Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

scarce (e.g., deserts) or seasonal (e.g., arctic). North-
ern species such as polar bears and reindeer build up
substantial depots of fat during the summer to pro-
vide reserves of nutrients during the winter. Such
species thus have substantial seasonal fluctuations in
the amount of adipose tissue in their bodies. Add-
itional reserves of adipose tissue are also accumulated
during pregnancy in most species to help support the
development of the fetus during the later stages of
pregnancy and to facilitate milk production. The use
of adipose tissue lipid is very important during early
lactation in dairy cows, for example, in which appe-
tite increases more slowly than milk production at the
beginning of lactation. It is also important for milk
production in some species of bears and seals that fast
during lactation.
0004 It is now apparent that adipose tissues are not
solely a store of fat. Subcutaneous adipose tissue will
act as insulation; adipose depots in the eye socket may
have a protective function. More importantly perhaps,
adipose tissue produces a number of biologically
active substances, e.g., prostaglandins, insulin-like
growth factor 1 and binding proteins, adipsin, cyto-
kines (e.g., tumor necrosis factor a), estrogens (pri-
marily estrone), and leptin. Some of these substances
are probably important for adipose tissue function
and development, but some have other roles. Adipose
tissue is the major source of estrogens in postmeno-
pausal women. The mammary gland grows in a bed
of adipose tissue and is thought to require factors
secreted by adipose tissue for its development.
Lymph nodes are located in adipose tissue depots
and in some species (e.g., guinea-pigs), at least, there
is an interaction between adipocytes and lymphoid
cells. Adipose tissue may have another role in defense
systems of the body as it secretes adipsin and several
other proteins involved in an alternative pathway of
complement production. Another important protein
produced by adipocytes is the cytokine tumor necro-
sis factor-a; production of this factor is normally low,
but it is markedly increased during obesity, when it
appears to play a major role in the development of
insulin resistance in the tissue, and hence noninsulin-
dependent diabetes.
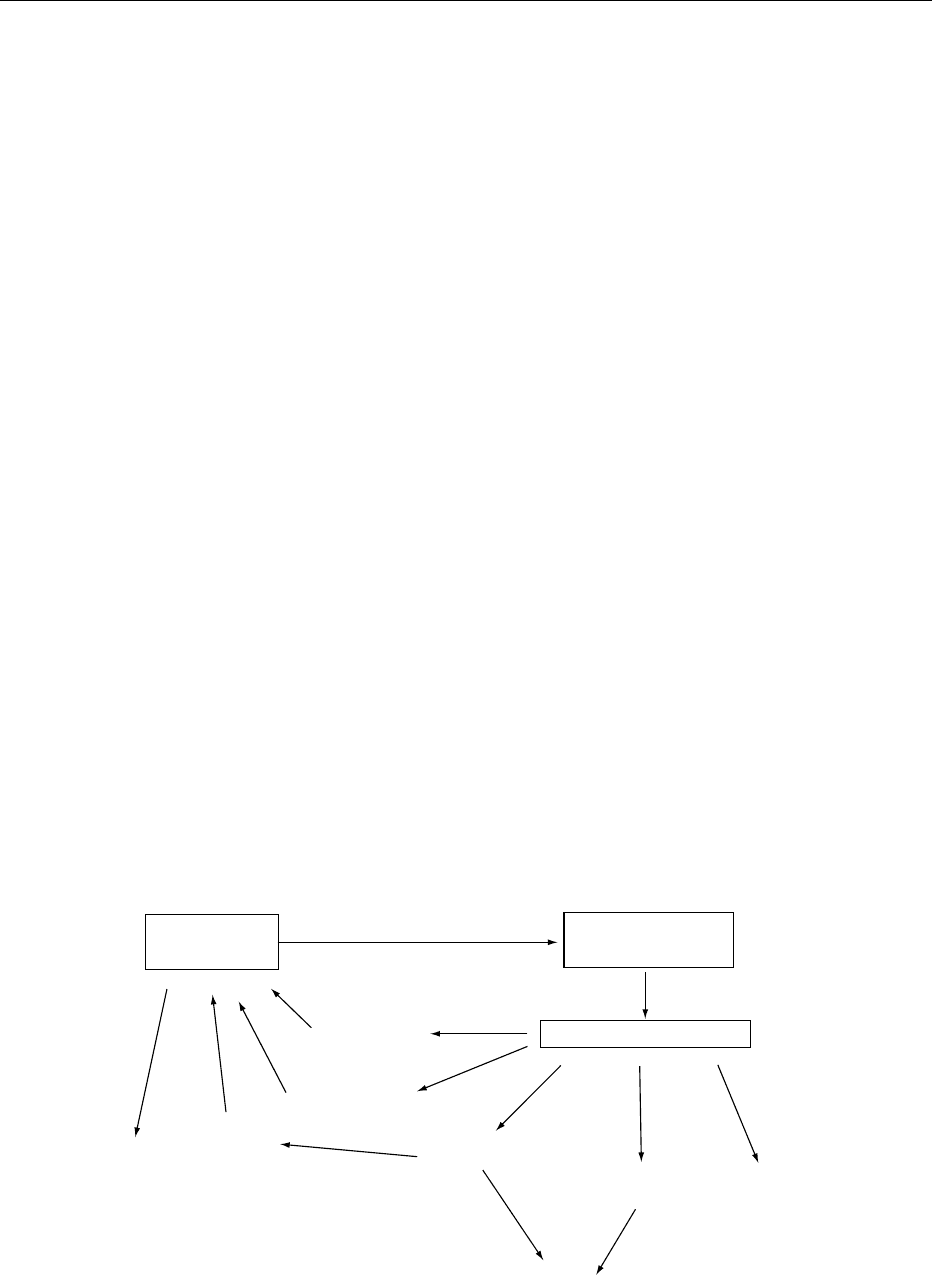
0005Perhaps the most important and interesting protein
secreted by adipocytes is leptin, which has a key role
in appetite control and energy balance (Figure 1).
Leptin was discovered only recently through studies
on the basis of a genetically obese strain of mice (ob/
ob mice); these mice produce a nonfunctional form of
leptin. Leptin is released into the blood and travels to
the brain, where there are leptin receptors in discrete
areas involved in appetite control. Low levels of lep-
tin in the blood increase appetite, whereas adminis-
tration of high doses inhibit appetite. Leptin not only
modulates appetite, but also increases energy expend-
iture, stimulating thermogenesis in brown adipose
tissue, suggesting a key role in the control of energy
balance in the body. Leptin synthesis is regulated
by insulin, glucocorticoids, and catecholamines, but
most interestingly, the concentration of leptin in the
blood in the fed state is proportional to the amount of
fat in the body; this led to the idea that leptin acts as a
‘lipostat,’ matching appetite to adiposity. However,
the leptin concentration in the blood is decreased by
fasting, and leptin is involved in the changes in
secretion of several pituitary hormones during
fasting. Thus, it has been suggested that the major
Adipocyte
LEPTIN
Hypothalamus
LEPTIN RECEPTORS
CNS, pituitary gland
Catecholamines
Insulin
Glucocorticoids
Immune system
(+ve)
(+ve)
(−ve)
Food intake
Thermogenesis
Energy balance
Reproductive
system
fig0001 Figure 1 Leptin production and function. CNS, central nervous system.
