Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


continuous belt, rotary wiper, and Rogueing glove
application. The Rope-wick device is a rig consisting
of a series of short, exposed nylon ropes, each end of
which is connected to a reservoir of herbicide solu-
tion. The solution passes into the ropes by both capil-
lary action and gravitational flow. As the applicator
moves through the weed-infested field, the chemical
on the soaked wicks is rubbed on to the tops of the tall
weeds but not on to lower-growing crop. Spray drift
is eliminated. The carpet roller, as the name suggests,
is a tractor mounted with a nylon carpeted roller
soaked with liquid herbicide through a delivery
system from a herbicide reservoir. The design is such
that herbicide will only be in contact with the tall
weeds without contacting the desired crop. A con-
tinuous belt consists of a V-belt, with sponges glued
on to it, passing through a herbicide-containing reser-
voir. An adjustable pressure wheel removes excess
herbicide from the sponges prior to its application
on to the weeds. This helps prevent herbicide from
contacting the crop. A rotary wiper applicator con-
sists of flexible arms that allows wiper rotation
around stationary objects to avoid injury to tree
trunks. A glove equipped with absorbent pads that
has continuous loading of herbicide solution from a
reservoir is known as the Rogueing glove application.
0011 A relatively new way of applying herbicide is
through a sprinkler system. Liquid herbicide is pumped
into the system and sprayed in a manner similar to a
water sprinkler system.
0012 Aerial spraying, using small aircraft or helicopters,
can cover a large field in a shorter time period.
Sprayers similar to those used in the ground operation
can be mounted on to the aircraft for application to
large areas.
0013 Considerable research has been conducted by chem-
ical companies to devise controlled-release formula-
tions, a technology which has been demonstrated to be
safe and effective in the drug industry. The three con-
trolled-release systems are: (1) the chemically bound
system, in which the herbicide is bound to a polymer;
(2) the microencapsulation system, where the herbi-
cide is coated by a polymer; (3) the matrix encapsu-
lation system, where the herbicide is dispersed within
a polymer matrix. This technology renders safer herbi-
cide handling, reduces environmental pollution, and
enhances herbicide selectivity.
0014 The treatment of seeds with herbicides is another
area of research currently under investigation. Cer-
tain herbicides can be applied to certain crop seeds
prior to planting. Once planted in the soil, the her-
bicide moves swiftly away from the seed to the
surrounding area to inhibit weed growth. This ap-
proach is economical and convenient to use. It also
has a lesser tendency to pollute the environment.
Safety Implications
0015Ideally, herbicides are chemicals designed to cause
injury only to undesirable weeds. Due to the biological
differences between plants and animals, herbicides
have low acute toxicity towards humans. However,
most chemicals tend to have more than one metabolic
effect on different living organisms. It is such unex-
pected secondary or side-effects of chemicals that
cause major concern. Before the approval for
marketing of any herbicide by the government, chem-
ical companies must conduct extensive studies on the
effectiveness of new herbicides. In addition, studies on
metabolic fate and toxicity to animal species and
plants are conducted to insure that the herbicide is
safe to be used in the field. The cost of these testing
procedures is tremendous. The specific requirements
for herbicide registration depend upon the law of the
country in which they are used but, in general, herbi-
cides may not be used without registration by some
governmental agency that carries out extensive
reviews of the studies and tests performed by the
chemical companies. It is also the duty of the vendor
to provide users with information about all precau-
tions and safe handling procedures of the herbicide.
Adherence to those instructions by users is of utmost
importance for the avoidance of harmful exposure.
0016The health effects of herbicides and their environ-
mental impacts have been a major concern of the
public. The health and safety issues associated with
herbicide use affect not only consumers, but also
farmers, formulators, applicators, and field workers,
as well as the users of home and garden products.
Groundwater contamination by agricultural chem-
icals has been an issue worldwide and will continue
to be important in the years to come. Restrictions on
the use and banning of certain herbicides are on the
rise. Development of biodegradable herbicides with
low residue levels combined with improved delivery
systems can help protect the environment.
0017Depending on the conditions, exposure to herbi-
cides at a high concentration can be fatal. Precautions
in handling herbicides should be strictly followed to
avoid unnecessary exposure. Safety training of all
personnel designated to handle herbicides is impera-
tive, and refresher safety training should also be con-
ducted periodically to keep personnel up-to-date on
all safety issues.
0018Equipment for application should be inspected fre-
quently to insure that it is functioning properly. Mal-
functioning equipment should be repaired prior to
usage. Emergency procedures covering spillage or ac-
cidental poisoning should be established and strictly
followed. There should be health surveillance pro-
grams for workers to monitor and insure worker
PESTICIDES AND HERBICIDES/Types, Uses, and Determination of Herbicides 4485

well-being. Personal protective equipment and
clothing should be checked for leaks periodically
and should be kept clean. Good record-keeping of
herbicide inventory and application is necessary to
account for usage.
0019 In order to avoid inhalation of herbicides during
application, workers should wear respirators. There
are many different kinds of respirators, namely the
chemical cartridge respirators, powered air-purifying
respirators, canister respirators, supplied-air respir-
ators, and self-contained breathing apparatus. There
are also certain herbicides that can be absorbed by the
skin; users should handle these with due caution and
should wear appropriate protective clothing, includ-
ing face shields.
0020 Care should be given in the storage and transport of
herbicides to minimize spillage. In case spillage occurs,
proper decontamination should be performed imme-
diately. Maintenance of all equipment for dispensing
herbicides should be done routinely. Warnings should
be given in advance to alert others of possible herbi-
cide drift during application, and warning signs and
restricted-entry signs should be posted to prevent
others from entering the treated areas.
