion content is not signifi-
cantly changed when a small amount of acid or base
is added to that solution. The reason that buffer
solutions resist appreciable changes in pH can be
best illustrated by an example. If a small amount of
hydrochloric acid is added to a buffer solution com-
posed of acetic acid and sodium acetate, the protons
from the hydrochloric acid would associate with the
acetate ions to form unionized molecules of acetic
acid. As the newly formed acid molecules ionize, the
equilibrium would shift towards forming more
hydronium ions (eqn (1)). This would result in only
a very slight increase in pH.
0018 Similarly, the addition of a small amount of sodium
hydroxide to the same buffer solution would have
little effect on pH. Hydroxide ions from the sodium
hydroxide would combine with hydronium ions
in the equilibrium mixture, forming undissociated
molecules of sodium hydroxide. More of the acid
molecules would then dissociate to replace the hydro-
nium ions lost; though a new equilibrium system
would be created, it would produce only a minimal
effect on pH.
0019 The quantity of acid or base that a buffer solution is
capable of consuming before a change in pH is real-
ized is termed the ‘buffering capacity.’ The buffering
capacity is defined as the number of moles of strong
acid or base required to increase the pH by one unit in
1 l of buffer solution. The buffering capacity of a
solution is greatest at its pK
a
value where the concen-
trations of acid and conjugate base are equal.
Analytical Methods
0020 Quantitative determinations of acidity play an im-
portant role in ensuring food product quality and
stability. Information obtained on acid levels can
help in detecting cases of food adulteration, moni-
toring fermentation processes, and evaluating the
organoleptic properties of fermented foods. pH
determination, titratable acidity, chromatographic
methods, and capillary electrophoresis are proced-
ures commonly employed by the food industry to
determine food acids. (See Adulteration of Foods:
Detection.)
pH Determination
0021 pH can be measured by two techniques: colorimetric
and potentiometric. The colorimetric method involves
adding a suitable indicator to a solution and matching
the color of the solution to a standard solution con-
taining the same indicator. This method can estimate
pH to the nearest 0.1 pH unit.
0022A more accurate technique and the one most fre-
quently employed, the potentiometric method, uses a
pH meter to determine hydrogen ion concentration.
The two electrodes of the meter – a calomel reference
electrode and a glass indicator electrode – are im-
mersed in the solution, of known temperature,
whose pH is to be measured. The electrode potential
of the indicator electrode is linearly related to changes
in hydrogen ion concentration and therefore pH.
Titratable Acidity
0023The total concentration of acid in a solution can be
determined by titration. The titration process is per-
formed by placing in a flask a known volume of acid
solution whose concentration is unknown. To the
flask, a few drops of indicator, e.g., phenolphthalein,
which is colorless in acid solutions and pink in basic
solutions, is introduced. A base solution of known
concentration is then gradually added until the acid
is completely neutralized. This point is indicated
when the solution permanently changes color. The
concentration of acid can then be calculated from
the volume of base solution used.
0024The value obtained, called titratable acidity, is an
estimate of the total acid in the solution. It accounts
for both the free hydronium ions present in the equi-
librium mixture and the hydrogen ions released from
undissociated acid molecules. For weak acids, the
titratable acidity is different from the actual acidity
(hydrogen ion concentration), since these compounds
exist largely in the undissociated state in solution. For
strong acids, however, titratable acidity and actual
acidity are virtually the same, since strong acids and
their salts are completely ionized in solution.
Chromatographic Methods
0025Gas chromatography (GC) and high-performance
liquid chromatography (HPLC) have almost entirely
replaced paper and thin-layer chromatography as
methods for identifying and quantifying food acids.
0026Gas Chromatography GC has been used to analyze
organic acids in fruit and fruit juice. Analysis involves
preparing volatile derivatives such as methyl esters of
the organic acids, prior to their injection into the gas
chromatograph. Derivatives are chromatographed on
a nonpolar stationary phase column and detected by a
flame ionization detector.
0027By use of GC, malic acid has been shown to be a
major constituent of many fruits, including apples,
pears, grapes, peaches, and nectarines, and significant
ACIDS/Properties and Determination 11

levels of citric acid have been found in citrus fruits
such as orange, lemon, and grapefruit, and in non-
citrus fruits, including pears, nectarines, cherries,
and strawberries. (See Chromatography: Gas Chro-
matography.)