24 ADIPOSE TISSUE/Structure and Function of White Adipose Tissue
role of leptin may be in adaptation to fasting and
acting as a signal of too little rather than too much
adipose tissue. Leptin appears to be required for
normal functioning of the immune system and also
for reproductive function. Indeed, a lack of leptin
may well be the main reason for the failure of the
menstrual cycle in anorexics and very lean athletes.
This makes good physiological sense as it insures that
females do not become pregnant, unless they have
adequate reserves of adipose tissue lipid.
0006 Adipose tissue thus has a variety of functions, in
addition to being an energy store. While the accumu-
lation of adipose tissue lipid reserves provides a buffer
against starvation, and some degree of adiposity is
important for the various other functions of the
adipose tissue described above, there is a cost in that
additional body mass decreases speed and agility and
so increases the chance of succumbing to predation.
Thus, in most wild animals for which food is gener-
ally plentiful, there are usually only small amounts of
adipose tissue (predation rather than starvation being
the greatest threat to mortality). In such species, it
seems likely that the leptin system, and probably
other systems, will be acutely tuned to maintain the
minimal amounts of adipose tissue needed. In gen-
eral, it is only species living in environments where
the availability of food is erratic or seasonal that
accumulate large amounts of adipose tissue since,
for these species, starvation is a greater threat than
predation. In such species, the leptin system must be
modulated to allow the accumulation of adipose
tissue lipid. It would also appear that the leptin
system can be readily subverted in humans and also
domestic pets for excess adiposity is becoming a
major problem.
0007 In addition to white adipose tissue, there is also
another form, brown adipose tissue, which differs
morphologically and biochemically, and has an
important role in thermogenesis.
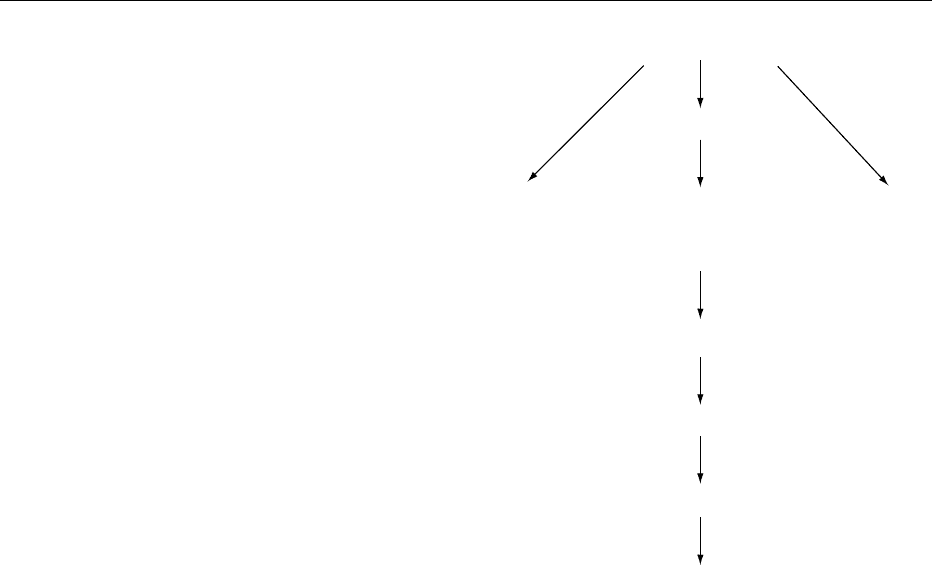
Development of Adipose Tissue
0008 Adipose tissue develops both by accretion of lipid in
adipocytes and by increases in the number of adipo-
cytes. Mature adipocytes are thought to be unable to
divide; rather, they are produced from a pool of pre-
cursor cells within the tissue. The sequence of events
in the formation of mature adipocytes (Figure 2)is
still partly speculative, and much has been gleaned
from studies of certain cell lines (e.g., ob17 and 3T3
L1 cells), which will differentiate and develop into
adipocytes in cell culture. Current thinking envisages
a pluripotent stem cell that can give rise to muscle and
bone cells as well as adipocytes. Once committed to
adipocyte formation, this cell is termed an adipoblast.
This is envisaged (it has not been isolated) as an
undifferentiated cell, devoid of lipid droplets but
able to proliferate. At some point, these cells begin
to differentiate, acquiring, in stages, the enzymes and
other proteins characteristic of adipocytes. Once dif-
ferentiated, these cells can begin to accumulate lipid,
which appears at first as a series of small droplets
within the cell. As these become larger, they fuse to
form the single lipid droplet characteristic of mature
adipocytes. Both differentiating cells and cells with
several small lipid droplets (multilocular phase) are
often referred to as preadipocytes, the term adipocyte
usually being used to describe cells with a single lipid
droplet. Multilocular adipocytes are very similar in
appearance to mature brown adipocytes, and it was
once thought that the brown adipocyte was a stage in
the development of the white adipocytes. It is now
recognized that this view is incorrect, except possibly
for a few special cases (e.g., the perirenal adipose
tissue depot of newborn lambs).
0009Adipocytes begin to appear in the fetus about half
way through gestation, developing in small clumps
around blood vessels. Within a depot, both the
number and size of adipocytes increase in phases
(Figure 3). In addition, it is now clear that devel-
opment is not synchronized in all depots; abdom-
inal depots in general develop earlier than those
Stem cell
Commitment
Muscle-cell
precursors
Bone-cell
precursors
Adipocyte
precursors
(adipoblasts)
Proliferation
Differentiation
Lipid accumulation
Mature, fat-filled
adipocytes
fig0002Figure 2 Adipocyte development.
