Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

0008 Insecticides Insecticides are represented by a variety
of different chemical classes involving a variety of
mechanisms of toxic action on insects as well as
mammals such as humans. Examples of mechanisms
of action include metabolic interference of the ner-
vous or muscle systems, desiccation, and sterilization.
Common types of insecticides include the chlorinated
hydrocarbons, organophosphates, carbamates, and
pyrethroids.
0009 The first major synthetic class of insecticides, the
chlorinated hydrocarbons, was developed during the
1930s and 1940s. Representative members of this
insecticide class include DDT, aldrin, dieldrin, and
chlordane. The chlorinated hydrocarbons are very
potent nerve toxins to insects, and their initial use
led to significant improvements in insect control.
Their high insect potency, combined with generally
low mammalian toxicity, provided excellent selectiv-
ity and insect control that was further enhanced by
their high environmental persistence. In subsequent
years, however, their resistance to environmental
decay, coupled with their widespread continuing
use, resulted in environmental build-up and food-
chain magnification that resulted in significant envir-
onmental and ecological damage. Today, very few
chlorinated hydrocarbon insecticides remain regis-
tered for use in the USA, because of environmental
concerns, although their use may still be significant in
many areas of the world. Many recent studies have
also associated chlorinated hydrocarbon insecticides
with potential adverse effects on human and nontar-
get organism fertility and reproduction arising from
enzyme-inducing or estrogenic properties of the
chemicals.
0010Historically, many of the uses of chlorinated hydro-
carbon insecticides were replaced by the organophos-
phate and carbamate insecticides. In contrast to the
chlorinated hydrocarbon insecticides, the organo-
phosphates and carbamates break down quite rapidly
in the environment following application. They also
have a far greater acute toxicity in mammals. The
organophosphates and carbamates are derived as
esters of two very different chemical families but
share a common mechanism of toxicological action
tbl0002 Table 2 Common pesticide classes and representative
examples
Pesticide Examples
Insecticides
Chlorinated hydrocarbons Dicofol, methoxychlor, DDT,
aldrin, chlordane
Organophosphates Parathion, malathion,
chlorpyrifos, azinphos-
methyl
Carbamates Aldicarb, carbaryl, carbofuran
Pyrethroids Permethrin, cypermethrin
Herbicides
Triazine Atrazine, simazine, cyanazine
Phenoxy 2,4-D, 2,4,5-T, MCPA
Quaternary ammonium Paraquat, diquat
Benzoic acids Dicamba
Acetanilides Alachlor, metolachlor
Ureas Linuron
Fungicides
Inorganic Sulfur
Ethylenebisdithiocarbamates Maneb, mancozeb, zineb
Chlorinated phenols Pentachlorophenol
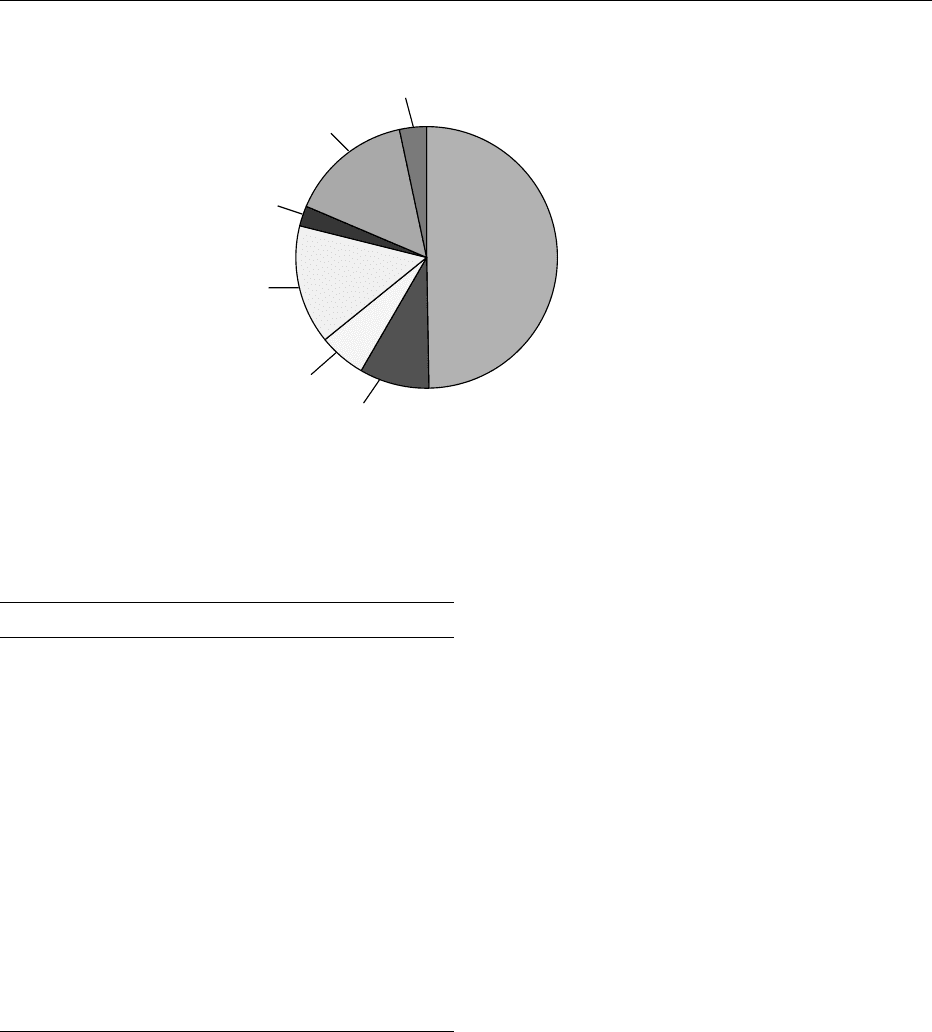
Herbicides/Plant growth regulators
213 million kg
49.8%
Insecticides/Miticides
37 million kg
8.7%
Fungicides
24 million kg
5.6%
Fumigants/Nematicides
64 million kg
14.9%
Other (Conventional)
11 million kg
2.6%
Other (Non-conventional)
14 million kg
3.2%
Sulfur/Oil
65 million kg
15.3%
Total does not equal 100% due to
rounding
fig0001 Figure 1 Agricultural use of pesticides in the USA, 1997.