Specific Examples of Uses
0021 Weeds are usually defined as undesirable plants. Weeds
are often the primary concern of farmers, because
they cover many millions of productive acres that
could be used to grow beneficial crops. In the past,
farmers controlled weeds by manually removing them
from the crops. The ancient Romans killed weeds
with salt. With farms of small size, manual weed
control, such as hand hoeing and pulling, mowing,
burning, and machine tillage, is feasible. However,
with large farms such labor is extensive and costly.
As the size of the farms increased and synthetic herbi-
cides were introduced, farmers began to rely on herbi-
cides to control weeds.
0022 Generally, there are three different types of treat-
ment for the application of herbicides. They are
preplanting, preemergence, and postemergence treat-
ments. Preplanting treatment takes place prior to
planting; preemergence treatment is done after
planting but preceding the crop or weed emergence;
and postemergence treatment is performed after the
emergence of the crop or weeds.
0023 2-4-Dichlorophenoxyacetic acid (2,4-D) was one
of the first synthetic herbicides introduced to control
broad-leafed weeds in cereal crops and pastures. 2,4-
D is an effective systemic herbicide and is selective for
broad-leafed plants but not grasses. It can be used as
either a pre- or a postemergence herbicide for corn,
but only as postemergence for sorghum. 2,4-D is
highly versatile and is used on a variety of crops
such as wheat, barley, oats, rice, and sorghum.
During the Vietnam war, Agent Orange, a 50: 50
mixture of 2,4-D and 2-4-5-Trichlorophenoxyacetic
acid (2,4,5-T), was used extensively over the terrain
in Vietnam as a defoliant to clear the way for US
troops. 2,4,5-T is usually contaminated with dioxin,
a highly toxic chemical compound and known car-
cinogen. It is due to this notorious contaminant that
Agent Orange has been blamed for various illnesses
and reproductive problems among those who came in
contact with the defoliant in Vietnam.
0024Paraquat is used as a preemergence treatment for
sugar beet. Simazine, on the other hand, is used both
as a preplant and as a preemergence treatment
for corn. S-ethyl dipropylthiocarbamate (EPTC) is
incorporated into the soil as a preplant treatment
for potatoes.
0025Herbicides have made it possible to grow more
food on less land with less labor and at lower cost.
Herbicides are also used to control aquatic weeds
which impede water flow in irrigation canals and
drainage systems, interfere with fishing, or promote
insect-breeding grounds.
Stability in the Environment
0026For herbicides to exert their effects on weeds, they
must be relatively stable in the treated environment.
However, the stability of the chemical creates a burden
on the environment, especially for those herbicides
that find their way into aquifers and contaminate
drinking water sources or remain on the crops at the
time they reach consumers. In order to insure that the
newer generation of herbicides do not linger on after
accomplishing their task, research is directed towards
synthesizing biodegradable compounds. The ideal
herbicide is one that degrades to harmless chemicals
after it performs its function and therefore does not
persist in nature. Carbamates are one such class of
chemicals specifically designed with that goal in mind.
Analysis of Residues in Foods
0027There is increasing awareness among consumers of
the hazard of chemical contamination of food and
drinking water. There is particular concern over the
implications of food contamination by herbicide resi-
dues. Analysis for herbicide residues in food requires
methods that identify not only parent structures, but
also their metabolites and degradation products in a
variety of food matrices. Certain food crops are per-
ishable and therefore cannot wait for lengthy analysis
to establish the suitability for consumption. Thus,
rapid analytical technology is needed. Multiresidue
4486 PESTICIDES AND HERBICIDES/Types, Uses, and Determination of Herbicides

methods, which can detect the presence of many herbi-
cides at once, are the methods of choice for determin-
ing the presence of a multiple number of herbicides
and their degradation products in a food sample.
0028 An analytical process consists of several major steps:
the sample preparation, the extraction, the clean-up,
the determinations, and the confirmation. These steps
are common to the determination of other agrochem-
ical residues, including pesticides, and are discussed in
detail in the following article. The basic operation of
sample preparation is to separate physically food or
plant parts and to chop and blend them. The essence of
the extraction process is to remove the target herbicide
from the other components in the sample matrix. The
main function of the clean-up procedure is to remove
interfering constituents, usually by selective partition-
ing into organic solvents followed by an adsorption or
size exclusion chromatographic purification step. The
determination step includes separation of the purified
samples through thin-layer chromatography, gas chro-
matography, or liquid chromatography techniques
followed by the detection procedure using a variety of
specific detectors for the targeted compound. For con-
firmation purposes, the analyte is further subjected
to mass spectrometric analysis. Recently, successful
attempts have been made in using gas chroma-
tography–mass spectroscopy (GCMS) as a primary
screening method. The GCMS screening technique
provides simultaneous results for both the detection
and the confirmation of the targeted compound in the
sample matrix. This one-step procedure will be the
method of choice as it offers both rapid and definitive
data. (See Chromatography: Thin-layer Chromatog-
raphy; Gas Chromatography; Mass Spectrometry:
Principles and Instrumentation.)
0029 Improvements to existing analytical technology
are well underway to reduce the time- and solvent-
consuming extraction and clean-up steps. Supercrit-
ical fluid extraction, which is based on the solvent
properties of gases such as carbon dioxide at its critical
pressure and temperature, can selectively remove the
targeted compound from the complex food matrix in a
short time. Through such approaches the recovery of
the compound can be easily accomplished.