ADIPOSE TISSUE/Structure and Function of White Adipose Tissue 25
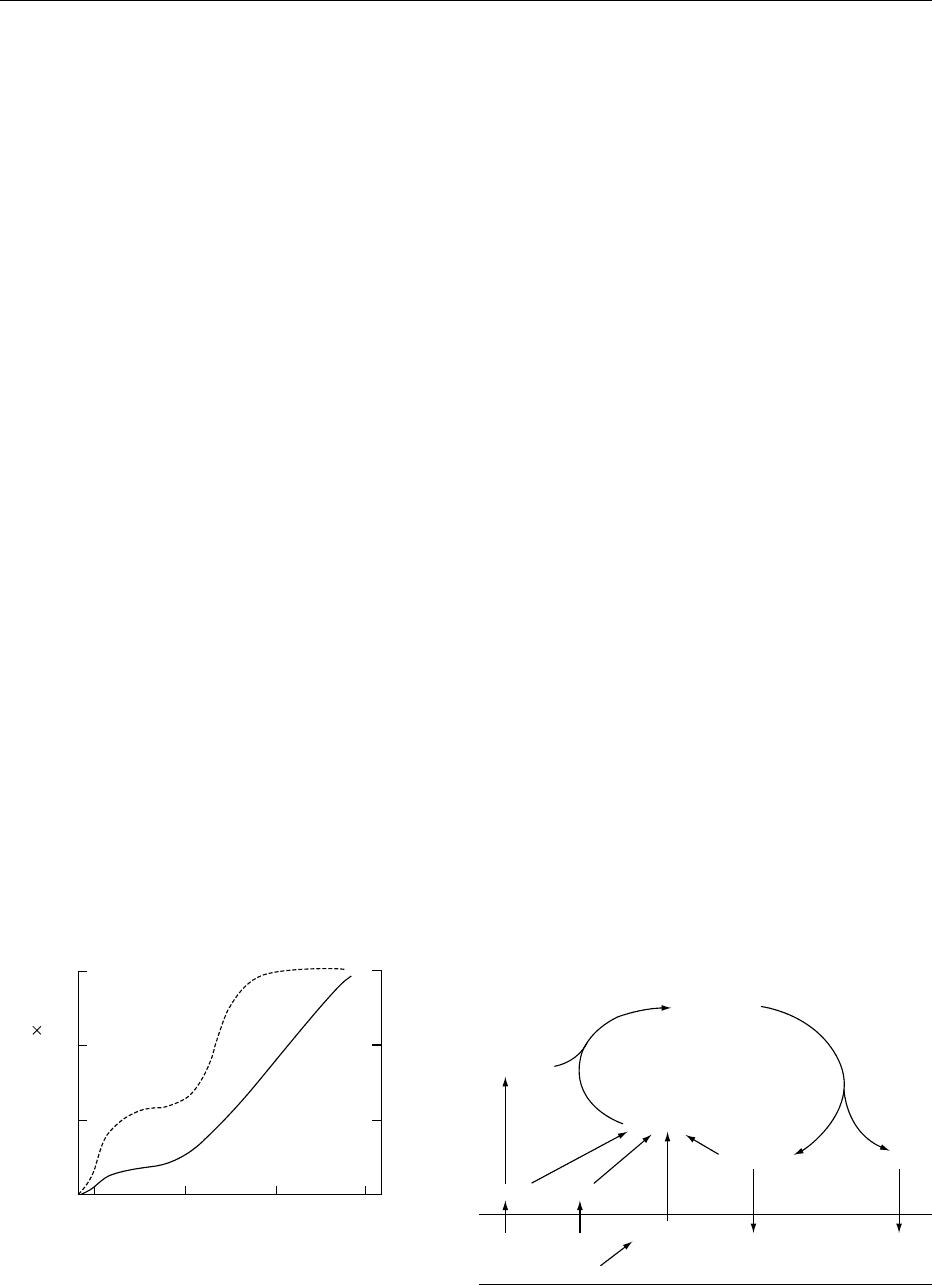
associated with the musculature. In most species, the
fetal stage is a period of active proliferation but little
hypertrophy, so that cells are small at birth (about 10
pl in volume). The suckling period usually results in
rapid hypertrophy and hyperplasia; this is followed
by a more quiescent period when muscle growth pre-
dominates. When the rate of muscle growth begins
to slacken, nutrients are diverted into adipose
tissue, and the fattening phase begins. This phase is
associated with marked hypertrophy, due to lipid
deposition, in most depots and further hyperplasia,
especially in the carcass depots. During the fattening
phase, depot-specific differences in adipocyte size
appear. Adipocytes do not increase in size indefin-
itely; once a maximum is reached (about 1–3 nl,
depending on species), this seems to trigger the for-
mation of new adipocytes from the precursor pool.
The view prevalent in the 1970s that all hyperplasia
occurred in young animals, including humans, is now
thought to be invalid.
0010 A great deal of research has gone into identifying
the hormones and other factors that promote the
proliferation and differentiation of adipocyte precur-
sor cells. At present, the picture is far from clear, in
part because of probable species differences and also
because much of the work has involved the use of cell
lines that do not all appear to have identical hormo-
nal requirements for development. A variety of pep-
tide growth factors (e.g., insulin-like growth factor 1,
fibroblast growth factor, platelet-derived growth
factor, epidermal growth factor) can stimulate pre-
adipocyte proliferation, whereas insulin, thyroid hor-
mones, and glucocorticoids appear to be important
for differentiation of preadipocytes into adipocytes
in a variety of species. Glucocorticoid hormones and
also testosterone are thought to have important roles
in site-specific development of adipose tissue. Deriva-
tives of arachidonic acid (an essential fatty acid) such
as 15-deoxy-D
12,14
-prostaglandin J
2
are also thought
to have a major role in adipogenesis, acting via the
recently discovered (and inappropriately named!) per-
oxisome proliferator-activated receptor-g. Growth
hormone has a complex role, stimulating insulin-like
growth factor 1 production in adipose tissue and hence
proliferation of preadipocytes and in addition may be
required for the cells to become ‘committed’ to differ-
entiation. In addition to positive effectors, tumor ne-
crosis factor a and transforming growth factor b can
inhibit differentiation. In contrast to hyperplasia,
much more is known about the control of hyper-
trophy, for this is dependent on the metabolic rates of
the pathways of lipid synthesis and degradation.
Deposition and Mobilization of Fat
0011The synthesis of triacylglycerol (esterification) re-
quires a supply of fatty acids and glycerol 3-phos-
phate (Figure 4). The latter is mostly synthesized
from glucose. Fatty acids, however, may be synthe-
sized de novo within the cell or obtained from blood
triacylglycerols. Fatty acids can be synthesized in adi-
pocytes from a variety of precursors, including glu-
cose, acetate, lactate, and some amino acids. Glucose
is quantitatively the most important in man and some
laboratory species (e.g., rats, mice), whereas acetate is
most important in ruminants. Liver is also an import-
ant site of fatty acid synthesis in many mammals and
is the major site of fatty acid synthesis in birds (avian
adipocytes have essentially no capacity for fatty acid
synthesis) and also in humans on a typical Western
diet. Some of the fatty acids synthesized in the liver
are incorporated into very-low-density lipoprotein
9
6
3
0
B
200 400 600
1500
1000
500
0
Mean cell volume (pl)
Number of subcutaneous
adipocytes / sheep ( 10
−9
)
Days
fig0003 Figure 3 Developmental changes in adipocyte number (broken
line) and mean cell volume (solid line) of sheep subcutaneous
adipose tissue from 25 days before birth (B) until 600 days after
birth.