PESTICIDES AND HERBICIDES/Toxicology 4495

in both insects and mammals that involves inhibition
of cholinesterase enzymes that can disrupt the ner-
vous system.
0011 A newer class of insecticides, the pyrethroids, are
synthetic derivatives of pyrethrins (natural extracts
from chrysanthemums) that are made to be more
light-stable than their natural predecessors and thus
more effective as insecticides. The pyrethroids were
introduced in the 1970s and have broad-spectrum
activity against many insects while possessing a
much lower mammalian toxicity than the organo-
phosphates or carbamates. The pyrethroids are fre-
quently used in agricultural pest control, but their
use is still limited by their environmental lability,
relatively high cost, and their tendency to lose
effectiveness through the development of insect
resistance.
0012 Herbicides Several different types of herbicides
exist (Table 2), and each type has its own mechanism
of toxic action on weeds. Since weeds and mammals
differ dramatically in terms of metabolic function,
herbicides targeting specific metabolic pathways in
weeds frequently have little effect, and therefore a
low relative toxicity, in mammals including humans.
0013 The timing of herbicide application may determine
significantly whether the potential exists for con-
sumers to be exposed to herbicide residues in foods.
Some herbicides (preplant herbicides) are applied
before a crop is planted, whereas others (preemergent
herbicides) are applied after planting but prior to
the appearance of weeds, and others (postemergent
herbicides) are used after the weeds have germinated.
Some herbicides have broad-spectrum activity that
makes them toxic to most types of plant life, in-
cluding the crop being produced. One such example
is glyphosate. Recent developments in genetic engin-
eering have led to the development of varieties of
soy, corn, and cotton that are resistant to glyphosate,
meaning that glyphosate applications made while the
crop is growing will control weeds without affecting
the crop.
0014 Other herbicides, such as the phenoxy herbicides
(2,4-D, MCPA) are more selective in their toxicity;
they are toxic to broad-leaf plants but not to narrow-
leaf plants such as grasses. Some epidemiological
studies have suggested links between agricultural
worker exposure to phenoxy herbicides and certain
types of cancer.
0015 Fungicides A wide number of different types of fun-
gicides are available for use in agriculture (Table 2).
Fungicides control molds and other plant diseases by
interfering with the growth and/or metabolic pro-
cesses of fungal pests. In addition to improving crop
yields, fungicides may provide human health benefits
by reducing the production of mycotoxins (naturally
occurring toxins produced by fungi living on the food
crop) such as aflatoxins and fumonisins.
0016Pesticides suspected as carcinogens The potential
carcinogenic (cancer-causing) effects of many pesti-
cides have generated considerable public concern and
regulatory scrutiny. Although the consumption of
foods containing pesticide residues has not been cor-
related with the development of human cancers, some
epidemiological studies have linked the occupational
use of specific pesticides with the development of
cancers. Most pesticides considered as carcinogens,
however, owe their status as suspected carcinogens to
the results of long-term animal toxicology studies in
rodents such as rats and mice.
0017The carcinogenic potential of pesticides and other
chemicals is typically determined through long-term
animal bioassay studies. In these studies, animals are
frequently given a high dose (the maximum tolerated
dose, or MTD, that may cause considerable toxicity
but does not reduce life expectancy), a lower dose
(commonly one-half to one-fourth of the MTD),
and a control (zero) dose. Relatively high doses of
chemicals are given to the animals to maximize the
potential to identify toxicological effects such as
tumor production.
0018The amounts of pesticides that cause cancer in
animal studies frequently exceed the amounts that
humans are exposed to from the diet by several orders
of magnitude. It has been argued that many pesticides
considered to be carcinogens receive such classifica-
tion because of the high doses used in the long-term
animal studies and that the results of such studies may
not be applicable to human exposure to much lower
levels of the pesticides. As an example, it is well
established that high-dose toxicity may lead to in-
creased rates of cell proliferation that in itself is linked
to the development of cancer in laboratory animals.
Human exposure at lower levels of exposure would
not be expected to trigger such a response and, as a
result, would not be expected to lead to the develop-
ment of cancer.
0019Long-term animal studies are also prone to incon-
clusive and/or contradictory results among different
test animals and under different conditions. As a
result, the EPA has developed a weight-of-the-evidence
evaluation scheme based upon results of any human
data and animal testing to determine the likelihood
of a pesticide to be carcinogenic. A list of several
pesticides considered by the EPA to be ‘probable’
carcinogens is provided in Table 3. Some pesticides
may receive classification a ‘possible’ carcinogens,
whereas many others are considered noncarcinogens.
4496 PESTICIDES AND HERBICIDES/Toxicology

Regulating and Monitoring Pesticide Residues in
Foods
0020 The use of pesticides does not necessarily imply that
food residues will occur. Many pesticides are applied
to nonfood crops, whereas others such as broad-spec-
trum herbicides could damage or eliminate a crop if
misapplied. The timing of the pesticide application is
another important factor; many pesticides are applied
to food crops prior to the development of edible
portions of the crop, whereas other pesticides used
on food crops may not result in residues, because of
rapid environmental degradation between the time of
application and the time of harvest.
0021 Pesticide residue regulation In cases where the use
of a pesticide on a food crop may present the potential
to leave a residue in the USA, a maximum permitted
allowable residue level, or tolerance, is established.
Tolerances are specific to combinations of pesticides
and commodities; it is possible for the same pesticide
to have different tolerance levels established on dif-
ferent commodities, and several different pesticide
tolerances for distinct pesticides may be established
on the same commodity.
0022 Although it may seem counterintuitive, pesticide
tolerances are not based upon safety but rather repre-
sent the maximum expected residue of a pesticide on
a particular commodity resulting from the legal use of
a pesticide. The maximum levels are determined from
the results of controlled field studies performed by the
pesticide manufacturer using the ‘worst legal case’
conditions such as the maximum recommended ap-
plication rate, maximum number of applications per
growing season, and harvesting at the minimal antici-
pated time following harvest. Pesticide manufacturers
typically petition the EPA to establish the tolerances
at or slightly above the highest levels determined from
the controlled field studies. As such, the values
selected for tolerances are determined solely on the
basis of agricultural practices but not as a result of
human health risk assessments. Pesticide tolerances
therefore should be considered to represent enforce-
ment tools to determine whether pesticide applica-
tions may have been made in accordance to legal
requirements; in cases where residues exceed the es-
tablished tolerances, it is likely that such residues
resulted from misapplication of the pesticides. Such
a finding, however, rarely constitutes an ‘unsafe’ resi-
due according to standard toxicological criteria.