0030 The use of antibodies as analytical tools is a
common practice in clinical laboratories. Antibodies
have been recently developed for identifying and
quantifying herbicides. Antibodies can be isolated
from the plasma of an immune-challenged animal or
from a hybridoma cell line. An antibody that is spe-
cifically generated from a compound will have high
selectivity towards that compound even in the midst
of other interfering components and can bind to it
tightly to form a complex. Therefore, by attaching a
tracer to the antibody molecule, one can quantify the
amount of antibody complex present, which is also an
indication of the amount of the compound in the
sample. A variety of tracers are available, for example
radioisotopes, fluorescent molecules, and so forth.
One of the disadvantages of the immunoassay is
that the length of time to generate the specific anti-
body is relatively long. Typically, it takes approxi-
mately a year to develop. However, once it is
generated, the immunoassay can be performed in
less than half an hour. The triazine immunoassay
is now available commercially, and, like most im-
munoassays, it is specific, sensitive, rapid, and cost-
effective. (See Immunoassays: Principles.)
0031One of the modes of action of herbicides is by
inhibiting photosynthesis. The Hill reaction is one of
the processes in the photosynthetic pathway; there-
fore, a screening technique based on the inhibition of
the Hill reaction can be a useful tool in detecting
herbicides like triazines and carbamates.
See also: Chromatography: Thin-layer Chromatography;
Gas Chromatography; Immunoassays: Principles; Mass
Spectrometry: Principles and Instrumentation;
Pesticides and Herbicides: Residue Determination
Further Reading
Ashton FM and Crafts AS (1981) Mode of Action of
Herbicides, 2nd edn. New York: Wiley.
FDA (1968) Pesticide Analytical Manual, 2nd edn, vol. 1.
Methods Which Detect Multiple Residues. Washington,
DC: US Department of Health Services, Food and Drug
Administration.
Klingman GC, Ashton FM and Noordhoff LJ (1982) Weed
Science: Principles and Practices, 2nd edn. New York:
Wiley.
McWhorter CG and Gebhardt MR (eds) (1987) Methods of
Applying Herbicides. Monograph Series of The Weed
Science Society of America, no. 4. Champaign, IL: The
Weed Science Society of America.
Newton M and Knight FB (1981) Handbook of Weed and
Insect Control Chemicals for Forest Resource Managers.
Beaverton: Timber Press.
Residue Determination
S Nawaz, Central Science Laboratory, Sand Hutton,
York, UK
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001Continued population growth has led to an increased
demand on the world’s natural resources. Pesticides
are widely used to help increase the yield and improve
PESTICIDES AND HERBICIDES/Residue Determination 4487

the quality of crops. Pesticides are categorized
according to their mode of action and include insecti-
cides, herbicides, fungicides, acaricides, nematicides,
and rodenticides. Pesticides are also used as plant
growth regulators and for public-health purposes.
Global sales of pesticides during 1996 were estimated
at over US$30 billion. Herbicides, insecticides, and
fungicides accounted for 48, 28, and 18%, respect-
ively, of the total sales (see Table 1). There are over
900 chemicals registered for plant protection pur-
poses in the European Union (EU) alone. In addition
to the existing pesticides, there are an ever increasing
number of new chemicals being granted approval.
0002 As pesticide use can leave undesirable residues,
various national and international authorities regu-
late the use of pesticides and set maximum residue
levels (MRLs) in crops. An MRL is the maximum
concentration of a pesticide and/or its toxic metabol-
ites legally permitted in food commodities and animal
feeds. If pesticides are properly applied at the recom-
mended rates, and crops are only harvested after
the appropriate time intervals have elapsed, residue
levels are not expected to exceed MRLs. The residue
levels in foods derived from commodities that comply
with the respective MRLs are also intended to be
toxicologically acceptable. In the EU, the regulation
of the agrochemical industry, and the setting of MRLs
is currently being harmonized across all member
states by the EC. In the USA, the Environmental
Protection Agency is responsible for such regulation.
The Codex Alimentarius Commission (FAO/WHO)
has published tables of MRLs which have official
status across the world and are used to aid inter-
national trade. The monitoring of the residues in
foods is often at the microgram per kilogram level
or lower and has to be supported by strict analytical
quality-control standards, so that the analysis pro-
duces unequivocal, precise, and accurate residue
data. Before samples are analyzed, the analyst has to
demonstrate that the intended analytical method can
achieve adequate specificity, sensitivity, linearity, ac-
curacy, and precision at the relevant analyte concen-
tration and in appropriate matrices. The calibration
solutions must be prepared using certified reference
standards. Residue analyses normally include the
metabolites, isomers, and other related compounds
included in the MRL definition. Many methods can
determine a large number of residues in a single ana-
lytical run; these multiresidue methods are in common
use and help reduce the total cost of analysis.
Sampling
0003A representative sample consists of a large number of
randomly collected units. It is not always possible to
collect large samples because of the cost of transpor-
tation and the practicalities of sample handling in the
laboratory. Monitoring of pesticide residues for MRL
compliance involves analysis of a composite sample,
made up of a number of individual units. Recent
research has shown that pesticide residues in individ-
ual units of fruit and vegetables can exhibit an ex-
tremely skewed distribution, and this is likely to add
to the difficulty of taking a representative sample. The
guidelines for obtaining composite samples for MRL
compliance monitoring are published by the Codex
Alimentarius Commission and are summarized in
Table 2. In addition to checking for MRL compliance,
residue analyses are also carried out to investigate
other issues such as cases of misuse or the deliberate
poisoning of wildlife or domestic animals. In such
instances, a more targeted sampling regime may be
adopted, and a qualitative analysis may suffice.