ADIPOCYTE
Glycerol 3-
phosphate
Triacylglycerol
Fatty acids
Fatty acids
Fatty acids
Glycerol
Glucose Acetate
BLOOD
VLDL,
Chylomicron
fig0004Figure 4 Pathways for synthesis and hydrolysis of triacyl-
glycerol in adipocytes. VLDL, very-low-density lipoprotein.
26 ADIPOSE TISSUE/Structure and Function of White Adipose Tissue

(VLDL) triaclyglycerols for transport to adipocytes
and other tissues. Dietary fatty acids are also in-
corporated into triacylglycerols in the intestinal cells
and secreted as another form of lipoprotein, called
chylomicrons. Triacylglycerols are essentially insol-
uble in water and so cannot be taken up directly by
adipocytes from blood lipoproteins; thus, the fatty
acids are released by the action of the enzyme lipo-
protein lipase. This enzyme is synthesized in adipo-
cytes and then secreted, after which it migrates to the
inner surface of the cells lining the blood capillaries.
Whereas most of the fatty acids released by the action
of lipoprotein lipase are taken up by the adipocytes,
some are released into the blood and used by other
tissues. The relative importance of de novo synthesis
and lipoprotein lipase activity as a source of fatty
acids for fat synthesis depends on the diet and the
species. When animals are fed high-fat diets, chylo-
micron lipids are the major source. When animals are
fed diets rich in carbohydrates, the major source be-
comes VLDL lipids or de novo fatty acid synthesis in
adipocytes, depending on whether adipocytes or the
liver are the major site of fatty acid synthesis in the
species.
0012 Once synthesized within the adipocyte, triacylgly-
cerols are stored in the lipid droplet. Fatty acids are
released from them when required by the action of the
enzyme hormone-sensitive lipase (distinct from lipo-
protein lipase). This enzyme cleaves two molecules of
fatty acids to yield a monoacylglycerol that is then
hydrolyzed to glycerol and fatty acid by a separate
enzyme. Essentially all the glycerol is released from
the cell as it cannot be metabolised by adipocytes.
Some fatty acids, however, are usually reesterified,
and so the ratio of fatty acid to glycerol leaving the
cell is normally less than the theoretical 3:1. Released
fatty acid is bound to albumin in the blood and trans-
ported to the liver and other tissues. Fatty acid ester-
ification and triacylglycerol hydrolysis (lipolysis)
occur continuously, i.e., there is a continual turnover
of adipocyte triacylglycerol. Net accretion or loss of
lipid thus depends on the relative rates of these two
processes.
Regulation of Adipose Tissue Metabolism
0013 Both lipid synthesis and hydrolysis are under complex
hormonal control. Hormones regulate the amounts of
key enzymes and other proteins involved, as well as
their activities. In addition, the ‘signal transduction’
systems (a series of reactions transmitting hormone-
induced signals to targets in the cell), through which
hormones achieve their effects, are also subject to
endocrine control themselves, and changes in the
ability of adipocytes to transmit such signals are an
important part of the adaptations to some physio-
logical states (e.g., lactation).
0014Regulation of fatty acid synthesis depends on the
precursor. For glucose, control begins at the point of
entry into the cell where its transport is dependent on
a specific carrier protein (transporter); the major glu-
cose transporter of adipocytes is called ‘glut 4.’ Insu-
lin stimulates glucose transport both by promoting
recruitment of glut 4 into the plasma membrane and
by increasing its activity. Within the cell, glucose is
initially phosphorylated and then metabolized by a
long series of reactions, some in the cytosol, some in
the mitochondria, to produce acetyl coenzyme A
(CoA) in the cytosol. Several enzymes, in particular
phosphofructokinase and pyruvate dehydrogenase,
have key roles in controling this flux. Insulin, for
example, activates pyruvate dehydrogenase. For acet-
ate, the control is much simpler as its initial reaction
results in the production of acetyl CoA. The conver-
sion of acetyl CoA to fatty acid is catalyzed by two
enzymes, acetyl CoA carboxylase and fatty acid
synthetase. The former is thought to be the most
important enzyme controling flux. Both the amount
of acetyl CoA carboxylase and its activation status (it
is an enzyme that exists in active and inactive forms in
the cell) change markedly with physiological, nutri-
tional, and pathological condition. The amount and
activity, for example, are decreased by fasting, high-
fat diets, diabetes, and lactation. Insulin increases
both the amount and activity of the enzyme. These
effects of insulin are antagonized by growth hor-
mone. Catecholamines and glucagon also cause in-
activation of the enzyme and hence a fall in the rate of
fatty acid synthesis.
0015Insulin increases the synthesis and secretion of lipo-
protein lipase; this effect is accentuated by glucocor-
ticoids. Gastric inhibitory polypeptide also increases
lipoprotein lipase activity; this effect is likely to be
important for promoting fat deposition in animals
eating high-fat diets as such diets stimulate secretion
of this hormone. Thus, insulin and certain gut hor-
mones increase fat synthesis by increasing the supply
of fatty acids for esterification. Insulin also promotes
glycerol 3-phosphate formation, in part at least, by
increasing glucose uptake by adipocytes. The rate of
fatty acid esterification itself may not be stimulated
directly by hormones but varies directly with fatty
acid availability. Curiously, adipocytes secrete adipsin
and two related proteins, which interact in the
presence of chylomicrons, to produce acylation-
stimulating protein, which then acts on adipocytes
to stimulate esterification and glucose uptake.
