Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


0030 The indicator used must have a color at the pH of
the test solution intermediate between the acidic and
basic forms.
0031 The approximate pH of the solution is first dete-
mined using a ‘universal,’ or full-range, indicator,
when an appropriate indicator for the measurement
can be chosen. A quantity of the test solution is
measured into a tube and a measured amount of
indicator added. Tubes of buffer solutions covering
the pH range of the indicator are treated in the same
way. The colors of the tubes are viewed through the
length of the tube against a white background. The
accuracy of the method will depend on the differences
of pH of the buffer solutions. These are typically 0.2,
and the pH of the unknown solution can then be
measured to within +0.1.
0032 The Lovibond Comparator The need to use buffer
solutions is eliminated by using a Lovibond 2000
comparator, in which the color of the test solution is
compared with colored glasses. Standard colored
glass rings are fitted into a disk that can be rotated
and the colored glasses compared with the test solu-
tion. For accurate results, the sample solution and
discs must be viewed against north daylight or in
white light. Indicator discs for 14 different indicators
are available. Special disks are available for the deter-
mination of the pH of blood.
0033 With the Nesslerizer attachment, the solution is
viewed through the depth of the liquid instead of
through its thickness. The proportion of indicator
therefore can be considerably reduced, resulting in
a significant gain in accuracy, particularly when
unbuffered or slightly buffered solutions are being
tested.
0034 Sources of error There are a number of possible
sources of error when making colorimetric
measurements.
1.
0035Slightly buffered solutions. As indicators are
weak acids or bases they will change the pH of
the solution if it is unbuffered or only slightly
buffered. Samples of relatively pure water, and
solutions of a salt of a strong acid and strong
base are the most frequently encountered solutions
of this type. It is recommended that an appropriate
pH meter is used for such solutions.
2.
0036Salt effect. As discussed earlier, equilibrium con-
stants depend on the activities of the components
rather than concentrations, and it is only in very
dilute solutions that activity equals concentration.
Buffer solutions normally used have ionic
strengths of up to 0.2 M. If the solution under
test has a lower ionic strength than the buffer, the
determined pH will be too small. The salt correc-
tion that must be applied is also affected by the
types of ion present and the indicator used.
3.
0037Effect of colloids. Colloid particles in the solution
may preferentially absorb either the acid or the
alkaline form of the indicator, giving totally
erroneous results.
4.
0038Effect of proteins. pH values obtained in the
presence of proteins are unreliable. The effect of
a protein on the determination depends on both
the type of protein and the indicator used. Usually,
the effect is greater when the protein is on the acid
side of its isoelectric point.
5.
0039Effect of finely divided particles. Chemical reac-
tions may occur between the indicator and
particles in the solution, giving erroneous results.
6.
0040Colored solutions. Color comparison is not
possible if the test solution is colored.
7.
0041Nonaqueous solvents. Literature data on indica-
tors apply to aqueous solutions; the indicator equi-
libria, hence the color, will be different in other
solvents. The color of an indicator in, say, a water–
alcohol mixture should be taken as only a rough
guide to the true pH.
tbl0002 Table 2 Some pH indicators and their color changes and ranges
Indicator Color in acid solution Color inalkaline solution pHrange pK
In
Thymol blue (acid) Red Yellow 1.2–2.8 1.7
Bromophenol blue Yellow Blue 2.8–4.6 4.0
Methyl orange Red Yellow 3.1–4.4 3.7
Methyl red Red Yellow 4.2–6.3 5.1
Litmus Red Blue 5.0–8.0
Bromothymol blue Yellow Blue 6.0–7.6 7.0
Phenol red Yellow Red 6.8–8.4 7.9
Thymol blue (base) Yellow Blue 8.0–9.6 8.9
Phenolphthalein Colorless Red 8.3–10.0 9.6
Universal
a
Red, orange, yellow Green, blue, violet 3.0–11.0
Full-range
a
Red, orange, yellow Green, blue, violet 1.0–14.0
a
These are mixtures of indicators; they give a continuous color change over a wide pH range.