Sample Preparation/Subsampling
0004Samples should be analyzed without any delay, as
some pesticide residues may degrade rapidly. If
tbl0001 Table 1 Major classes of chemicals used as pesticides
Type of chemical Examples and their primary uses
Benzimidazole Benomyl (F), carbendazim (F),
thiabendazole (F)
Bipyridylium Diquat (H), paraquat (H),
Carbamate Aldicarb (I, N), carbaryl (I),
carbofuran (A, I, N)
Dithiocarbamates Mancozeb (F), ziram (H), thiram (H)
Organochlorine DDT (I), lindane (I), endosulfan (I)
Organophosphorus Chlorpyrifos, malathion (I, A),
parathion (I)
Pyrethroids Permethrin (I), cyfluthrin (I)
Substituted phenyl ureas Diuron, linuron, monolinuron (H)
Triazine Atrazine (H), simazine (H)
A, acaricide; F, fungicide; H, herbicide; I, insecticide; N, nematicide.
tbl0002Table 2 Codex guidelines for collection of representative
samples
Sample type Minimumweight of sample (kg)
Small or light products
(e.g., berries, peas, spinach)
1
Medium sized products
(e.g., apples, carrots, potatoes)
1 (at least 10 units)
Large size products
(e.g., melons)
2 (at least 10 units)
Dairy products
(e.g., cheese, butter)
0.5
Meat, poultry, fish 1
Oils and fats 0.5
Cereals and cereal products 1
4488 PESTICIDES AND HERBICIDES/Residue Determination

immediate analysis is not possible, storage of samples
at 20
C may help minimize the degradation pro-
cess. Typically, a 20–50-g portion (subsample) is re-
quired for analysis. In order to obtain representative
subsamples, it may be necessary to grind/mill and
thoroughly mix the whole sample, so that any resi-
dues present are evenly distributed. This process is
especially important because residue levels can ex-
hibit a high degree of variability between individual
units. Some pesticides are known to degrade during
the processing of fruit and vegetable samples at ambi-
ent temperature. Milling frozen food samples in the
presence of excess solid CO
2
(dry ice) has been shown
to minimize the losses of most pesticide residues
during the process.
0005 Domestic mills (e.g., coffee grinder) as well as more
specialized mills are used to grind samples of cereals,
nuts, and pulses. Manual methods, such as cone and
quarter or mechanized devices such as the riffle div-
ider can be used to obtain representative subsamples
from such samples. Samples of animal tissues are
minced and mixed thoroughly before subsampling.
For pesticides (e.g., organochlorines) that accumulate
in the fatty tissue of animals, visible layers of fat may
be removed for direct analysis. Preparation of homo-
genous fruit and vegetable samples prior to sub-
sampling may be carried out using domestic food
processors and blenders. However, larger specialized
mechanical bowl choppers are more suitable for large
samples, and heavy-duty choppers may be required to
process frozen samples.
Extraction
0006 The extraction step involves the quantitative transfer
of pesticide residues from the food matrix into solv-
ent(s). The efficiency of extraction process depends
on the physicochemical properties of the solvent(s)
and analytes. Important factors include pH, polarity,
temperature, sample/solvent ratio, presence of water,
and degree of analyte/matrix binding. Most extrac-
tion procedures employ organic solvents in the pres-
ence of water. The presence of water is critical for
extraction of pesticides from cereals and cereal prod-
ucts, as it helps reduce the binding between residues
and matrix. Samples of cereals, nuts, pulses, fruit, and
vegetable samples are extracted by simple homogen-
ization with an organic solvent. The most commonly
used solvents include ethyl acetate, acetone, acetoni-
trile, hexane, methanol, and dichloromethane. For
multiresidue extraction methods, it is not possible to
establish the optimum extraction conditions for all
residues with differing physical and chemical proper-
ties so the choice of solvent polarity is usually a
compromise. Although the presence of water may
aid the extraction process, most polar pesticides are
partitioned into the water phase if the organic solvent
used is not water-miscible. Addition of anhydrous
sodium sulfate prior to extraction can overcome
this problem. The analysis of nonpolar pesticides in
fatty products involves extraction of fat using non-
polar solvents such as hexane, n-pentane, or light
petroleum. After the evaporation of the solvent, the
fat is redissolved in an organic solvent prior to the
clean-up and determination steps.
0007Other methods used for residue extraction include
soxhlet, where samples are exposed to solvent vapor
that is condensed and vaporized repeatedly to
exhaustively extract analyte(s). Supercritical fluid ex-
traction (SFE) methods use a gas under high pressure
and above the critical temperature to extract the resi-
dues. This technique is more widely used for samples
with low moisture content (cereals) where sample/
analyte binding is more common.
0008For residues that are not suited to multiresidue
extraction methods, dedicated extraction methods
may be used for single or small groups of closely
related pesticides. These methods utilize physical
and chemical properties of analyte and solvent to
carry out selective extraction of the analyte from
matrix. A number of pesticides are normally analyzed
using single residue methods and include formeta-
nate, fluazifop, 2,4-D, formetanate, propamocarb,
and maleic hydrazide.
Clean-up
0009Sample extracts not only contain the target analyte(s)
but may also contain coextractives, such as plant
pigments, proteins and lipids. These coextractives
may have to be removed prior to instrumental analy-
sis to avoid possible contamination of instruments
and to eliminate compounds that interfere during
the determination step. To achieve low detection
limits, sample extracts may also require a concentra-
tion step, which can be incorporated in the clean-up
procedure. Clean-up procedures can lead to losses of
residues and increases in the cost of analysis, and can
reduce the sample throughput. Therefore, a number
of methods utilize minimal clean-up and instead rely
on the selectivity of the detector(s).