0028 High-performance Liquid Chromatography HPLC
is used more extensively than GC to determine or-
ganic acids because the technique requires little or no
chemical modification to separate these nonvolatile
compounds. Separation is usually done on either
a reversed-phase C8 or C18 column or a cation-
exchange resin column operated in the hydrogen
mode. Acids are detected by either refractive index
(RI) or ultraviolet (UV) detectors. RI detection re-
quires prior removal of any sugars present that poten-
tially can interfere with quantification; sugar removal
is not required for UV detection at 220–230 nm.
0029 Adulteration of a commercial cranberry juice drink
was detected using HPLC when the test yielded dif-
ferent results for organic acids, sugars, and anthocya-
nin pigments than those obtained for a standard juice
drink. Atypical citric and/or malic acid contents and
presence of a natural colorant, probably grape skin
extract, confirmed that the drink was adulterated.
0030 In wine-making, HPLC is used to monitor concen-
trations of tartaric, malic, succinic, citric, lactic, and
acetic acids, which contribute tartness and stability to
the finished product. A common approach involves
using a column containing a strong cation exchange
resin and eluting the sample with dilute sulfuric
acid; the eluant is then analyzed for acids by RI detec-
tion. This column has the additional advantage of
permitting the simultaneous detection and quantifica-
tion of ethanol and the monitoring of wine for adul-
teration with methanol. Organic acids in wine can
also be separated using ion chromatography with a
conductivity detector. (See Chromatography: High-
performance Liquid Chromatography.)
Capillary Electrophoresis
0031 A relatively new technique, capillary electrophoresis,
is also useful for separating and quantifying organic
acids in food systems. This technique utilizes an elec-
trical field to separate molecules on the basis of their
charge and size. Small volumes of sample, usually a
few nanoliters, are injected on to a fused silica capil-
lary tube, which is usually less than 1 m in length and
50 mm in internal diameter. The ends of the tube are
placed in electrolyte reservoirs containing electrodes.
A voltage in the range of 20–30 kV is delivered to the
electrodes by a power supply and causes the charged
molecules to move. Because organic acids are nega-
tively charged, they migrate away from more neutral
or positively charged molecules, such as sugars and
phenols, respectively. Acids are detected by a UV
detector, and the signal is sent to a data collector.
The resulting separation is graphically represented
as an electrophoregram.
Enzymatic Analysis
0032Enzyme assays provide another means of analyzing
acids. For example, an enzymatic assay of l-malic
acid uses an NAD(P)-linked malic enzyme and in-
volves spectrophotometrically measuring the absorb-
ance of NADPH, a reaction product, at 340 nm.
See also: Adulteration of Foods: Detection;
Chromatography: High-performance Liquid
Chromatography; Gas Chromatography; pH – Principles
and Measurement
Further Reading
Fennema OR (ed.) (1979) Food Chemistry. Principles of
Food Science, Part 1. New York: Marcel Dekker.
Lehninger AL (1975) Biochemistry, 2nd edn. New York:
Worth.
Macrae R (1988) HPLC in Food Analysis. London:
Academic Press.
Pomeranz Y and Meloan CE (1978) Food Analysis: Theory
and Practice. Westport: AVI.
Suye S, Yoshihara N and Shusei I (1992) Spectrophotomet-
ric determination of l-malic acid with a malic enzyme.
Bioscience, Biotechnology, and Biochemistry 56(9):
1488–1489.
Natural Acids and Acidulants
J D Dziezak, Dziezak & Associates, Ltd., Hoffman
Estates, IL, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Acids, or acidulants as they are also called, are com-
monly used in food processing as flavor intensifiers,
preservatives, buffers, meat-curing agents, viscosity
modifiers, and leavening agents. This article discusses
the functions that acidulants have in food systems and
reviews the more commonly used food acidulants.
Functions of Acidulants
0002The reasons for using acidulants in foods are numer-
ous and depend on what the food processor hopes to
accomplish. As outlined above, the principal reasons
12 ACIDS/Natural Acids and Acidulants

for incorporating an acidulant into a food system
are flavor modification, microbial inhibition, and
chelation.
Flavor Modification
0003 Sourness or tartness is one of the five major taste
sensations: sour, salty, sweet, bitter, and umami (the
most recently determined). Unlike the sensations of
sweetness and bitterness, which can be developed by a
variety of molecular structures, sourness is evoked
only by the hydronium ion of acidic compounds.