PH – PRINCIPLES AND MEASUREMENT 4505
Electrochemical Method
0042 In order to measure pH by the electrochemical
method, the emf of a reference cell is determined
(see earlier). In this cell, the potential of one electrode
(the hydrogen electrode) changes with pH, and the
potential of the second, reference, electrode does not.
Historically, the hydrogen gas electrode was used as
the pH electrode, but the discovery by Cremer in
1906 that the potential difference across a glass mem-
brane depends on the H
þ
ion concentration on either
side of it led to the development of the glass electrode.
This is the electrode that is now always used for
measuring pH.
0043 Glass electrode In its simplest form, the glass
electrode consists of a thin glass bulb containing a
solution of constant pH, usually hydrochloric acid or
a phosphate buffer with potassium chloride. In this
solution is an electrode, usually silver coated with
silver chloride.
0044 The exchange of ions in the glass membrane for H
þ
ions in the solutions on either side of it to form silanol
(
—
—
—
Si-OH) layers is the major factor in determining
the pH response of the electrode, and special sodium
and lithium glasses are used. As this surface layer is
crucial to the correct functioning of a glass electrode,
the pH-sensitive tip is stored in a pH 7 buffer solu-
tion, preferably containing 0.1 mol dm
3
potassium
chloride. As it is most important that the tip does not
dry out, commercial electrodes are supplied with a
storage teat containing the buffer plus potassium
chloride solution.
0045 Glass electrodes give a good linear response in the
pH range 2–9, and up to pH 14 if the salt concen-
tration in the test solution is not too high.
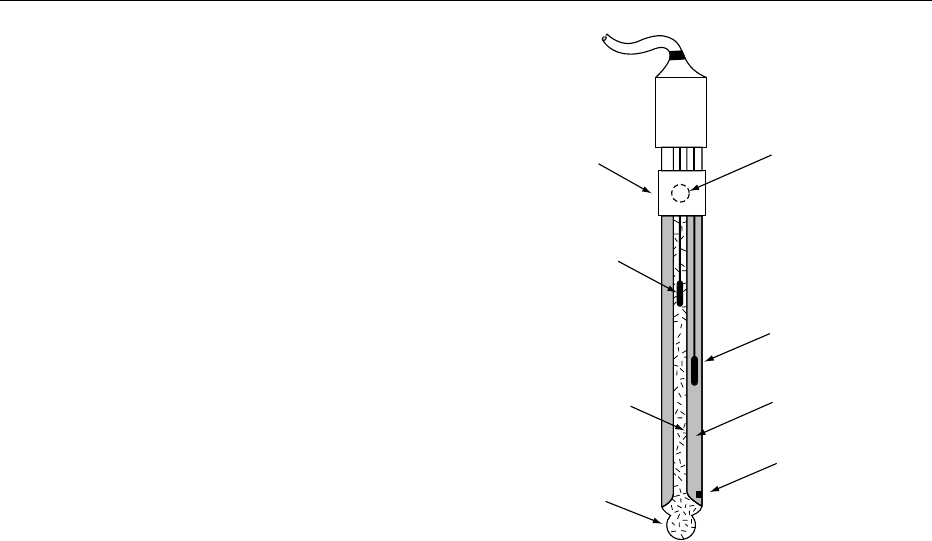
0046 Combination electrodes Historically, the separate
glass and reference electrodes were placed side by
side in the test solution. Such electrodes are still avail-
able but are now used for special applications. Almost
all pH measurements are made with combination
electrodes in which the glass and reference electrodes
are combined together. Although there are many
details that vary from one electrode to another, the
basic design is as shown in Figure 1. The glass mem-
brane is at the bottom and is surrounded by the
reference electrode. This is usually a silver/silver
chloride electrode (silver wire coated with silver
chloride in saturated potassium chloride solution),
and the reference–test solution junction is at a porous
glass frit near the bottom of the electrode. The potas-
sium chloride in the reference cell slowly bleeds
through the frit into solutions being tested and must
be topped up, when necessary, through the filling hole
near the top of the electrode.