0010A number of analytical procedures employ liquid–
liquid extraction for clean-up of sample extracts
by selective partitioning of analytes between two
immiscible solvents. This technique is commonly
used during the analysis of fatty samples. Liquid–
liquid extraction is not easy to automate and requires
the use of large amounts of solvents. (See Analysis of
Food.)
PESTICIDES AND HERBICIDES/Residue Determination 4489

0011 Adsorption chromatography is used in many resi-
due laboratories for the clean-up of sample extracts.
This process involves either:
.
0012 retention of analyte(s) on a chromatographic
column while the coextractives are unretained:
(the analyte(s) are then selectively eluted from
adsorption medium); or
.
0013 retention of coextractives on a chromatographic
column while the analyte(s) are unretained.
0014 A number of materials are available for adsorption
chromatography, including alumina, florisil, cellu-
lose, diatomaceous earth (celite), carbon (charcoal,
graphite), silver nitrate, and silica. Alumina and silica
are effective for the clean-up of fatty samples for
organochlorine (OC) pesticide residue analysis. Silver
nitrate is used for the removal of sulfur-containing
intreferences. Carbon has a high affinity for plant
pigments and is particularly suitable for the clean-up
of green leafy vegetable extracts with a high chloro-
phyl content.
0015 Chemically modified silica sorbents are widely
used for the clean-up of sample extracts. These
materials are prepared by the reaction of silanol
groups on silica surfaces with silane reagents to
form esters containing required functional groups.
These sorbents are used in cartridges, disks, mem-
brane filters, and impregnated fiber tips. Some
chemically modified silica and other sorbents used
for clean-up of sample extract are given in Table 3.
0016 Gel-permeation chromatography (GPC) or size-
exclusion chromatography separates molecules on
the basis of their molecular size. Large molecules
(e.g., lipids, pigments, and polymeric coextractives)
elute faster than smaller molecules such as pesticide
residues. The reproducibility, suitability to automa-
tion, and compatibility with a wide range of pesticide/
matrix combinations make GPC a popular clean-up
method in many laboratories worldwide. The disad-
vantages of the technique include the use of large
quantities of solvents, limited sample throughput,
and incomplete separation of high-molecular-mass
pesticides from coextractives. (See Chromatography:
Principles.)
Chromatography and Determination
0017The final stage of the pesticide residue analysis pro-
cedures involves the chromatographic separation and
instrumental determination. Where chromatographic
properties of some pesticides are affected by sample
matrix, calibration solutions should be prepared in
sample matrix. The choice of instrument depends on
the physiochemical properties of the pesticide(s) and
the sensitivity required. As the majority of pesticides
are relatively volatile, gas chromatography (GC) has
proved to be an excellent technique for pesticide
determination and is by far the most widely used. A
typical chromatogram from a multiresidue method is
shown in Figure 1.(See Chromatography: Gas Chro-
matography.)
0018Most residue methods employ splitless injection of
1–3 ml of the sample extract. Cold on-column injec-
tion is employed when the pesticides are likely to
breakdown in a hot injection port. A wide range of
GC column types are used for residue analysis, and
the choice depends on the physicochemical properties
of the analytes. Fused silica capillary columns are
most widely used during analysis of pesticides (see
Table 4). Typical capillary columns are 25–30 m
long with an internal diameter range of 0.1–0.5 mm
and a stationary phase of 0.1–10 mm thickness. Non-
polar stationary phases are used for the separation of
nonpolar pesticide residues such as OC and pyreth-
roids. Similarly, more polar pesticide residues (e.g.,
methadimophos) are separated on relatively polar
columns. The conventional semiselective detectors
are widely used for residue analysis. Electron-capture
detectors (ECDs) are utilized for halogenated pesti-
cides (OCs and pyrethroids). Nitrogen phosphorus
detectors are used for organophosphorus (OP) and
nitrogen-containing pesticides. Flame-photometric
detectors (FPDs) are used for OP and sulfur-
containing pesticides, while atomic emission detect-
ors can be used for a wide range of pesticides.
0019Gas chromatography–mass spectrometry (GC–
MS) has been the predominant technique for the con-
firmation of pesticide residues in the past. Relatively
inexpensive bench-top instruments have made the
technique more widely available for routine screening
in recent years. The resolving power of GC coupled
with the specificity of mass spectroscopy provides the
most effective means of pesticide residue analysis.