Pesticide tolerances, therefore, should be viewed as
enforcement tools but not as standards of safety.
0023Before the EPA grants a tolerance, human health
risk assessments are performed to determine the con-
ditions for acceptable pesticide use. Such conditions
include the listing of commodities on which a specific
pesticide may be used, the target pests controlled by
the pesticide, application requirements, and the ac-
ceptable interval between application and harvest. In
the EPA’s assessment of acceptable levels of consumer
exposure to pesticides, it considers potential human
exposure from all registered (and proposed) uses of the
pesticide. If the resulting risk is deemed to be excessive,
the EPA will not allow tolerances to be established for
specific commodities. If the risks are considered to be
acceptable, the tolerances are established, as discussed
in the preceding paragraph.
0024The processes that the EPA uses to determine the
acceptability of dietary pesticide risk are quite com-
plicated and subject to ongoing evolution to meet
the needs of new regulations, improved toxicology
testing, and advances in computational methods.
0025The first step in evaluating the consumer risks from
pesticide residues involves making estimates of the
amount of consumer exposure. The maximum legal
exposure to the pesticide is frequently calculated by
assuming that (1) the pesticide is always used on all
food items for which it is registered and/or proposed
for registration, (2) all residues on the food items
will be present at the established or proposed
tolerance levels, and (3) there will be no reduction
in residue levels resulting from postharvest effects
such as washing, cooking, peeling, processing, and
tbl0003 Table 3 Some pesticides considered to be ‘probable’
carcinogens by the US Environmental Protection Agency
Pesticide Pesticide type
Acetochlor Herbicide
Aciflourfen sodium Herbicide
Alachlor Herbicide
Amitrol Herbicide
Cacodylic acid Herbicide
Chlorothalonil Fungicide
Creosote Wood preservative
Cyproconazole Fungicide
Fenoxycarb Insect growth regulator
Folpet Fungicide
Heptachlor Insecticide
Iprodione Fungicide
Lactofen Herbicide
Lindane Insecticide
Mancozeb Fungicide
Maneb Fungicide
Metiram Fungicide
Oxythioquinox Insecticide
Pentachlorophenol Fungicide
Pronamide Herbicide
Propargite Insecticide
Propoxur Insecticide
Terrazole Fungicide
Thiodicarb Insecticide
TPTH Fungicide
Vinclozolin Fungicide
PESTICIDES AND HERBICIDES/Toxicology 4497

transportation. This approach leads to the calculation
of the theoretical maximum residue contribution
(TMRC). Although studies have indicated that the
TMRC values may overestimate the actual consumer
exposures by factors of 100–100 000, the TMRC
provides a starting point from which to estimate con-
sumer risks.
0026 In practice, the TMRC is compared with estab-
lished toxicological criteria such as the reference
dose (RfD) or the acceptable daily intake (ADI) that
represent, following analysis of animal toxicology
data and extrapolations to human health, the daily
exposure levels that do not constitute an appreciable
level of risk. In cases where the EPA determines that
exposure to a pesticide at the TMRC is below the RfD
or ADI, the risks for the pesticide are typically
deemed to be negligible, and the EPA allows toler-
ances to be established for the pesticide on specific
commodities. For pesticides that are considered as
potential carcinogens, the EPA also requires that the
quantitative carcinogenic risk to the pesticide be
below one excess cancer per million using models
that calculate possible human risks from low levels
of exposures to potentially carcinogenic pesticides.
Such models are developed from the results from
long-term studies on animals given high doses of the
pesticides. In cases where the exposures at the TMRC
exceed the RfD or ADI, or in cases where the carcino-
genic risks at the TMRC exceed one excess cancer per
million, the EPA may adopt a refined risk assessment
that more accurately expresses exposures. Such re-
finements may include adjustments of actual pesticide
use, the use of more realistic pesticide residue data,
and consideration of postharvest effects that may
significantly reduce residue levels prior to consump-
tion. In cases where the refined exposure estimates are
below the RfD or ADI and where the carcinogenic
risks at the refined exposure estimates are below one
excess cancer per million, the EPA will typically allow
tolerances to be established.
0027 The processes by which the EPA determines human
risks from pesticide exposure became more compli-
cated following the passage and adoption of the Food
Quality Protection Act (FQPA) of 1996. It is clear that
the new requirements of FQPA will require scientists
to develop improved methods for assessing risks to
pesticides and that once such methods are adopted,
the regulatory requirements may be much more strin-
gent and may lead to reductions in the amounts and
types of pesticides that may be used on food crops.
0028 Prior to FQPA, the EPA allowed tolerances to be
established on a chemical-by-chemical basis and con-
sidered only exposures resulting from dietary path-
ways. FQPA now requires the EPA to establish
tolerances only when the risks posed by pesticides
represent a ‘reasonable certainty of no harm.’ In de-
termining what constitutes this ‘reasonable certainty
of no harm,’ the EPA must now consider the aggregate
exposure to pesticides from dietary, drinking water,
and residential sources as well as the cumulative ex-
posure from pesticides possessing a common mechan-
ism of toxic action. As such, the EPA may consider the
risks from entire families of chemicals rather than the
risks from individual chemicals. Another important
provision of FQPA is the so-called 10 factor, which
requires the EPA to consider applying an additional
10-fold uncertainty factor in cases where infants or
children may be more susceptible to the toxicological
effects of pesticides than adults. The application of the
full 10 factor would result in a subsequent reduc-
tion in the RfD by a factor of 10.
0029Internationally, many countries adopt the Codex
Alimentarius maximum residue limits (MRLs),
which, like the US tolerances, exist primarily as en-
forcement tools to determine if pesticide applications
are made following good agricultural practices. Al-
though many US tolerances and Codex Alimentarius
MRLs are identical, there are many cases in which
the US tolerances are more restrictive and many
others in which the Codex Alimentarius MRLs are
more restrictive.
0030Pesticide residue monitoring Authority In the US,
the Food and Drug Administration (FDA) has the
primary responsibility for enforcing tolerances in do-
mestic and imported foods. Domestic food samples
are frequently collected near the source of production
or at the wholesale level, whereas imported food
samples are typically taken at the point of entry into
the US. The types and quantities of samples taken by
FDA are determined by a variety of factors such as
regional intelligence on pesticide use, the dietary im-
portance of specific foods, information on the
amount of foods that enter interstate commerce, and
pesticide use patterns. Samples are analyzed using
multiresidue methods capable of detecting over 200
individual pesticides.