0047For many purposes, this electrode is perfectly satis-
factory, but under adverse conditions, the positive
flow of reference electrolyte is reversed, and the
sample solution contaminates the reference cell. This
can be avoided by using a double-junction reference
system in which the reference cell is connected
through a glass frit with an intermediate electrolyte,
which is in turn in contact, through a glass frit, with
the test solution. Such an electrode also eliminates
clogging owing to precipitation of silver chloride at
the glass frit. Also, the intermediate electrolyte can be
changed if that being used reacts with the test solution
at the glass frit junction.
0048Special glass electrodes
Amplified electrode The major experimental
difficulty in measuring the emf of a pH cell is the
high electrical resistance of the glass membrane, com-
monly 10–500 MO. This requires a high-impedance
millivoltmeter and short, screened connections to the
electrode. One solution to this problem is a glass
electrode with a built-in amplifier (Hanna Instru-
ments), where the high-impedance circuitry is in an
integrated circuit encapsulated at the top of the elec-
trode together with a mercury battery. This electrode
is ideal for industrial pH measuring and monitoring
Filling
hole cover
Glass (pH)
electrode
element
Glass (pH)
electrode
electrolyte
pH-sensitive glass bulb
Reference
junction
Reference
electrolyte
Reference
electrolyte
filling hole
Reference electrode
element
fig0001Figure 1 Combination pH electrode. Reproduced from pH:
Principles and Measurement, E ncycl opaed i a of Food Science,
Food Technology and N utrition, Macrae R, Robinson RK and Sadler
MJ (eds), 1993, Academic Press.
4506 PH – PRINCIPLES AND MEASUREMENT

as long, unshielded cables can be used from the
electrode to the pH meter.
0049 Other electrodes pH glass and combination elec-
trodes of many types are available; these include
electrodes in plastic bodies (for durability), micro-
electrodes for small volumes of test solution, elec-
trodes resistant to proteins, and flat electrodes for
pH measurement at surfaces.
0050 pH meters A pH meter is a high-impedance milli-
voltmeter that is designed to convert millivolts to pH.
There are many types available at a range of prices.
Temperature compensation is important, and many
meters have a temperature probe facility for this pur-
pose. Many meters contain microprocessors and are
very easy to use.
0051 To make a pH measurement, it is necessary to
calibrate the meter first. This is done by using two
buffer solutions that, ideally, span the pH of the test
solution. After rinsing the electrode, this is placed in
the test solution and the pH read from the meter.
0052 Accurate pH measurements can be made without
difficulty on test solutions containing dissolved acids,
bases or salts. If, however, the sample is virtually pure
water (e.g., tap, rain, or boiler-feed water) pH meas-
urements are unreliable. This is because (1) the glass
electrode requires a long time to stabilize in a low-
ionic-strength solution; (2) the solution has a
low buffering capacity and is therefore susceptible
to drift as atmospheric carbon dioxide is absorbed;
(3) the low conductivity of the solution allows the
pick-up of electromagnetic noise; and (4) there is
electrical noise created at the reference junction.
Special low-conductivity electrode buffer kits have
been developed (Russell pH Ltd) for such measure-
ments. In these kits, the glass electrode is of extremely
low resistance, and the calibration errors are reduced
by using a buffer of low ionic strength.
0053Very small, portable pH meters are now available. A
good example is the Piccolo (Hanna Instruments).
This meter weighs only 100 g and uses an interchange-
able amplified electrode in a plastic container. It has a
built-in temperature sensor that compensates for tem-
peratures from 0 to 70
C, and it measures pH in the
range 1–13 with an accuracy and resolution of +0.01.
See also: Canning: Principles; Colloids and Emulsions;
Spectroscopy: Visible Spectroscopy and Colorimetry;
Spoilage: Chemical and Enzymatic Spoilage; Molds in
Spoilage; Yeasts in Spoilage
Further Reading
Anonymous (1987) pH Values, 8th edn. Poole, UK: BDH.
Bates RG (1973) Determination of pH. Theory and
Practice, 2nd edn. New York: John Wiley.
Covington A (1989) pH and its Measurement. London:
Royal Society of Chemistry (a chemistry cassette and
workbook).
Galster H (1991) pH Measurement (Fundamentals,
Methods, Applications, Instrumentation) New York:
Wiley-VCH.