0016The enzyme controling lipolysis, hormone-sensitive
lipase, exists in active and inactive states in the fat
cell. Glucagon and adrenaline (epinephrine), and also
ADIPOSE TISSUE/Structure and Function of White Adipose Tissue 27
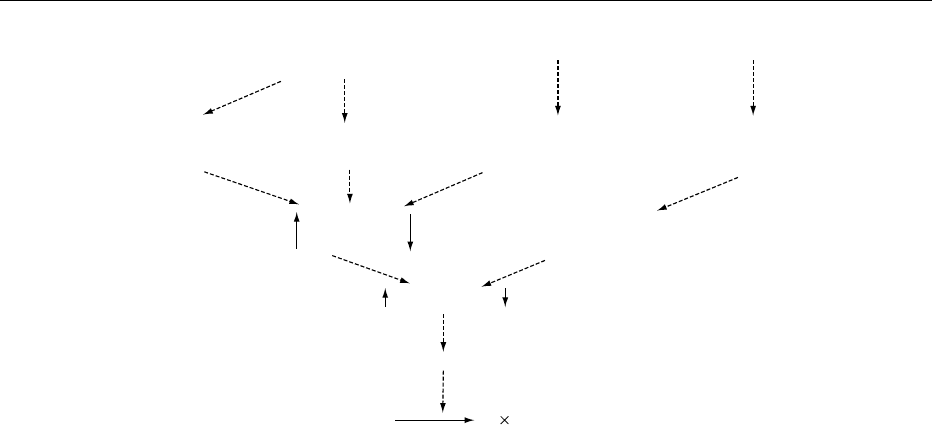
noradrenaline (norepinephrine) (which is released
from nerve endings of the sympathetic nervous system
within the tissue itself), interact with specific receptor
proteins in the plasma membrane (Figure 5). This
causes activation of a key enzyme, adenylate cyclase,
which synthesizes cyclic adenosine monophosphate
(cAMP). Increased concentrations of cAMP both ac-
tivate hormone-sensitive lipase and promote its
movement from the cytosol to the surface of the
lipid droplet, resulting in increased lipolysis. This
stimulatory mechanism is attenuated by several in-
hibitory systems. Adenosine and prostaglandin E
2
,
which are both produced within adipose tissue, inter-
act with their own receptors, leading to inhibition of
adenylate cyclase. Curiously, adrenaline and nor-
adrenaline can both activate and inhibit adenylate
cyclase. They activate adenylate cyclase by interact-
ing with b-adrenergic receptors and inhibit by inter-
acting with a
2
-adrenergic receptors. The effect of
adrenaline and noradrenaline on lipolysis will thus
depend in part on the relative number of b- and a
2
-
adrenergic receptors in the adipocytes. There is con-
siderable site- and gender-specific variation in the
ratio of a
2
-tob-adrenergic receptor number of adi-
pocytes in some species. For example, in women,
intraabdominal adipocytes have a ratio of about
1:1, whereas subcutaneous femoral and gluteal adi-
pocytes have a ratio of about 10:1 a
2
-:b-adrenergic
receptors. This ratio is thought to be responsible
for the very poor lipolytic response to catecholamines
of these subcutaneous adipocytes in women and
hence the relatively large size of these cells com-
pared with adipocytes elsewhere in the body. In add-
ition to the above, insulin activates the enzyme,
cAMP-phosphodiesterase, which catalyzes the deg-
radation of cAMP and so reduces its concentration.
The rate of lipolysis then will depend on the concen-
tration of a whole range of hormones, locally pro-
duced factors, and neurohumoral transmitters
(substances, such as noradrenaline, which are re-
leased by nerve endings in tissues). In addition, the
ability of the ‘signal transduction’ system to transmit
signals varies with age and with physiological state.
For example, during lactation, when fat is often mo-
bilized to support milk production, the system can
become more responsive to agents that promote lipo-
lysis. Thyroid hormones, glucocorticoids, sex ster-
oids, and growth hormone all act on one or more
components of the signal transduction system,
altering its ability to respond to stimulatory and/or
inhibitory agents.
0017Adipose tissue metabolism is thus under complex
control. In general, insulin promotes fat synthesis and
inhibits lipolysis, whereas catecholamines and
glucagon inhibit synthesis and promote lipolysis. In
addition, steroid hormones, thyroid hormones, and
growth hormone act to modulate the effects of insulin
and catecholamines, in part at least, by modifying the
ability of the signal transduction systems to transmit
signals.
Composition of Stored Fat
0018Triacylglycerols comprise about 95% of adipose
tissue lipid; the remainder includes diacylglycerols,
phospholipids, unesterified fatty acids, and choles-
terol. The fatty acid composition of the triacylglycer-
ols shows species variation (Table 1), but oleic and
Adrenaline
Noradrenaline
Prostaglandin E
Prostaglandin E
receptor
Insulin
Insulin
receptor
β-Adrenergic
receptor
α
2
-Adrenergic
receptor
Adenylate
cyclase
Cyclic AMP
phosphodiesterase
Cyclic AMP
Hormone-sensitive lipase
Triacylglycerol 3 fatty acids + glycerol
fig0005 Figure 5 Control of triacylglycerol hydrolysis (lipolysis) by the catecholamines (adrenaline and noradrenaline) and insulin. AMP,
adenosine monophosphate; ", #, activity/concentration increased or decreased by stimulus, respectively.
28 ADIPOSE TISSUE/Structure and Function of White Adipose Tissue

palmitic acids are major components in all species.
The proportions of polyunsaturated fatty acids (lino-
leic and linolenic) are usually low in adipose tissue
from ruminant animals and higher in chicken and pig
adipose tissue. This reflects the dietary supply; as
described above, fatty acids are derived both from
dietary lipid (via chylomicrons) and from de novo
synthesis (which produces palmitic acid). There is
some capacity for chain elongation of palmitic acid
to produce stearic acid, and for desaturation, which
converts palmitic to palmitoleic and stearic to oleic
acids, but the tissue cannot synthesize linoleic or
linolenic acids. In simple-stomached species, such as
humans and pigs, varying the fatty acid composition
of the diet will alter the fatty acid composition of
adipose tissue lipids. For ruminant animals, however,
dietary polyunsaturated fatty acids are mostly hydro-
genated in the rumen to produce oleic and stearic
acids. The small amount of linoleic and linolenic
acids escaping this fate is conserved for essential func-
tions (membrane synthesis, prostaglandin produc-
tion), so that adipose tissue lipids (and milk fat)
normally contain little linoleic or linolenic acids.
This is ironic, for linolenic acid is the major fatty
acid of the ruminant diet. If hydrogenation in the
rumen is avoided (e.g., by coating dietary lipid with
formaldehyde-treated casein), large quantities of
these polyunsaturated fatty acids are absorbed, pro-
ducing adipose tissue rich in linoleic and linolenic
acids.
0019 Minor changes in the fatty acid composition occur
during development, and there are minor differences
between adipose tissue depots, but these are small
compared with the changes that can be elicited by
dietary manipulation.
See also: Fats: Production of Animal Fats; Fatty Acids:
Properties; Hormones: Adrenal Hormones; Pituitary
Hormones; Obesity: Etiology and Diagnosis; Fat
Distribution
Further Reading
Bjorntorp P (1991) Adipose tissue distribution and func-
tion. International Journal of Obesity 15: 67–81.
Flier JS (1995) The adipocyte: storage depot or node on the
information superhighway? Cell 80: 15–18.
Flint DJ and Vernon RG (1993) Hormones and adipose
tissue growth. In: Pang PKT, Scanes CG and Schreibman
MP (eds) Vertebrate Endocrinology: Fundamentals and
Biomedical Implications, pp. 469–494. Orlando, FL:
Academic Press.
Friedman JM and Halaas JL (1998) Leptin and the regula-
tion of body weight in mammals. Nature 395: 763–770.
Gregoire FM, Smas CM and Sul HS (1998) Understanding
adipocyte differentiation. Physiological Reviews 78:
783–809.
Mohammed-Ali V, Pinkey JH and Coppack SW (1998)
Adipose tissue as an endocrine and paracrine organ.
International Journal of Obesity 22: 1145–1158.
Pond CM (1992) An evolutionary and functional view of
mammalian adipose tissue. Proceedings of the Nutrition
Society 51: 367–377.