A number of ionization techniques are available for
GC–MS instruments and include electron impact (EI)
and chemical ionization (CI). EI impact ionization
tbl0003 Table 3 Examples of sorbents used for clean-up of sample
extracts
Nonpolar Polar Ion exchange
Octadecyl (C18) Florisil SCX
benzenesulfonylpropyl
Octyl (C8) Diol (2OH) PRS sulfonylpropyl
Ethyl (C2) NH
2
aminopropyl Water’s Oasis
TM
divinylbenzene:
vinylpyrrolidione
copolymer
Polypropylene Silica SAX
trimethylaminopropyl
4490 PESTICIDES AND HERBICIDES/Residue Determination
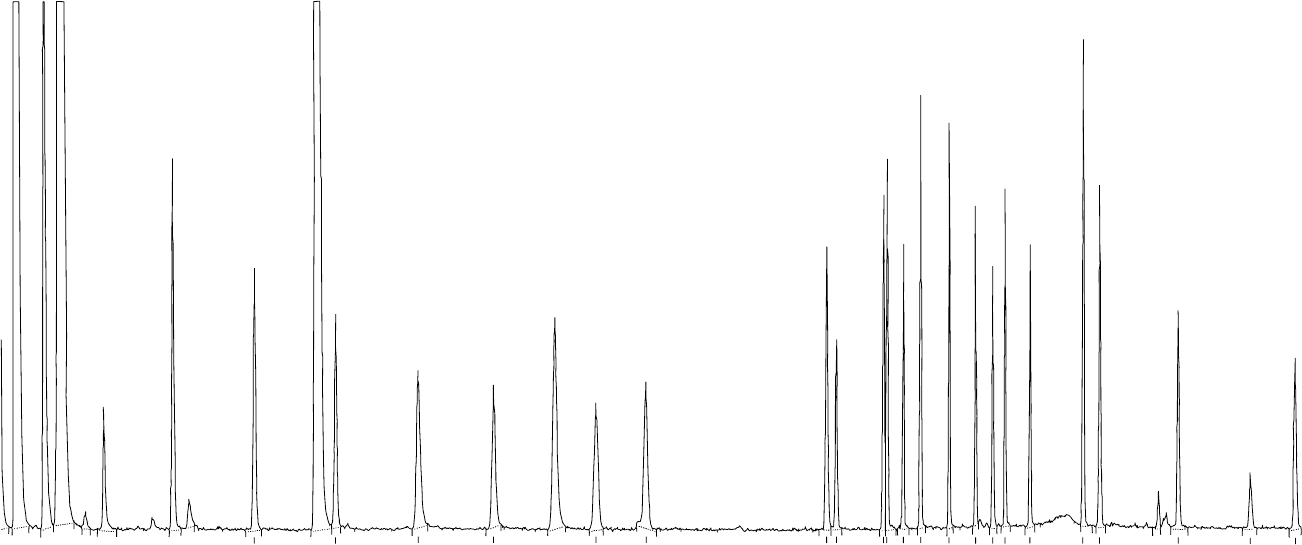
9.79
11.36
12.98
14.43
16.48
17.45
21.02
21.21
22.14
22.21
22.86
23.42
23.92
24.25
24.49
24.97
26.01
26.33
27.85
29.22
30.08
22.53
fig0001 Figure 1 Chromatogram (GC–FPD) obtained from an injection of cauliflower matrix matched calibration solution containing 0.3 ng of each of following pesticides (and their retention times
in minutes): heptenophos (9.79), ethoprophos (11.36), monocrotophos (12.98), dimethoate (14.43), fonofos (16.48), diazinon (17.45), parathion-methyl (21.02), malaoxon (21.21), fenitrothion
(22.14), pirimiphos-methyl (22.21), malathion (22.53), parathion (22.86), pirimiphos-ethyl (23.42), mecarbam (23.92), methidathion (24.25), tetrachlorvinphos (24.49), profenofos (24.97), ethion
(26.01), triazophos (26.33), pyridaphenthion (27.85), azinphos-methyl (29.22), and pyrazophos (30.08).

leads to a greater degree of fragmentation of molecules
compared with CI. Hence, CI provides a greater sensi-
tivity, but EI provides more spectral information. The
detection systems most widely utilized in residues an-
alysis are based on either the quadrupole or ion-trap
principle. The quadrupole instruments have limited
sensitivity in the scan mode compared with the ion-
trap instruments. However, by operating in the
selected ion mode, adequate sensitivity can be
achieved on quadrupole detectors. A small quadru-
pole mass selective detector can typically detect over
100 pesticides in food extracts at the relatively high
levels (typically 0.2 mg kg
1
) using full scan spectra,
reducing to lower levels (typically 0.01 mg kg
1
) in the
selected ion mode. The ion-trap detectors have a
higher inherent sensitivity, and this allows screening
of clean sample extracts for a wide range of pesticides
at low levels (typically 0.05 mg kg
1
) in full scan
mode. The major advantage of the ion-trap instru-
ments is that the characteristic ions can be selected
and then further fragmented to provide added specifi-
city (MS–MS). (See Chromatography: Combined
Chromatography and Mass Spectrometry.)
0020 High-performance liquid chromatography (HPLC)
is increasingly being used for the determination of
pesticide residues, as it is especially suited to the
analysis of nonvolatile, polar, and thermally labile
residues that are difficult to analyze using GC. The
resolution achieved on HPLC can be comparatively
low, and therefore, the use of selective detection
systems may be necessary for reliable residue analysis.
Ultraviolet (UV) spectroscopy is the most common
choice for detection of (OP) residues in environmen-
tal samples (e.g., soil, water). Although UV detection
is not a very selective technique, it is commonly used
for screening purposes due to its low cost, simplicity,
and wide application range. Elimination of interfer-
ences and optimized chromatography are essential
prior to detection in order to enhance the selectivity
of UV-based methods. The use of diode array
detectors can further enhance the selectivity of UV-
detection procedures. Fluorescence detection offers a
greater selectivity and sensitivity than UV. Pesticides
with inherent fluorescence include dimethoate,
ethoxyquin, azinphos methyl, phosalone, thiabenda-
zole, and carbendazim. With the exception of thia-
bendazole and carbendazim, this technique is not
widely used in pesticide residue analysis, as methods
based on inherent fluorescence have a poor sensitivity
compared with other methods available. Precolumn
and postcolumn reaction systems can be employed
with HPLC methods, which can help improve the
chromatographic separation and detection of ana-
lytes. A number of pesticides (e.g., N-methyl carba-
mates, glyphosate, and phenylurea herbicides) are
analyzed after derivatization to enable fluorescence
detection. The electrochemical detectors are used for
a number of pesticide residues (e.g., captan) in rela-
tively clean samples.