PHENOLIC COMPOUNDS
M Murkovic, Graz University of Technology, Graz,
Austria
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Background
0001 Polyphenols play an important role in plants as well
as in foods. The main uses of polyphenols in foods are
as colorants and antioxidants. A large amount of
research on polyphenols has focused on their anti-
oxidant properties, since they are thought to have
positive effects on chronic degenerative diseases
(cataracts, age-related macular degeneration, central
neurodegenerative diseases, and diabetes mellitus),
cardiovascular disease, and cancer. Polyphenols are
also used as antioxidants in the food industry to
increase shelf-life, which can be limited due to deteri-
oration because of oxidation reactions, especially fat
oxidation. Not only is oxidized food a problem
because of the resultant rancid aroma. It can also
have implications on human health, since increased
exposure to free radicals can also increase the risk of
these degenerative diseases.
0002The several thousand polyphenols that have been
described in plants can be grouped in several classes.
Distinction between these classes is drawn first on
the basis of the number of constitutive carbon atoms
and then in the light of the structure of the basic
skeleton. In addition, besides simple soluble forms
mainly found in vacuoles, there are also polymerized
forms of varying solubility like the tannins or the
completely insoluble lignins. The groups of polyphe-
nols dealt with in this chapter comprise anthocyanins,
PHENOLIC COMPOUNDS 4507
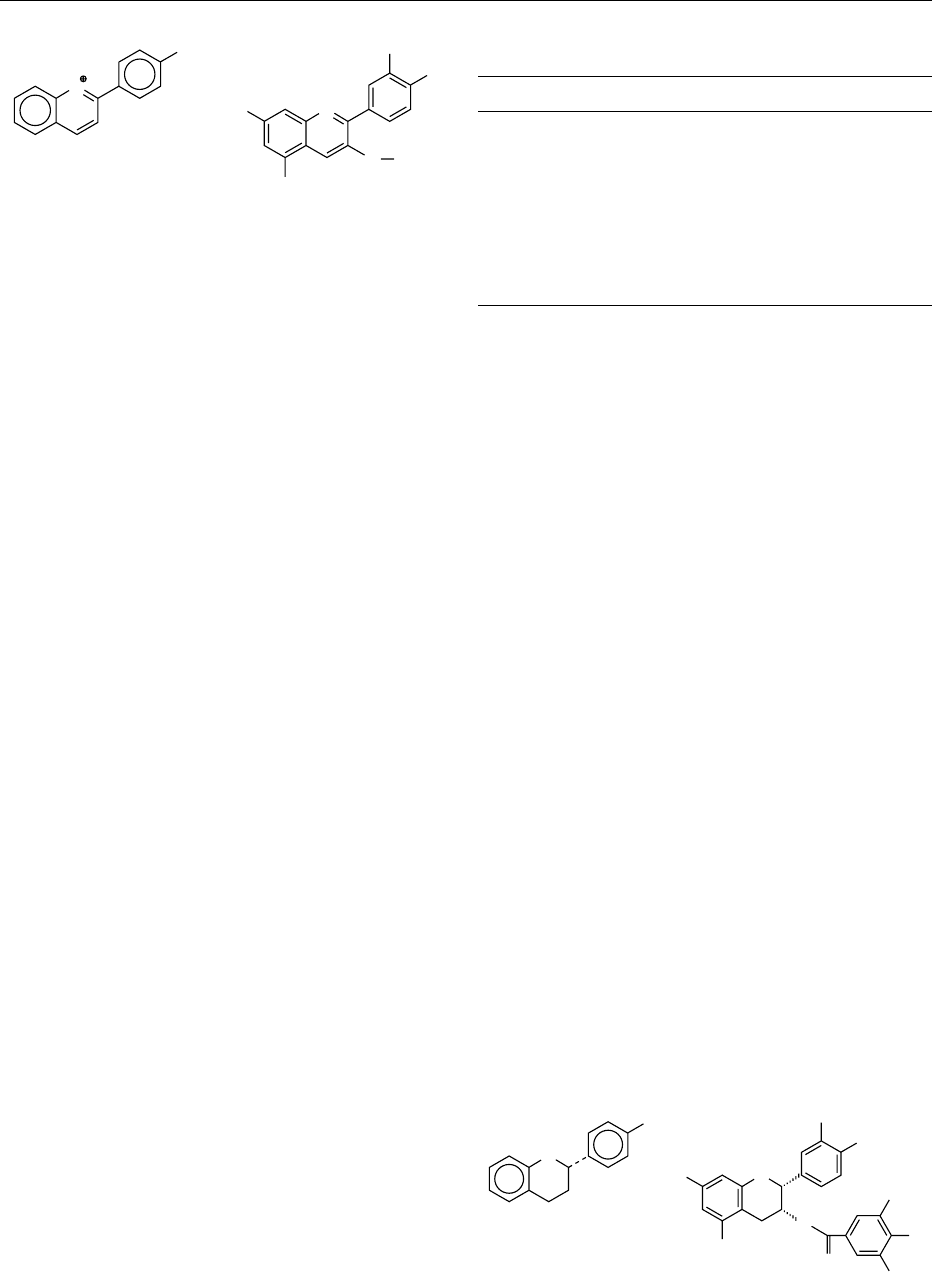
flavan-3-ols, isoflavones, flavones and flavonols,
hydroxybenzoic acids, and hydroxycinnamic acids.
Anthocyanins
0003 Anthocyanins are the most important group of water-
soluble plant pigments visible to the human eye.
With a few exceptions, e.g., betalains, they are
universal plant colorants and largely responsible for
the cyanic colors of flower petals and fruits. They
may also occur in roots, stems, leaves, and bracts,
accumulating in the vacuoles of epidermal or subepi-
dermal cells.
0004 The main positions of hydroxylation are 3, 5, and 7
in the A ring and 3
0
and 5
0
in the B ring (see Figure 1).
Methoxyl groups can be attached to 3
0
and 5
0
.In
some cases, the 3-hydroxyl group is removed, and a