Spiegelman BM and Flier JS (1996) Adipogenesis and
obesity – rounding out the big picture. Cell 87: 377–389.
Vernon RG (1992) Control of lipogenesis and lipolysis. In:
Buttery PJ, Boorman KN and Lindsay DB (eds) The
Control of Fat and Lean Deposition, pp. 59–80. Oxford:
Butterworth-Heinemann.
Vernon RG, Barber MC and Travers MT (1999) Present
and future studies on lipogenesis in animals and human
subjects. Proceedings of the Nutrition Society 58:
541–549.
Structure and Function of
Brown Adipose Tissue
M J Stock*, St George’s Hospital Medical School,
Tooting, London, UK
S Cinti, Universita degli Studi di Ancona, Ancona, Italy
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Brown Adipose Tissue
0001Brown adipose tissue (BAT), or brown fat, is a small
but highly specialized tissue, the main function of
which is to produce heat (thermogenesis). This func-
tion requires a good blood supply and a dense popu-
lation of mitochondria – two features that account for
its reddish brown color and distinguish it from white
adipose tissue (WAT) (see Figure 1). It is found in
most mammals, particularly in the neonate, and
plays an important role in the control of body
temperature during exposure to the cold. There is
tbl0001 Table 1 Fatty acid composition of adipose tissue
triacylglycerols (representative values)
Fattyacids (g per 100 g of total fattyacids)
Fattyacid Humans Pig Sheep Chicken
Myristic 4 1 3 1
Palmitic 23 26 22 26
Palmitoleic 5 3 4 6
Stearic 6 13 20 7
Oleic 49 42 39 40
Linoleic 9 13 3 19
Linolenic 1 2 2 1
Other 3 7
*Author deceased.
ADIPOSE TISSUE/Structure and Function of Brown Adipose Tissue 29
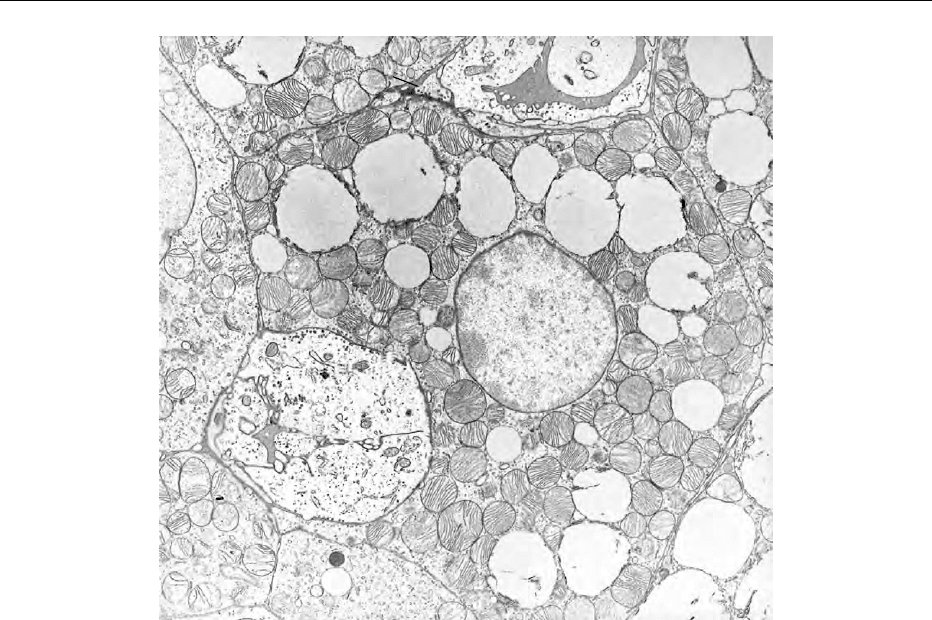
evidence indicating that it is also involved in the
regulation of energy balance. The tissue was first
described some 300 years ago, but its thermogenic
function was not recognized until the early 1960s,
and only during the 1980s did its capacity for thermo-
genesis and its unique metabolism come to be fully
appreciated. (See Thermogenesis.)
Location
0002 BAT is most obvious in small mammals, hibernators,
and neonates, and is usually found around the
kidneys, heart and aorta, along the intercostal
muscles and sternum, in the axilla, in the subcutane-
ous inter- and subscapular regions, and deep within
the neck, around the main arteries and veins. This
distribution suggests that the tissues act as a jacket
to heat the major organs and warm the blood passing
from the periphery into the trunk. The distribution
varies considerably between species, and some (e.g.,
dog, human) have little or no interscapular BAT,
whereas in others (e.g., rodents), the interscapular
depot may account for 20–30% of the total. BAT
rarely exceeds 2–3% of body mass, and is present in
such small quantities in large adult mammals that it is
often impossible to detect visually. In spite of this,
BAT has been identified histologically in human
adults up to the age of 80 years or more, and bio-
chemical tests suggest that it might retain its thermo-
genic activity. BAT depots often contain white
adipocytes, and some WAT depots may contain
brown adipocytes, but these can be difficult to see.
Histology and Development
0003Brown adipocytes appear polygonal under the micro-
scope, with a diameter of 10–25 mm, compared with
20–150 mm for white adipocytes. The adipocytes are
organized in discrete lobules, surrounded by connect-
ive tissue, extensive blood vessels and numerous sym-
pathetic nerves terminating on the adipocytes and
blood vessels. Unlike white adipocytes, the nuclei
are spherical and located centrally, and the lipid is
stored in small, multilocular droplets. Between the
droplets and packing the cytoplasm are numerous,
well-developed mitochondria that possess distinctive
and regular cristae, often traversing the width of the
mitochondrion. The endoplasmic reticulum (particu-
larly the rough reticulum) and Golgi apparatus are
relatively small, and lysosomes, peroxisomes, and
clusters of glycogen granules are often present; adja-
cent cells are usually connected by gap junctions.
CAP
N
L
P
m
CAP
P
P
L
m
m
L
fig0001 Figure 1 Electron micrograph of brown adipose tissue showing the typical features of a highly thermogenic tissue, i.e., a dense
population of well-developed mitochondria, lipid droplets, rich nerve (sympathetic) and blood (capillaries) supply. m, mitochondria; L,
lipid droplets; CAP, capillary; N, nerve fiber; P, precursor.
30 ADIPOSE TISSUE/Structure and Function of Brown Adipose Tissue

0004 Cytogenic studies indicate that brown adipocytes
are derived from stem cells closely associated with
vascular structures, and it is now generally agreed
that these are distinct from stem cells that give rise to
white adipocytes. Mature brown adipocytes cannot
undergo mitosis, and the recruitment (hyperplasia)
seen during cold adaptation occurs by cytogenesis
and mitosis of newly differentiated brown adipocytes.