0021The on-line combination of HPLC and mass spec-
troscopy (HPLC–MS) offers a high sensitivity and
specificity, and its use in the field of pesticide residues
analysis is growing. There are a number of ionization
techniques used to interface HPLC with MS ana-
lyzers, of which the most widely used are electrospray
and atmospheric pressure chemical ionization. The
HPLC–MS methods use soft ionization techniques
which typically produce protonated or deprotonated
pseudomolecular ions. Therefore, the chromato-
graphic data do not provide structural information
except when the ions produced are subjected to
successive fragmentation (MS
n
). (See Chromatog-
raphy: High-performance Liquid Chromatography.)
Derivatization
0022Some pesticide residues require derivitization to en-
hance the extractability, clean-up or subsequent chro-
matographic resolution and determination steps. For
example, pesticides with hydroxy groups are not
suited to GC analysis, and such an analysis may be
possible only after derivatization to esters. Further-
more, esters of certain functional groups can enhance
the detection process, e.g., pentafluorobenzyl deriva-
tives produce a high response on the ECD.
0023Dithiocarbamate pesticides break down to carbon
disulfide (CS
2
) during analytical procedures. These
residues are determined after treatment of samples
with acidic tin (II) chloride. Any dithiocarbamate
residues in the sample break down to produce CS
2
gas, which can be trapped in the reaction chamber. An
aliquot of the gas (headspace) in the reaction chamber
is analyzed for CS
2
using GC–FPD. Alternatively, the
gas produced can be absorbed into a 2,2,4-trimethyl
tbl0004 Table 4 GC stationary phases commonly used for pesticide
analysis (listed in increasing order of polarity)
Stationary phase Typicaluses
100% methyl silicone (DB1) Nonpolar pesticides
5% phenyl, 95% methyl silicone
(DB-5)
Multiresidue screening
35% phenyl 65% methyl silicone
(DB-35)
EPA method 608
50% phenyl 50% methyl silicone
(DB-17)
Polar organophosphorus
pesticides
14% cyanopropylphenyl 86%
methyl silicone (DB1701)
Organochlorine pesticides
50% cyanopropylphenyl 50%
methyl silicone (DB-225)
Polar pesticides
Poly(ethylene) glycol (DB-wax) Polar pesticides
4492 PESTICIDES AND HERBICIDES/Residue Determination

pentane, and an aliquot of the liquid layer is then
analyzed using GC. This approach is more robust
and more amenable to GC–MS analysis compared
with the headspace procedure.
0024 Some pesticides containing sulfur may oxidize to
form sulfoxide and sulfone derivatives before or
during analysis. These products are also toxic and
are included in the residue definition for the monitor-
ing purposes. These residues are analyzed after com-
plete conversion of the pesticide and its sulfoxide to
the corresponding sulfone and thus enable combined
measurement of the pesticide, its sulfoxide, and
sulfone residues. The conversion step involves the
treatment of sample extracts with potassium perman-
ganate in the presence of 2-methyl propan-2-ol. The
sulfone formed is then extracted into an organic
solvent and analyzed by GC.
Other Techniques
0025 Enzyme-linked immunosorbent assay (ELISA)
methods are used for rapid screening of an individual
or a group of closely related pesticides. These
methods require little or no sample clean-up, require
no expensive instrumentation, and are suitable for
field use. ELISA methods are especially suitable
for residue analyses that are not possible using
multiresidue methods. ELISA kits are available for
a number of pesticides, including 2,4-D, aldicarb,
carbendazim, thiabendazole, chlopyrifos, diazinon,
endosulfan, and metalaxyl.
Confirmation
0026 For regulatory purposes, it is essential that pesticide
residues be unequivocally confirmed using MS. How-
ever, if an MS method is not available, the sample
extract is reanalyzed using a different chromato-
graphic column and/or a different detection system
to confirm the initial results.
Emerging Techniques
0027 There are continued improvements in the design of
instruments available for residue analysis. The use
of GC injectors capable of injecting large volumes
can enable determination at low levels. The use of
fast GC, which utilizes an improved design for
heating the columns, can enable faster chromato-
graphic runs and thus enable quicker analysis. Im-
provements in GC instrumentation have enabled
precise control over temperature and gas flow rate.
The use of electronic pressure control devices can
enable more reproducible chromatographic runs,
thus improving the quality of data. These advances,
coupled with more sophisticated software, can enable
more reproducible chromatography, with a typical
retention time variation of 0.01 s. Improvements in
MS instruments will continue to enhance the selectiv-
ity of methods.
See also: Chromatography: Principles; High-
performance Liquid Chromatography; Gas
Chromatography; Mass Spectrometry: Principles and
Instrumentation; Applications; Pesticides and
Herbicides: Types of Pesticide; Types, Uses, and
Determination of Herbicides; Toxicology
Further Reading
Anon (1991) Concerning the placing of PPPs on the
market, Council Directive 91/414/EEC, Brussels:
Official Journal No. L 230. Luxembourg: Office of
Official Publications of the European Communities.
Cairns T and Sherma J (eds) (1992) Emerging Strategies for
Pesticide Analysis. Boca Raton, FL: CRC Press.
Chapman JR (1993) Practical Organic Mass Spectroscopy,
A Guide for Chemical and Biochemical Analysis.
Chichester, UK: John Wiley.
Codex Alimentarius (1993) Codex Commission Guide-
lines, Recommended Method of Sampling for Determin-
ation of Pesticide Residues, vol. 2, Section 3. Rome:
Food and Agriculture Organization of the United
Nations.