few structures are known that have a hydroxyl group
attached to C-6 of the A ring. The anthocyanidins
that are commonly found in fruits are pelargonidin,
cyanidin, delphinidin, peonidin, petunidin, and mal-
vidin. These are all hydroxylated at the positions 3, 5,
and 7. Normally, anthocyanins occur as 3-monosides,
3-biosides, and 3-triosides as well as 3,5-diglycosides
and, in some cases, 3,7-diglycosides. The carbo-
hydrates that are attached to the positions 3, 5, and
7 comprise glucose, galactose, rhamnose, arabinose,
and xylose.
0005 An example of a glycoside is cyanidin 3-rutinosid
(6-O-a-l-rhamnosyl-d-glucose), which is very com-
mon in fruits, most commonly associated with
cyanidin 3-glucoside. Other glycosides are cyanidin
3-sambubioside (2-O-b-d-xylosyl-d-glucose), which
is present in elderberry, redcurrant, and black
raspberry, and cyanidin 3-sophoroside (3-O-b-d-
glucosyl-d-glucose), the main pigment in raspberries,
loganberries, boysenberries, and mangosteen. The
latter also occurs in redcurrants and sour cherries,
but as a minor component (see Table 1).
0006 The color-active form of the anthocyanins shows
an absorption maximum at about 520 nm. This is due
to the flavylium cation structure, which occurs only in
an acidic environment. An increase in pH above 4
leads to a substantial fading of color. Other color
changes occur during oxidation of the anthocyanins,
leading to the browning of foods.
Flavan-3-ols
0007Flavan-3-ols are components in the structure of
proanthocyanidins (condensed tannins) as their
monomers. Flavan-3-ols have been obtained from
several tree species (Quercus, Castandea, Acacia,
Eucalyptus, etc.). Four main flavan-3-ols are found
in fruits. Two are orthodiphenols hydroxylated in the
3
0
and 4
0
positions in the B-ring ([þ]-catechin and
[]-epicatechin), and two are trihydroxylated in
the 3
0
-, 4
0
-, and 5
0
-positions ([þ]-gallocatechin and
[]-epigallocatechin). C-2 and C-3 represent two
centers of asymmetry in the molecule in such a way
that the four mentioned flavan-3-ols in fruits are
grouped in two pairs of diastereomers whose absolute
configurations are as follows: 2R in all cases and
either 3S for (þ)-catechin and (þ)-gallocatechin or
3R for ()-epicatechin and ()-epigallocatechin (see
Figure 2).