The first appearance of differentiated BAT cells varies
between species, and in some neonates (e.g., guinea-
pig, rabbit, puppy, lamb), the tissue is well developed
and functional at birth. In other species (e.g., rats,
mice), the tissue is not fully functional at birth, but
becomes thermogenically active within a few days. By
contrast, the Syrian hamster is born without BAT, and
it takes about 2 weeks for the tissue to develop, during
which time, the animal is essentially poikilothermic.
Morphology is highly dependent on age, strain, envir-
onment, and various physiological and pathological
conditions. Brown adipocytes will transform grad-
ually into what look like white adipocytes during
prolonged inactivity.
Innervation
0005 The innervation of BAT is another feature that distin-
guishes it from WAT, since the metabolic activity of
the tissue is almost entirely determined by the release
of noradrenaline at sympathetic nerve terminals on
the brown adipocytes. In some depots (e.g., rodent
interscapular BAT), the sympathetic nerves enter as
obvious bundles. This makes experimental techniques
such as surgical sympathectomy and nerve stimula-
tion and recordings relatively easy to undertake,
although there can be problems in distinguishing be-
tween effects on adipocytes and those on the vascular
supply. The parenchymal sympathetic fibers innervat-
ing adipocytes and arterioles release mainly nor-
adrenaline, and this explains why the tissue content
and turnover of noradrenaline are high; noradren-
aline turnover is a good index of sympathetic acti-
vation in response to various environmental and
dietary stimuli. Apart from noradrenaline, histamine,
adenosine, and various peptides may modulate the
sympathetic activation of BAT. Neuropeptide-Y
(NPY) is found colocalized with noradrenaline in
perivascular sympathetic nerve endings, and the
depletion of sensory peptides – CGRP (calcitonin
gene-related peptide) and Substance P – by capsaicin
suggests that the tissue contains afferent fibers.
Blood Supply
0006 The high oxygen supply required to support thermo-
genesis is provided by an extensive network of vessels,
estimated to be four to six times denser than that in
white adipose tissue. The vascular supply can support
a blood flow in excess of 20 ml per gram of tissue per
minute; during maximal stimulation in cold-adapted
rodents, this relatively small mass of tissue can receive
over 30% of cardiac output. Blood flow increases
result partly from the vasomotor activity of the
sympathetic nerves, but also from autoregulatory
increases caused by sympathetic activation of meta-
bolism and the release of metabolites. Aerobic heat
production can be so intense that the oxygen supplied
in arterial blood is almost completely extracted, and
the venous blood appears desaturated. The small
amounts of oxygen remaining probably represent
blood that bypassed the capillary network via
arteriovenous anastomoses (i.e., vascular shunts).
These vascular shunts, of which there are many, prob-
ably act to convect the heat generated away from the
tissue, thereby avoiding thermal damage (BAT tem-
peratures can rise to over 44
C). The thermogenic
capacity of BAT can be determined from measure-
ments of blood flow and oxygen extraction, and esti-
mates of up to 500 W kg
1
can be compared with
values of only 60 W kg
1
for the maximal aerobic
power of skeletal muscle. (See Exercise: Muscle.)
Metabolism
0007The exceptional heat-producing capacity of BAT is
due to its mitochondria, which possess a 32-kDa
polypeptide called uncoupling protein (UCP). This is
now known as UCP1, since two other, similar mito-
chondrial proteins (UCP2 and UCP3) have been dis-
covered, but UCP1 is unique to BAT mitochondria
and is responsible for the only significant, physio-
logical example of uncoupled oxidative phosphoryl-
ation in mammalian metabolism. UCP forms a proton
conductance channel in the mitochondrial inner
membrane, and dissipates the proton electrochemical
gradient generated by oxidation of substrates via the
electron transport system. This has the effect of un-
coupling oxidation from the phosphorylation of ADP
(adenosine diphosphate) to ATP (adenosine triphos-
phate), thereby dissipating the energy released as
heat, as well as increasing the rate of oxidation due
to the loss of respiratory control.
0008The proton conductance pathway is under inhibi-
tory control by purine nucleotides (e.g., ADP, ATP,
GDP), which bind to UCP, and is activated following
sympathetic activation of the adipocyte b-adrenergic
receptors, which also stimulate lipolysis and the
release of free fatty acids from the triglyceride drop-
lets. These fatty acids provide the principal fuel
for thermogenesis. The rapid activation of the
proton conductance pathway following sympathetic
ADIPOSE TISSUE/Structure and Function of Brown Adipose Tissue 31

stimulation can be detected by measuring the mito-
chondrial binding of purine nucleotides – usually
GDP (guanosine diphosphate) – in vitro, whereas
chronic, adaptive changes in thermogenic capacity
depend on immunoassay of mitochondrial UCP con-
centrations.
0009 High rates of oxidation in any tissue require
adequate levels of all the enzyme systems of inter-
mediary metabolism, and BAT is particularly well
endowed with those required for glycolysis, the tri-
carboxylic acid cycle, and the mitochondrial electron
conductance chain. Since fatty acids are the main fuel
for thermogenesis, adenyl cyclase activity and the
subsequent cascade that leads to the intracellular
release of fatty acids from stored triglyceride are
prominent features of BAT metabolism. However,
the lipid stored in the multilocular droplets is not
sufficient to sustain thermogenesis for long periods,
and brown adipocytes then rely on their remarkable
capacity for lipogenesis. In cold-adapted rats and
mice, the lipogenic capacity of BAT is high enough
to account for a major fraction of the amount of
dietary carbohydrate that the animal converts to
lipid. As well as the fatty acids supplied de novo by
lipogenesis, the high level of lipoprotein lipase allows
BAT to take up fatty acids released by the hydrolysis
of circulating triglycerides.
0010 In addition to the normal complement of respira-
tory enzyme systems, brown fat cells also contain
peroxisomes, and these proliferate during chronic
stimulation of the tissue. Peroxisomal oxidation of
substrates is not linked to phosphorylation, and
could therefore make a contribution to cellular
thermogenesis. However, the contribution is prob-
ably very small, and their function may be more to
do with controling levels of free radicals as well as
the cytosolic metabolism of fatty acids that are not
preferentially metabolized by mitochondria. Another
interesting feature of BAT metabolism is the presence
of an enzyme, 5
0
-deiodinase, that converts thyroxine
(T
4
) to the physiologically active hormone, triiodo-
thyronine (T
3
). The enzyme is under sympathetic
control, and its activity can increase several hun-
dred-fold in cold-adapted animals. The T
3
produced
is more than sufficient to saturate the nuclear recep-
tors, and it is possible that much of the T
3
is exported
and exerts effects on other tissues. (See Hormones:
Thyroid Hormones.)