0031The US Department of Agriculture (USDA) also has
a role in pesticide residue monitoring, as it is respon-
sible for the monitoring of meat, poultry, and egg
products. It also conducts the Pesticide Data Program
(PDP), which collects residue data on fruits, vege-
tables, and processed foods. Findings from the
USDA’s PDP are considered to be more representative
of the actual food supply than those collected from the
FDA’s regulatory monitoring programs and are com-
monly used by EPA to aid in its risk-assessment efforts.
0032US monitoring of pesticide residues also occurs at
the state level. The largest state pesticide regulatory
and monitoring program exists in California, and
4498 PESTICIDES AND HERBICIDES/Toxicology
several other states, including Texas and Florida, have
significant pesticide-monitoring programs.
0033 Residue findings The most recent FDA pesticide
monitoring residue data are available for 1999.
During that year, FDA analyzed 9438 food samples
for pesticide residues. More samples were taken from
imported foods (6012 samples, or 63.7%) than from
domestic foods (3426 samples or 36.3%).
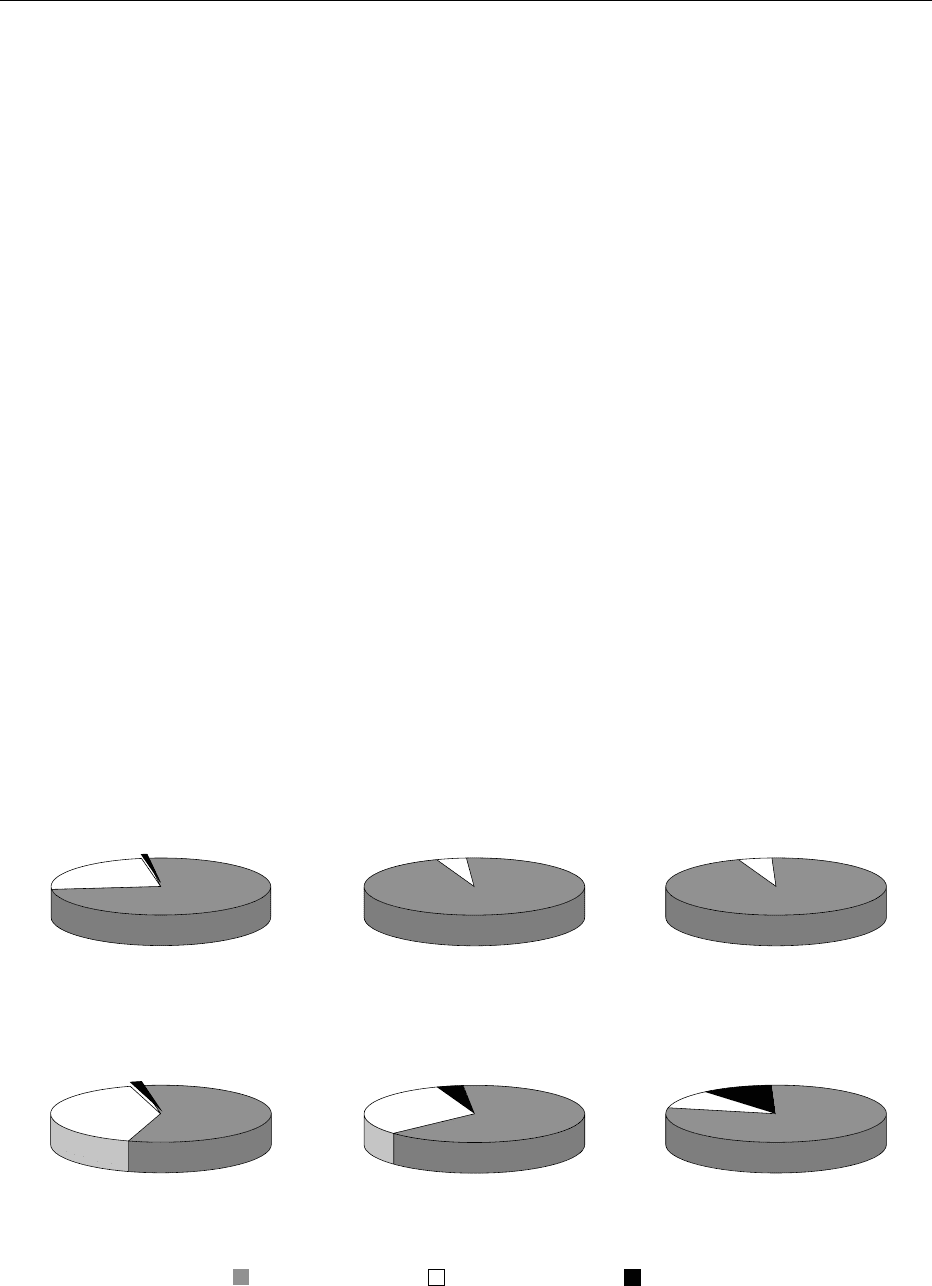
0034 The results of FDA’s 1999 monitoring of pesticide
residues in imported foods are shown in Figure 2.
Overall, 65.0% of the samples showed no detected
residues, and violations were identified in 3.1% of the
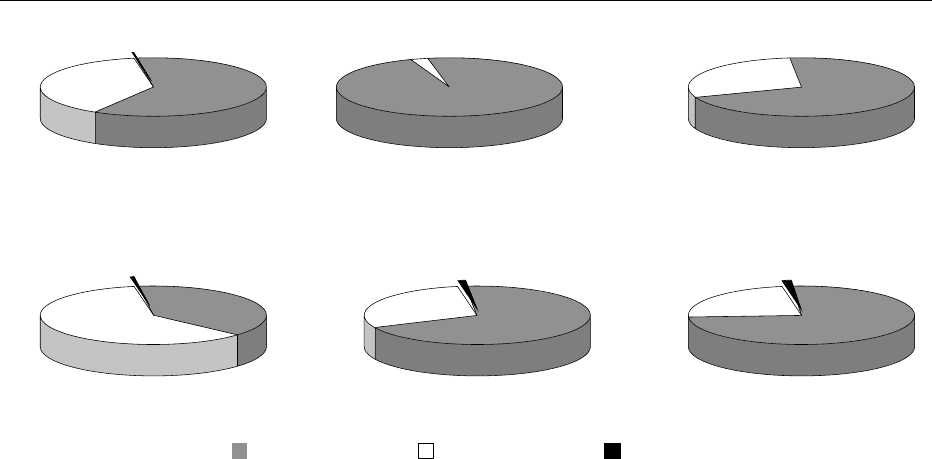
samples. Figure 3 shows the comparable results from
domestic foods where 60.2% of the samples showed
no detectable residues, and violations were present in
0.8% of the samples.