0008Unlike in other classes of flavonoids, monomeric
flavan-3-ols are generally found in free rather than
glycosylated or esterified form in fruits (see Table 2).
One exception is the case of the ()-epicatechin-3-O-
gallate identified in grapes. This ester has been found
in several red grape cultivars.
O
O
+
OH
OH
OH
HO
O Glucose
OH
A
5
7
3
5
3
B
fig0001 Figure 1 Structure and possible positions of hydroxylation and
methoxylation of anthocyanidins (cyanidin-3-O-glucoside).
tbl0001Table 1 Anthocyanidin content of selected vegetables and
fruits (mg per 100 g fresh weight)
Food Totalanthocyanidin content
Fruits
Blueberry 25–500
Elderberry 300–500
Strawberry 30–50
Cherry, sweet 350–450
Grapes, red 30–750
Vegetables
Cabbage, red 20–40
Onion, red 10–20
O
O
OH
OH
OH
OH
OH
OH
HO
O
O
OH
A
5
7
3
5
3
B
fig0002Figure 2 Structure and possible positions of hydroxylation and
methoxylation of flavans; (()-epicatechin 3-gallate).
4508 PHENOLIC COMPOUNDS

Isoflavones
0009 The isoflavones are a distinctive but large subgroup
of flavonoids. These compounds possess a 3-phenyl-
chroman skeleton that is biogenetically derived by
rearrangement of the flavonoid 2-phenylchroman
system (see Figure 3).
0010 Despite the restricted distribution of the isoflavo-
noids in the plant kingdom, the structural variation is
surprisingly large. This arises not only from the
number and complexity of substituents on the basic
3-phenylchroman system but also from the different
oxidation levels in this skeleton and the presence of
extra heterocyclic rings. Within the flavonoids, the
isoflavones are the largest group of naturally occur-
ring structures.
0011 The enzyme that is responsible for the transform-
ation of the flavones to the isoflavones is named
isoflavone synthase. This enzyme catalyzes the
isomerization of the two flavones (2S)-naringenin
or (2S)-liquiritigenin to the isoflavones genistein and
daidzein, respectively.
0012 Soy foods have received considerable attention for
their role in disease prevention, especially in relation
to heart disease, osteoporosis, and cancer. The re-
search interest in soy focuses on establishing the
physiological effects of isoflavones. The occurrence
of the isoflavonoids is restricted to only a few plants
within the family of the leguminosae and some non-
legume dicotyledons. Soybeans and soyfoods are
practically the only nutritionally relevant dietary
sources of isoflavones. Isoflavones are weak estrogens
in that they bind to estrogen receptors, but they also
have important nonhormonal properties as well. Ini-
tial speculation that soyfoods, and in particular iso-
flavones, might promote bone health was based on
the estrogenic properties of isoflavones. In ovariecto-
mized rodents, isoflavones retard bone loss almost as
effectively as estrogen. Most soyfoods are rich in iso-
flavones and favorably affect bone turnover and
spinal bone mineral density in perimenopausal and
postmenopausal women (see Table 3).
Flavones and Flavonols
0013Flavones are flavonoids characterized by a nonsatu-
rated 3-C chain and have a double bond between C-2
and C-3, like flavonols, with which they differ by the
absence of hydroxyl in the 3-position. It appears that
this simple difference in structure between flavones
and flavonols has very important consequences in the
biogenesis, physiological, and pharmacological roles,
and the phylogenetic and chemotaxonomic significa-
tion of these compounds. Flavones are widely distrib-
uted among the higher plants in the form of aglycones
or glycosides (see Figure 4).