Functions of BAT
Thermoregulation
0011 Shivering is an acute response to cold exposure and
not a particularly effective mechanism for protecting
the body against hypothermia. As a consequence,
many animals resort to a form of heat production
called nonshivering thermogenesis (NST), which,
unlike shivering, can be sustained without fatigue
and disruption of locomotor activity or sleeping
behavior. NST appears as an adaptive response to
chronic cold exposure in many mammals, but
particularly in small animals where heat losses are
greater due to the large surface area relative to body
mass. The high degree of surface heat loss and imma-
ture neuromuscular development also explain why
the neonates of most mammalian species (including
humans) depend on NST to maintain body tempera-
ture until shivering, locomotor activity and other
behavioral thermoregulatory responses develop. A
third group is the hibernators, who rely on NST for
the rapid rewarming that occurs during arousal.
0012Depending upon the species, NST can raise heat
production by 100–300% above that in a warm,
thermoneutral environment, and is associated with
large increases in the activity of the sympathetic ner-
vous system. Pharmacological blockade (particularly
with b-adrenergic antagonists) can inhibit completely
the cold-induced rise in heat production, and demon-
strates the dominant role of the sympathetic nervous
system in mediating NST. The effector tissue is BAT,
and a considerable body of evidence now exists to
link BAT function to NST. For example, the capacity
for NST is inversely proportional to age, bodyweight,
and acclimation temperature, and this coincides with
histological, physiological, and biochemical indices
of BAT activity. Conversely, deacclimation and de-
creased NST is associated with a parallel decline in
BAT activity. Perhaps the most convincing evidence
comes from in vivo measurements of BAT oxygen
consumption, which, in spite of enormous technical
difficulties, have shown that the tissue can account
for well over 60% of NST. Even this may be an
underestimate, since it is not possible to measure the
contribution of all the numerous, small and diffuse
BAT depots.
Energy-balance Regulation
0013Evidence linking BAT to energy-balance regulation
comes mainly from studies on laboratory rodents
that represent examples of two extremes of metabolic
efficiency. At one extreme, there are normal, young
rats and mice that fail to become obese in spite of an
excessive energy intake, and at the other extreme,
there are examples of obesity developing in rats and
mice (e.g., genetic and hypothalamic obesities), even
when energy intake is normal. The explanation for
these differences appears to depend on an adaptive
form of heat production called diet-induced thermo-
genesis (DIT), which is absent or defective in obese
32 ADIPOSE TISSUE/Structure and Function of Brown Adipose Tissue

animals, but provides a mechanism whereby normal
animals can adjust energy expenditure to compensate
for energy consumed in excess of requirements. DIT
can produce increases in total heat production of
60–70%, and account for up to 90% of the excess
energy consumed by hyperphagic rats. In rats feeding
normally, the level of DIT is low, but sufficient to
control energy balance by compensating for errors
in the control of energy intake.
0014 The control and metabolic origins of DIT are iden-
tical in almost every respect to NST, although cold is a
more potent stimulus and produces more dramatic
changes than dietary stimuli. As a consequence, the
changes in sympathetic activity, BAT hypertrophy
and hyperplasia, mitochondrial proliferation, guano-
sine diphosphate binding and UCP concentration in
rats exhibiting DIT are smaller than those seen in
cold-adapted rats. However, these changes in BAT
function are sufficient to account for up to 80% of
the diet-induced changes in thermogenic capacity
seen in hyperphagic rats. By contrast, BAT is usually
atrophied and relatively inactive in obese rodents,
although it will respond to exogenous noradrenaline,
and the animals retain the capacity to adapt to the
cold and exhibit NST. This suggests that the defective
DIT in these obese rodents is due to a failure of the
sympathetic activation of BAT, rather than a defect
in BAT itself. This contrasts with what is seen in a
transgenic mouse bearing a ‘toxigene’ that causes a
genetic ablation of BAT. These mice fail to exhibit
NSTand DIT, and become obese – sometimes without
eating any more than normal. (See Obesity: Etiology
and Diagnosis.)
Other Functions
0015 In addition to cold- and diet-induced thermogenesis,
there are several pathological conditions in which
BAT has been implicated as a source of increased
heat production. Fever, sepsis, and cancer cachexia
are three examples where increased sympathetic acti-
vation of BAT is thought to be at least partly respon-
sible for the hypermetabolic response seen in animal
models of these conditions, and often involve cyto-
kines such as the interleukins. Patients with pheo-
chromocytoma (adrenomedullary tumor) have very
high circulating levels of adrenaline and noradren-
aline, and it is thought that the elevated heat produc-
tion in this condition is due to the stimulatory effect
of these catecholamines on BAT; the best examples of
active BAT in human adults have been seen in patients
with pheochromocytoma.
0016 In spite of increased energy intakes, pregnant rats
and mice show little or no change in BAT activity, but
during lactation, the tissue atrophies, and its sympa-
thetic activation and thermogenic capacity decline to
levels seen after sympathectomy or fasting. Similar
reductions can be seen in warm-adapted nonlactating
animals, which suggests that BAT thermogenesis de-
clines to compensate for the elevated heat production
associated with milk synthesis in the lactating mam-
mary glands. Increased heat production during exer-
cise could also account for the lower BAT activity
seen in exercise-trained animals. This is particularly
noticeable in cold environments, where exercise can
prevent many of the changes in BAT function associ-
ated with NST.
Control of BAT
Neural
0017The control over the sympathetic supply to the vari-
ous BAT depots originates from the hypothalamus,
which receives afferent information on thermal and
nutrient status from the periphery, as well as having
its own receptor mechanisms and pathways. One of
the main thermosensitive and thermoregulatory areas
is the preoptic/anterior hypothalamus (POAH), but
this is thought to modulate BAT thermogenesis via
inhibitory pathways that descend to the lower brain-
stem. The area that appears to exert a major influence
over BAT is one that has been classically associated
with the control of energy intake – the ventromedial
hypothalamus (VMH), often loosely referred to as the
‘satiety center’. Electrical stimulation of the VMH
increases BAT thermogenesis, whereas lesions cause
the tissue to atrophy, and the latter observation helps
explain why VMH-lesioned animals can become
obese without overeating. There are connections
between the VMH and other hypothalamic areas
concerned with feeding behavior (e.g., lateral hypo-
thalamus, paraventricular nucleus), and with the
POAH, which provide a neural basis for integrating
information on energy intake and body tempera-
ture, and modulate the level of NST and DIT
accordingly.
Hormonal
0018Adrenaline stimulates BAT thermogenesis, but it is
not as potent as noradrenaline, and in most physio-
logical situations, the circulating levels of adrenaline
are probably not sufficient to activate the tissue’s
b-adrenoceptors. However, views may change on
this in the light of recent, more sensitive measure-
ments that show that circulating levels of adrenaline
may have been previously underestimated. Although
thyroid hormones (T
3
and T
4
) are necessary to main-
tain BAT function, and T
3
is itself produced by the
tissue, hyperthyroidism suppresses BAT activity. This
is probably due to reduced sympathetic activation
ADIPOSE TISSUE/Structure and Function of Brown Adipose Tissue 33