0035 Pesticide residue violations commonly take one of
two forms. The most common form of a violative
residue results when residues of a pesticide are
detected on a commodity for which no tolerance has
been established. This type of violation may result
from application of the pesticide to the wrong com-
modity, uptake from soil contaminated from a prior
use of the pesticide on a different commodity, or drift
of a pesticide from an adjacent field. The other type of
violation results when residue levels are detected in
excess of the established tolerance. In 1999, 90% of
the import violations occurred where residues were
detected on commodities for which tolerances were
not established, and only 10% of the violations
represented residues in excess of the tolerance. For
domestic samples, 69% were detected on commod-
ities for which tolerances were not established, and
the remaining 31% of violations occurred when levels
exceeding tolerances were detected. Fruits and vege-
tables were responsible for the highest percentages of
residue detections and the greatest number of viola-
tions from both imported and domestic foods.
0036Results obtained from California’s 1997 Market-
place Surveillance Program were quite similar to
those of the FDA’s 1999 monitoring. This program
analyzed 5660 samples in 1997, with 62.2% of those
originating from California, 6.7% from other US
states, and the remaining 31.1% from other coun-
tries. The majority of samples (62.1%) showed no
detectable residues, whereas 36.7% of the samples
contained legal residues. Violations were detected in
1.2% of the samples, and only 12% of the violations
represented residues detected in excess of tolerances.
The violation rate from imported foods was 2.4%,
and the violation rate for domestic foods was 0.7%.
0037The USDA’s PDP analyzed 9125 food samples for
pesticide residues in 1999. Foods analyzed included
apples, cantaloupe, cucumbers, grape juice, lettuce,
oats, pears, spinach, strawberries, sweet bell peppers,
tomatoes, winter squash, and corn syrup. Most of
the samples (8637) were from fruits and vegetables,
with lower numbers of samples collected for oats
(332) and corn syrup (156). The majority of samples
(79%) was of domestic origin. Overall, 36% of the
samples contained no detectable residue, whereas
57.5%
1.8%
40.7%
Grains and grain products
276 samples
Milk/Dairy products/Eggs
22 samples
Fruits
2290 samples
Vegetables
2768 samples
No residue found Residue found
not violative
Residue found
violative
Other
358 samples
Fish/Shellfish
Other aquatic products
298 samples
23.9%
4.5%
95.5%
5.0%
95.0%
3.9%
64.8%
31.3%
10.6%
78.8%
10.6%
75.4%
0.7%
fig0002 Figure 2 Results of US Food and Drug Administration monitoring of imported foods for pesticide residues, 1999.
PESTICIDES AND HERBICIDES/Toxicology 4499
26% contained one residue, and 35% contained more
than one residue. Residues exceeding the tolerance
level were detected on 0.3% of the samples, and
residues of pesticides were detected on commodities
for which no tolerances of the pesticides were estab-
lished on another 3.7% of the samples.
Dietary Risks from Exposure to Pesticides in Food
0038 A common method used to discuss the potential
human health risks arising from the consumption of
pesticide residues in the diet is to report on the results
of regulatory monitoring programs, as has been done
in the previous paragraphs. Results indicate that a
large percentage of food samples contain no detect-
able residues and that violation rates are relatively
low, particularly for foods grown in the US. Fre-
quently, the recitation of such findings is used as
justification for a lack of significant human health
risks resulting from pesticide residues in foods.
0039 The problem with adopting such an approach is
that it ignores the important, yet confusing, fact that
pesticide tolerances are not safety standards but
rather represent the maximum legal residues expected
when pesticides have been used according to direc-
tions. Taking this a step further, violative residues
should not be considered to be unsafe residues in
most cases but merely represent cases in which pesti-
cides have been misapplied or have been transported
to commodities for which they are not registered.
Although cases of pesticide misuse have historically
resulted in a small number of incidents of acute
poisoning of people consuming tainted foods, it can
be reasonably argued that the vast majority of viola-
tive pesticide residues do not represent significant
health threats to consumers based upon common
toxicological and risk assessment criteria.
0040A more accurate approach for estimating human
dietary risks from pesticides is to use exposure data
derived from market basket surveys rather than regu-
latory monitoring data. The FDA, for example, per-
forms its Total Diet Study annually. This study uses a
market basket approach, with each market basket
containing more than 250 individual food items.
Foods are collected by FDA inspectors from three
cities in each of the four geographical regions of the
USA and are prepared for table-ready consumption
prior to analysis for pesticide residues. By combining
analytical results from estimates of typical consump-
tion rates of the various food items or their com-
ponents, it is possible to estimate the typical daily
exposure of members of the general population to
specific pesticides as well as to break the population
down further into subgroups defined by factors such
as age, gender, and geographical location.
0041Although the FDA no longer makes its dietary
pesticide exposure estimates from its Total Diet
Study available to the public, results reported from
the 1991 Total Diet Study indicate that, for most
pesticides in a variety of different population sub-
groups, the typical daily exposure estimates represent
only a small fraction (often less than 1%) of the
corresponding RfDs or ADIs. To put this into some
level of perspective, it should be noted that typical
RfDs are derived by first identifying the highest level
of exposure to a pesticide that causes no noticeable
0.2%
61.3%
38.5%
2.6%
97.4%
28.9%
71.1%
0.6%
38.8%
60.6%
1.2%
69.7%
29.1%
1.4%
75.5%
23.1%
Grains and grain products
468 samples
Milk/Dairy products/Eggs
116 samples
Fruits
1063 samples
Vegetables
1414 samples
No residue found Residue found
not violative
Residue found
violative
Other
147 samples
Fish/Shellfish
Other aquatic products
218 samples
fig0003 Figure 3 Results of US Food and Drug Administration monitoring of domestic foods for pesticide residues, 1999.