000 4 Each acid has a particular set of taste characteris-
tics, which include the time of perceived onset of
sourness, the intensity of sourness, and any lingering
of aftertaste. Some acids impart a stronger sour note
than others at the same pH. As a general rule, weak
acids have a stronger sour taste than strong acids at
the same pH because they exist primarily in the undis-
sociated state. As the small amount of hydronium
ions is neutralized in the mouth, more undissociated
acid (HA) molecules ionize to replace the hydronium
ions lost from equilibrium (eqn (1)). The newly
released hydronium ions are then neutralized until
no acid remains. Taste characteristics of the acid are
an important factor in the development of flavor
systems.
HA þ H
2
O ! H
3
O
þ
þ A
ð1 Þ
0005 As pH decreases, the acid becomes more undisso-
ciated and imparts more of a sour taste. For example,
the intense sour notes of lactic acid at pH 3.5 may be
explained by the fact that 70% of the acid is undisso-
ciated at this pH, compared with 30% for citric acid.
In addition to sourness, acids have nonsour charac-
teristics such as bitterness and astringency, though
these are less perceptible. At pH values between 3.5
and 4.5, lactic acid is the most astringent. Acids also
have the ability to modify or intensify the taste sen-
sations of other flavor compounds, to blend unrelated
taste characteristics, and to mask undesirable after-
tastesbyprolongingatartnesssensation.Forexample,
in fruit drinks formulated with low-caloric sweeten-
ers, acids mask the aftertaste of the sweetener and
impart the tartness that is characteristic of the natural
juice. In another example, in substitutes for table salt,
acids remove the bitterness from potassium chloride
and provide the salty taste of sodium chloride. Other
acids, such as glutamic and succinic acids, possess
flavor-enhancement properties. (See Flavor (Flavour)
Compounds: Structures and Characteristics; Sensory
Evaluation: Taste.)
000 6 Because acids are rarely found in nature as a single
acid, the combined use of acids simulates a more
natural flavor. Two acids that are frequently blended
together are lactic and acetic.
Microbial Inhibition
0007Acidulants act as preservatives by retarding the
growth of microorganisms and the germination of
microbial spores which lead to food spoilage. The
effect is attributed to both the pH and the concen-
tration of the acid in its undissociated state. It is
primarily the undissociated form of the acid which
carries the antimicrobial activity: as the pH is
lowered, this helps shift the equilibrium in favor of
the undissociated form of the acid, thereby leading to
more effective antimicrobial activity. The nature of
the acid is also an important factor in microbial inhib-
ition: weak acids are more effective at the same pH in
controlling microbial growth. Acids affect primarily
bacteria because many of these organisms do not grow
well below about pH 5; yeasts and molds, in compari-
son, are usually acid-tolerant. (See Spoilage: Bacterial
Spoilage; Molds in Spoilage; Yeasts in Spoilage.)
0008In fruit- and vegetable-canning operations, the
combined use of heat and acidity permits sterilization
and spore inactivation to be achieved at lower tem-
peratures; this minimizes the degradation of flavor
and structure that generally results from processing.
(See Canning: Principles.)
0009Acidification also improves the effectiveness of
antimicrobial agents such as benzoates, sorbates,
and propionates. For example, sodium benzoate –
an effective inhibitor of bacteria and yeasts – does
not exert its antimicrobial activity until the pH is
reduced to about 4.5. (See Preservation of Food.)
Blends of acids act synergistically to inhibit microbial
growth. For example, lactic and acetic acids have
been found to inhibit the outgrowth of heterofermen-
tative lactobacilli.
Chelation
0010Oxidative reactions occur naturally in foods. They
are responsible for many undesirable effects in the
product, including discoloration, rancidity, turbidity,
and degradation of flavor and nutrients. As catalysts
to these reactions, metal ions such as copper, iron,
manganese, nickel, tin, and zinc need to be present in
only trace quantities in the product or on the process-
ing machinery. (See Oxidation of Food Components.)
0011Many acids chelate the metal ions so as to render
them unavailable; the unshared pair of electrons in
the molecular structure of acids promotes the com-
plexing action. When used in combination with
antioxidants such as butylated hydroxyanisole, butyl-
ated hydroxytoluene, or tertiary butylhydroquinone,
acids have a synergistic effect on product stability.
Citric acid and its salts are the most widely used
chelating agents. (See Antioxidants: Natural Antioxi-
dants; Synthetic Antioxidants.)
ACIDS/Natural Acids and Acidulants 13