0014The edible portion of some foodstuffs has an un-
usually high concentration of quercetin. The concen-
trations of quercetin analyzed in hydrolyzed samples
from fruits and vegetables are 284–486 mg kg
1
in
onions, 110 mg kg
1
in kale, 32–45 mg kg
1
in French beans, 30 mg kg
1
in broccoli, 14 mg kg
1
in lettuce, and 8 mg kg
1
in tomatoes. The average
concentration of quercetin in fruits is 15 mg kg
1
,
with apples having the highest concentration (21–
72 mg kg
1
) (see Table 4). Parsley and thyme are
also major food sources of flavones.
tbl0003Table 3 Content of isoflavones in soy products (mg per 100 g
(ml) fresh weight)
Food Totalisoflavone content
Soya bean 60–400
Tofu 8–70
Soy flour 80–180
Soya milk 3–180
Soy sauce 1–8
tbl0002 Table 2 Content of flavanol monomers in selected vegetables
and fruits (mg per 100 g fresh weight)
Food Total flavan-3-olcontent
Apricot 25
Apple 8
Strawberry 4
Cherry, sweet 22
Grapes (skin) 19
Barley 3–4
Tea 18–35
a
a
Flavanols in g per 100 g of dry leaves.
O
O
OH
OH
HO
O
O
A
5
7
2
4
5
2
3
fig0003 Figure 3 Structure and possible positions of substitutions of
isoflavone (genistein).
O
O
OH
OH
OH
HO
O
Rutinose
OH
A
5
7
R
3
B
O
O
fig0004Figure 4 Structure and possible positions of substitutions of
flavones (R ¼OH in flavonols).
PHENOLIC COMPOUNDS 4509

Hydroxybenzoic Acids
0015 Hydroxybenzoic acids have a general structure of the
C6–C1 type derived directly from benzoic acid. Vari-
ations in structure lie in the hydroxylations and meth-
oxylations of the aromatic cycle. Four acids dominate
the family of these compounds: p-hydroxybenzoic
acid, vanillic, syringic, and protocatechuic acids.
The first three are constituents of lignin, from which
they are released by alkaline hydrolysis. Gentisic acid
has a particular pattern of hydroxylation, which can
be related to that of salicylic acid, but it is much less
common. Gallic acid is another well-known plant
acid which participates in the formation of hydrolyz-
able gallotannins. Its dimeric condensation product
(hexahydrodiphenic acid) and the related dilactone,
ellagic acid, are also common plant constituents.
0016 Hydroxybenzoic acids are commonly present in
bound form (see Figure 5). They are the component
of a complex structure like lignins and hydrolyzable
tannins. The content of hydroxybenzoic acids in
foods of plant origin is generally low (see Table 5),
but in some berries and vegetables such as onions and
horse-radish, the content of hydroxybenzoic acids
may be very high.
Hydroxycinnamic Acids
0017Among the fruit phenolics, hydroxycinnamic acid
derivatives play an important role because of both
their abundance and diversity. They all derive from
cinnamic acid and are essentially present as combined
forms of the four basic molecules p-coumaric, caffeic,
ferulic (see Figure 6), and sinapic acids. The free
forms of these acids are very rare in fruits. Two
main types of soluble derivatives have been identified:
first, those involving an ester bond between the carb-
oxylic function of phenolic acid and one of the alco-
holic groups of an organic compound, for example
chlorogenic acid, which has been identified in numer-
ous fruits; second, those involving a glycosidic bond
with one of the phenolic groups of the molecule (e.g.,
p-coumaric acid O-glucoside). The diversity of the
hydroxycinnamic acids encountered in plants and
particularly in fruits thus results from the nature of
the bonds and that of the molecules involved.
0018Hydroxycinnamic acids occur most commonly in
foods of plant origin. Of these, caffeic acid is the
predominant hydroxycinnamic acid in many fruits,
constituting over 75% of total hydroxycinnamic
acids found in plums, apples, apricots, blueberries,
and tomatoes. However, p-coumaric acid is the dom-
inant hydroxycinnamic acid of citrus fruits and pine-
apple. Hydroxycinnamic acids are widely present in
the bound form and are rarely found in free form.
Processing of fruits and vegetables (freezing, steriliza-
tion, and fermentation) contributes to the formation
of free hydroxycinnamic acids in such products (see
Table 6). Chlorogenic acid is found in many foods,
including apples, apricots, berries, peaches, pears,
plums, avocados, and carrots. Chlorogenic acid is
the key substrate for enzymatic browning, particu-
larly in apples and pears. Its content is reduced by
70% during browning of the pear skins.