4500 PESTICIDES AND HERBICIDES/Toxicology

signs of toxicity in laboratory animals and then div-
iding that level by an uncertainty factor (usually 100)
that presumably covers potential variability resulting
from the animal to human extrapolation and from
interhuman variability. Exposure at a level of 1% of
the RfD represents an exposure 10 000 times below
the level that does not produce noticeable effects
in the animals. Such findings provide an illustration
of why the majority of health professionals consider
the typical human health risks from pesticides in the
diet to be much lower than food safety risks posed by
such factors as microbiological contamination of
foods, nutritional imbalance, environmental contam-
inants, and naturally occurring toxins. The risks from
pesticides in the human diet are clearly not zero, since
consumption of tainted foods has caused documented
human illnesses throughout the world, and concerns
remain regarding potential long-term effects of diet-
ary pesticide exposure.
0042 It is also clear that the potential health benefits
resulting from pesticide use should be considered.
Pesticide use has resulted in increases in production
of a wide variety of food crops, which translates into
greater availability and lower consumer costs and
thus a greater potential consumption of agricultural
products. Epidemiological studies have clearly indi-
cated that diets rich in consumption of fruits, vege-
tables, and grains may significantly decrease one’s
risk of heart disease and certain types of cancer. The
US National Academy of Sciences, among other sci-
entific bodies, has concluded that the theoretical in-
creased risks from pesticide exposure resulting from
increases in consumption of fruits, vegetables, and
grains were greatly outweighed by the health benefits
of these foods.
See also: Cancer: Epidemiology; Carcinogens in the Food
Chain; Carcinogens: Carcinogenic Substances in Food:
Mechanisms; Food and Drug Administration; Food
Poisoning: Classification; Pesticides and Herbicides:
Types of Pesticide; Types, Uses, and Determination of
Herbicides
Further Reading
Fong WG, Moye HA, Seiber JR and Toth JF (1999) Pesti-
cide Residues in Foods: Methods, Techniques, and Regu-
lation. New York: John Wiley.
Hayes WJ and Laws ER (1991) Handbook of Pesticide
Toxicology. San Diego, CA: Academic Press.
National Research Council (1987) Regulating Pesticides
in Foods: The Delaney Paradox. Washington, DC:
National Academy Press.
National Research Council (1993) Pesticides in the Diets of
Infants and Children. Washington, DC: National Acad-
emy Press.
Tweedy BG, Dishburger HJ, Ballantine LG and McCarthy J
(1991) Pesticide Residues and Food Safety: A Harvest of
Viewpoints. Washington, DC: American Chemical Soci-
ety Symposium Series No. 446.
Winter CK (1992a) Pesticide tolerances and their relevance
as safety standards. Regulatory Toxicology and Pharma-
cology 15: 137–150.
Winter CK (1992b) Dietary pesticide risk assessment.
Reviews of Environmental Contamination and Toxicol-
ogy 127: 23–67.
Winter CK (2001) Contaminant regulation and manage-
ment in the United States: The case of pesticides. In:
Watson DH (ed.) Food Chemical Safety, Volume 1: Con-
taminants, pp. 295–313. Cambridge, UK: Woodhead.
Winter CK and Francis FJ (1997) Assessing, managing, and
communicating chemical food risks. Food Technology
47: 85–92.
PH – PRINCIPLES AND MEASUREMENT
D Webster, Formerly of University of Hull, Hull, UK
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Background
0001 pH measurements have been, and continue to be,
widely used as a rapid, accurate measure of the acid-
ity of fluids of all sorts. There are two methods
for measuring pH: colorimetric methods using
indicator solutions or papers, and the more accurate
electrochemical methods using electrodes and a
millivoltmeter (pH meter). The development of the
glass electrode, which is convenient to use in a variety
of environments, and the development of the pH
meter have enabled the widespread application of
pH measurement and control to take place. The de-
termination, and hence the control of pH, is of great
importance in the food industry.
Basic Theory
0002In water, molecules (H
2
O) are in equilibrium with
hydrogenions(H
þ
)andhydroxideions(OH
)(eqn(1)).
PH – PRINCIPLES AND MEASUREMENT 4501

H
2
O Ð H
þ
þ OH
:ð1Þ
This ionization is of great importance in the chemistry
of aqueous systems, as it enables water to give or take
H
þ
ions, as required, by other dissolved substances.
0003 Applying the law of mass action to eqn (1):
½H
þ
½OH
=½H
2
O¼constant ;ð2Þ
where [] indicates the concentration in units of moles
per cubic decimeter (mol dm
3
).
0004 In pure water and dilute solutions, the concentra-
tion of the undissociated water may be considered
constant, hence ½H
þ
½OH
¼K
w
, where K
w
is a con-
stant called the ionic product of water.
0005 The ionic product varies with temperature, but at
about 25
C, its value is 10
-14
mol
2
dm
-6
(K
w
(0
C) =
10
14.9
, K
w
ð25
CÞ¼10
14 :0
, K
w
ð60
C Þ¼10
13 :0
).
This means that in pure water, the ionization is
very small. As the concentrations of H
þ
ions and
OH
ions are equal in pure water, and as
H
þ
OH
½¼10
14
mol
2
dm
6
at 25
C, H
þ
(or
OH
½Þ¼10
7
mol dm
3
.
0006 To be strictly correct, eqn (1) should be written as
eqn (3):
2H
2
O Ð H
3
O
þ
þ OH
, ð3Þ
where the H
þ
ion is attached to a water molecule to
form the oxonium ion (H
3
O
þ
). However, the symbol
H
þ
will be used throughout this article.
0007 When, in a solution, there are an equal number of
H
þ
and OH
ions, the solution is said to be neutral,
when there is an excess of H
þ
ions (> 10
7
mol dm
3
at
25
C), it is acidic, and when there is an excess of OH
ions ([H
+
<10
7
mol dm
3
), it is basic or alkaline.
pH Scale
0008 Although concentrations of H
þ
and OH
ions in
acidic and alkaline solutions can be expressed in
these molar concentrations, a much more convenient
method was introduced by So
¨
rensen in 1909. He
proposed the use of the H
þ
ion exponent pH, defined
by the relationship pH ¼log
10
½H
þ
. (This may also
be expressed as ½H
þ
¼10
pH
). To be strictly correct,
this equation is pH ¼log
10
ð½H
þ
=½1), as a loga-
rithm must be dimensionless. From this, it can be
seen that pH does not have units – the often-used
expression ‘pH unit’ is wrong.