Codex Alimentarius (1996) Pesticide Residues in Food,
Maximum Residue Limits, vol. 2B. Rome: Food and
Agriculture Organization of the United Nations.
Fong WG (ed.) (1999) Pesticide Residues in Foods:
Methods, Techniques and Regulations. New York:
John Wiley.
Hill ARC and Reynolds SL (1999) Guidelines for in-house
validation of analytical methods for pesticide residues in
food and animal feeds. Analyst 124: 953–958.
Krause RT (1979) Resolution, sensitivity and selectivity of a
high performance liquid chromatographic post-column
fluorometric labeling technique for determination of
carbamate insecticides. Journal of Chromatography
185: 615–624.
Mellon F, Self R and Startin JR (2000) Mass Spectroscopy
of Natural Substances in Food. Cambridge: The Royal
Society of Chemistry.
Sherma J (1981) Manual of Analytical Quality Control for
Pesticide Residues and Related Compounds, USA: EPA
600/2–81–059. US Environmental Protection Agency,
Research Triangle Park.
The Working Party on Pesticide Residues (1997) Unit to
Unit Variation of Pesticide Residues in Fruit and Vege-
tables. York, UK: Pesticide Safety Directorate.
Tomlin C (ed.) (1997) The Pesticide Manual, A World
Compendium, 11th edn. Bracknell, UK: The British
Crop Protection Council.
Zooner P Van (1996) Analytical Methods for Pesticide
Residues in Foodstuffs, 6th edn. The Hague, The Neth-
erlands: Ministry for Public Health.
PESTICIDES AND HERBICIDES/Residue Determination 4493

Toxicology
C K Winter, University of California Davis, CA, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 The use of agricultural chemicals, collectively known
as pesticides, in the past several decades has led to
significant reductions in crop losses resulting from
insects, weeds, and plant diseases throughout the
world. The toxicological properties that pesticides
possess also present the potential for impacts upon
human health and upon the environment. As an
example, agricultural workers involved in the mixing,
loading, and/or application of pesticides as well as
those working in fields previously treated with pesti-
cides face potential health risks resulting from excess
exposure to the pesticides. Consumers are routinely
exposed to pesticide residues in their foods, and the
potential dietary risks from pesticide exposure have
been the subject of considerable government study,
regulation, and societal concern.
0002 This review focuses upon the toxicology of the
various types of pesticides used, how pesticide resi-
dues in foods are regulated, and the magnitude of
potential risks faced by consumers from pesticide
residues in the food supply.
Pesticides
Classification
0003 The US Federal Insecticide, Fungicide, and Rodenti-
cide Act defines a pesticide as ‘any substance or mix-
ture of substances intended for preventing, destroying,
repelling, or mitigating any pest, any substance or
mixture of substances intended for use as a plant regu-
lator, defoliant, or desiccant, and any nitrogen stabil-
izer....’ Under this broad definition, it is clear that a
variety of pesticide types exist to control a wide
number of different types of pests. A commonly held
perception is that pesticides refer primarily to agricul-
tural chemicals that control insects (insecticides).
According to the US definition, however, pesticides
also refer to chemicals that control plant diseases
(fungicides) and weeds (herbicides) as well as a variety
of other ‘pests’ (Table 1). For the purposes of consist-
ency, all types of pesticides, including herbicides, will
be considered under this broad umbrella in this article.
Pesticide Use
0004 According to the US Environmental Protection Agency
(EPA), approximately 2 billion kg of chemicals were
used as pesticides in the USA in 1997. It should be
noted that the majority of pesticide use was not for
agricultural purposes. For example, 53% of pesticide
use (by volume) involved chlorine or hypochlorites
used for disinfection of potable and wastewater
pools. ‘Conventional’ pesticides, defined as those de-
veloped or produced exclusively or primarily for use
as pesticides, accounted for the remaining 47% of
pesticide use by volume; 77% of this total was for
agricultural uses, and 12% represented industry/gov-
ernment/commercial use, with the remaining 11%
resulting from home and garden use.
0005Figure 1 shows the relative amounts of a variety of
pesticide types used in US agriculture in 1997. Nearly
half of the total volume of agricultural pesticide use
came from herbicides and plant growth regulators
(213 million kg), followed by sulfur/oils (65 million
kg) and fumigants/nematicides (63 million kg). Agri-
cultural insecticide use in 1997 was approximately 37
million kg, and 26 million kg of fungicides were used
for agricultural purposes.
0006In terms of trends, total agricultural pesticide use,
in terms of kg applied, has decreased slightly since
1979, with the largest drops in use representing in-
secticides, sulfur, and oils. The use of herbicides has
been relatively steady.
Toxicity
0007Hundreds of different pesticide active ingredients
are presently registered by the EPA, and nearly 300
pesticides are considered to have the ability to leave
residues on food crops. Some of the more common
classes of pesticides and some representative examples
are given in Table 2. A comprehensive review of the
toxicity of all pesticides is clearly beyond the scope of
this article, and those interested in more specific and/
or detailed pesticide toxicity information are encour-
aged to consult articles cited in the Further Reading
section.
tbl0001Table 1 Pesticide types and targets
Pesticide type Pest controlled
Acaricide Mites
Algacide Algae
Bacteriocide Bacteria
Defoliant Leaves
Fungicide Fungi
Herbicide Weeds
Insecticide Insects
Molluscicide Snails
Nematicide Nematodes
Rodenticide Rodents
4494 PESTICIDES AND HERBICIDES/Toxicology