0009 Using the pH scale, a change of 1 corresponds
to a 10-fold change in the H
þ
ion concentration,
a change of 2 corresponds to a 100-fold change,
etc. The pH scale has the advantage that all
solutions from 1 mol dm
3
acid to 1 mol dm
3
alkali can be expressed by positive numbers from 0
to 14. Thus, at 25
C, a neutral solution, which has
H
þ
¼ 10
7
mol dm
3
,hasapHof7,a1moldm
3
solution of a strong (completely ionized) acid such
as hydrochloric acid, which has H
þ
¼ 1 ¼ 10
0
mol dm
3
, has a pH of 0, and a 1 mol dm
3
solution
of a strong (completely ionized) alkali acid such as
sodium hydroxide, which has OH
½¼1 mol dm
3
,
hence H
þ
¼ K
w
= OH
½¼10
14
mol dm
3
, has a
pH of 14. pH values below 0 and above 14 occur in
solutions of strong acids and alkalis of strengths
greater than 1 mol dm
3
.
0010As K
w
varies with temperature, pH measure-
ments should be made at about 25
C. The pH of
a neutral solution at 0
C is 7.45 (K
w
¼ 10
14:9
,
H
þ
¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
10
14:9
p
¼ 10
7:45
mol dm
3
, and at 60
C,
the pH is 6.5 (K
w
¼10
13.0
,[H
þ
] ¼
ffiffiffiffiffiffiffiffiffiffiffiffiffiffiffi
10
13:0
p
¼
10
6.5
mol dm
3
).
0011To measure pH values, So
¨
rensen used the electro-
chemical cell:
Pt, H
2
ðgÞjSolution X jSalt bridge jHg
2
Cl
2
jHg:
This representation indicates a hydrogen electrode
(hydrogen gas passed over a platinum metal elec-
trode) in a solution X and a calomel electrode (metal-
lic mercury in contact with calomel (Hg
2
Cl
2
), in
contact with a chloride ion solution) separated by a
salt bridge (which allows ionic conduction to occur,
but prevents solution X and the chloride ion solution
from mixing). For such a cell, the difference in electro-
motive force (emf) (E
1
E
2
) between two cells in
terms of the H
þ
ion concentrations [H
þ
]
1
and [H
þ
]
2
of the two solutions is given by the equation
E
1
E
2
¼ðRT=FÞlog
e
ð½H
þ
2
=½H
þ
1
Þ, ð4Þ
where R is the gas constant, T the temperature, and F
the Faraday constant. If [H
þ
]
2
is 1 mol dm
3
, E
2
will
have a definite value, E
0
. Therefore, E
1
E
0
¼(RT/
F)log
e
ð1=½H
þ
1
Þ,orE
1
¼ E
0
þ (RT log
e
10=FÞ p
s
H
1
¼
E
0
þ 0.05916 p
s
H
1
V (at 25
C) (p
s
H is used here
for So
¨
rensen’s pH). So
¨
rensen assumed that H
þ
1
¼
a
1
M
1
where a
1
is the degree of dissociation of the acid
in solution 1, and M
1
is its molality. E
0
¼ 0.3376 V at
18
C.
0012After this initial work of So
¨
rensen, it was realized
that the emfs of cells depend on activity rather than
concentration, and other assumptions were incorrect.
So
¨
rensen and Linderstro
¨
m-Lang (1924) proposed
p
a
H ¼log
10
a
H
þ
¼log
10
H
þ
y
H
þ
, where a
H
þ
is
the activity, [H
þ
] is the concentration of the H
þ
ion
in mol dm
3
, and y
H
þ
is the activity coefficient of the
H
þ
ion on the molarity scale. (To be strictly correct, a
ratio of concentrations, as earlier, is needed here to
give a dimensionless quantity.) These p
a
H values can
be shown to be related to the original p
s
H values by
the relationship:
4502 PH – PRINCIPLES AND MEASUREMENT

p
a
H ¼ p
s
H þ 0 :04 :ð5 Þ
Operational Definition of pH
0013 In the previous section, some of the problems of the
theory of pH were discussed. Careful study has
shown that the numbers obtained depend, in a com-
plex manner, on H
þ
ion activity of electrolytes in
solution, and it became clear than an operational
definition of pH and a standard scale were needed
to unify the great variety of pH measurements being
made by research scientists and by industry and com-
merce. Hence, we now have an operational definition
that has been endorsed by the International Union of
Pure and Applied Chemistry. This definition is
pH ðXÞ¼pH ðSÞþðE
S
E
X
ÞF =RTlog
e
10, ð6 Þ
where E
x
is the emf of the galvanic cell.
Reference electrode jKCl solution jSolution X
jH
2
ðgÞ,Pt
and E
s
is the emf of the same cell with solution X of
unknown pH(X) replaced by solution S of standard
pH(S).
001 4 In practice, a glass electrode is almost always used
in place of the platinum/hydrogen gas electrode. The
reference electrode is usually mercury/mercury(I)
chloride (calomel), silver/silver chloride, or thallium
amalgam/thallium(I) chloride. The standard reference
pH is that of an aqueous solution of potassium
hydrogen-phthalate of molality 0.05mol kg
1
at
25
C. This has a pH of 4.005.
Food and pH
0015 There are many instances when the food scientist
needs to measure or control pH. For example, pH
control is vital in the clarification and stabilization
of fruit and vegetable juices, in the use and control of
enzymes and microorganisms, in the preparation
of foods by the fermentation of fruit or cereals, in
the control of the texture of jams and jellies, and in the
stability of the color and flavor of fruits.
001 6 The properties (e.g., emulsification and foaming
ability) of many colloidal systems are affected by pH
– these include proteins, pectins, and gums, all of
which occur in foods. Chemical reactions that can
occur in foods, particularly hydrolysis, are catalyzed
by hydrogen ions, and therefore can be controlled by
controlling the pH of the system. (See Colloids and
Emulsions.)
001 7 Hydrogen ions affect the rate of growth of molds,
yeasts, and, particularly, bacteria. Usually, there is a
pH value at which optimum growth occurs, above
and below which growth is inhibited. The relation-
ship of pH to sterilization by heat is of particular
significance during the canning of foodstuffs. (See
Canning: Principles; Spoilage: Bacterial Spoilage;
Molds in Spoilage; Yeasts in Spoilage.)
0018pH is a rough measure of the maturity of fruit.
Young fruits initially have a pH close to that of
the plant, but this rapidly decreases as the acidity
increases. The pH of acidic fruits such as lemons
and limes is about 2.0, and that of mildly acidic fruits
about 4.0. These values can be compared with vege-
tables, which have pH values of 5–6. The pH ranges
of a number of fruits and other foods is listed in
Table 1.(See Ripening of Fruit.)
Buffer Solutions
0019Buffer solutions are of prime importance in pH deter-
minations, as they are the standards for both colori-
metric and electrochemical methods of determining
pH.
0020Buffer solutions are solutions that resist a change in
H
þ
ion concentration (pH) when an acid or alkali is
added to them. They are made up of a weak acid or
weak base and its salt.
0021The equilibria in a solution of, say, a weak acid and
its sodium salt are shown in eqns (7) and (8):
tbl0001Table 1 pH values of some foods
Food pH
Limes 1.8–2.0
Lemons 2.2–2.4
Gooseberries 2.8–3.0
Plums 2.8–3.0
Pickles 3.0–3.4
Grapefruit 3.0–3.4
Oranges 3.0–4.0
Rhubarb 3.1–3.2
Cherries 3.2–4.0
Pineapples 3.4–3.7
Pears 3.6–4.0
Apricots 3.6–4.0
Tomatoes 4.0–4.4
Bananas 4.5–4.7
Cheese 4.8–6.4
Carrots 4.9–5.3
Spinach 5.1–5.7
Potatoes 5.6–6.0
Peas 5.8–6.4
Tuna 5.9–6.1
Corn 6.0–6.5
Salmon 6.1–6.3
Butter 6.1–6.4
Chicken 6.2–6.4
Drinking water 6.5–8.0
PH – PRINCIPLES AND MEASUREMENT 4503

HA Ð H
þ
þ A
ð7Þ
NaA Ð Na
þ
þ A
:ð8Þ
As HA is a weak acid, equilibrium (eqn (7)) at
moderate concentration will lie over to the left, i.e.,
as undissociated acid HA. The equilibrium of the
sodium salt (eqn (8)) is a source of A
ions. If acid
(i.e., H
þ
ions) is added to this weak acid and salt
solution, the H
þ
ions will be removed as they will
combine with the A
ions to form undissociated HA.
If base (i.e., OH
ions) is added, the OH
ions will be
removed as they will combine with the H
þ
ions to
form water and the equilibrium (eqn (7)) will move to
the right to supply the H
þ
ions required. Hence, the
concentration of H
þ
ions in the solution (i.e., the pH)
will not change significantly. This is the buffer effect.
0022 Common buffer solutions are made from potas-
sium hydrogenphthalate with hydrochloric acid
or sodium hydroxide (for pH 3–5), potassium
dihydrogenphosphate with sodium hydroxide (for
pH 6–8), and boric acid with sodium hydroxide
(for pH 8–10).
0023 Biologically, the buffer effect is often more import-
ant than the absolute value of pH. For example,
buffer chemicals affect the way in which enzymes
and microorganisms respond when the pH is
changed. Juices from plant tissue have the ability to
buffer pH changes to a greater or lesser degree, owing
to the presence of organic acids, acid–salt systems,
proteins, and acid phosphates.
Measurement of pH
Colorimetric Methods
002 4 Determination of pH using the color of acid–base
indicators is a very simple technique that can be
carried out rapidly and reproducibly. Approximate
pH values can be obtained very quickly using pH
papers. Under optimum conditions, accurate values
of pH can be obtained by colorimetric methods. No
doubt, these methods will be used for many years to
come, but must become less favored with the advent
of portable, cheap, easy-to-use electrochemical pH
meters. (See Spectroscopy: Visible Spectroscopy and
Colorimetry.)
0025 Color change of acid–base indicators Indicators are
natural or artificial dyestuffs that are weak acids or
bases (acids and bases that are only partially dissoci-
ated) and have different colors in their acidic and
basic forms.
0026 If the indicator is written as HIn and it dissociates
(eqn (9))
HIn Ð H
þ
þ In
, ð9Þ
the equilibrium (or ionization) constant is
K
In
¼½H
þ
½In
=½HIn ;ð10Þ
and
½H
þ
¼K
In
ð½HIn =½In
Þ:ð11Þ
Using pH ¼log
10
[H
þ
],
pH ¼ pK
In
þ log
10
ð½In
=½HInÞ; ð12Þ
pK
In
is log
10
K
In
and is called the indicator con-
stant. The ratio [In
]/[HIn] determines the color of
the indicator in the solution, and eqn (12), therefore,
directly relates the color to the pH of the solution.
0027Eqn (12) shows that when pH ¼ pK
In
, the concen-
tration of the indicator in each form is equal (i.e.,
½HIn¼½In
), and that at all pH values, both
the acid (HIn) and basic (In
) forms of the indicator
are present in the solution. Our eyes are unable
to detect the color of less than about 10% of one
form of the indicator in the presence of the other,
and the solution will appear to be the ‘acid’ color
when [In
] / [HIn] < 1/10, and the ‘alkaline’ color
when [In
] / [HIn] > 10. Therefore, the solution will
be the color of the acid form of the indicator until
pH ¼ pK
In
1, when the color will appear to change
until it is that of the alkaline form of the indicator
from pH ¼ pK
In
þ 1. That is, the color changes over
approximately 2 in terms of pH. In practice, the range
for the color change is about 1.6–2 in terms of pH;
this corresponds to a 40–100-fold change in the H
þ
ion concentration. By choosing indicators with ap-
propriate pK
In
values, the whole of the pH range
may be covered – a selection of some common pH
indicators is given in Table 2.
0028Colorimetric Measurements of pH
Indicator papers For approximate determination of
pH values, indicator papers may be used. Of
particular value are ‘nonbleeding’ indicator papers.
These contain dyestuffs strongly bound to the cellu-
lose, so that the dye does not bleed into the test
solution. These indicator papers are available,
covering very narrow pH ranges, enabling moder-
ately accurate pH values to be obtained very cheaply,
conveniently, and quickly.
0029Comparison method This is carried out by adding
similar quantities of an indicator to the test solution
and a reference solution, and if the two solutions have
the same color, they are assumed to have the same
pH. The accuracy of the method, therefore, depends
on the accuracy of the pH of the reference solution.
4504 PH – PRINCIPLES AND MEASUREMENT