Analysis of polyphenols
Sample Preparation
0019Prior to extraction, solid food samples have to be
homogenized or crushed using a mortar and pestle.
Soluble phenolic compounds are generally extracted
by means of alcohol–water mixtures. Extraction is
performed using a mixture of ethanol (80%) and
HO
3
5
COOH
HO
HO
HO
COOH
fig0005 Figure 5 Structure and possible positions of hydroxylation of
hydroxybenzoic acids (gallic acid).
HO
H
3
CO
COOH
HO
3
5
COOH
fig0006Figure 6 Structure and possible positions of hydroxylation of
hydroxybenzoic acids (ferulic acid).
tbl0004 Table 4 Content of flavonols and flavones in selected
vegetables and fruits (mg per 100 g fresh weight)
Food Total flavonol content
Apple 2–4
Apricot 3
Broccoli 9–11
Kale 32–60
Onion 35
Celery, leaf 95
Sweet pepper, red 1
tbl0005 Table 5 Content of hydroxybenzoic acids in selected fruits (mg
per 100 g fresh weight)
Food Totalhydroxybenzoic acid content
Blackberry 8–27
Blackcurrant 4–12
Raspberry 6–10
Strawberry 2–8
4510 PHENOLIC COMPOUNDS

water (20%) of the freeze-dried powder of the food to
be analyzed. Methanol can be used instead of ethanol,
and extraction with methanol gives good results, es-
pecially in the extraction of soluble phenolic com-
pounds from fruits. Oxidation is avoided by
working at low temperatures and, if necessary, by
the addition of antioxidants such as ascorbic acid or
butylated hydroxytoluene. To avoid enzymatic oxida-
tion from polyphenol oxidases, samples may need to
be heated to a temperature of more than 90
C for a
few minutes. These enzymes catalyze the oxidation of
phenols to quinones with subsequent nonenzymatic
rapid polymerization. These oxidases can also be in-
hibited by lowering the pH to below 4.0. Extraction
of anthocyanins is normally carried out under cold
conditions with methanol containing 1% of hydro-
chloric acid to obtain the flavylium cation, which is
stable under acidic conditions.
0020 For analysis of liquid samples like fruit juices, only
dilution, filtration, and, in some cases, clarification
are necessary. Carotenoids that can disturb the analy-
sis should be extracted using nonpolar solvents. To
remove the sugar moieties from the polyphenols,
the glycosides have to be hydrolyzed. The cleaving
off be carried out in acidic, basic, or enzymatic con-
ditions. The hydrolysis of anthocyanins to anthocya-
nidins is often necessary owing to the difficulties of
obtaining anthocyanin standards. This can be done
by refluxing the dry anthocyanins in methanol acid-
ified with 2 M HCl. Alkaline hydrolysis cleaves the
acylated portions of anthocyanins using 2 M NaOH.
During hydrolysis, care has to be taken that no poly-
phenols are destructed or rearrangement takes place
so that artifacts are analyzed.
0021 The water–alcohol extract obtained is a raw ex-
tract containing numerous nonphenolic substances
(carbohydrates, organic acids, proteins, pigments,
etc.). Before these crude extracts can be analyzed by
high-performance liquid chromatography (HPLC),
they should be purified using solid-phase extraction
methods. Chlorophylls and carotenoids can be re-
moved by applying conventional depigmentation
techniques with petroleum ether.
HPLC Analysis
0022The columns used for HPLC analysis in the literature
are almost exclusively reversed phase. Elution
systems are usually binary, with an aqueous acidified
polar solvent such as aqueous acetic acid, phosphoric
acid, or formic acid and a less polar organic solvent
such as methanol or acetonitril. Isocratic elution is
mainly used for routine analysis if only a few poly-
phenols are to be determined.
0023For detection and quantification after separation,
the absorption of ultraviolet or visible light is nor-
mally used. For flavonoids, two absorption bands are
characteristic: the first in the range of 240–285 nm,
which is believed to arise from the A-ring, and the
second with a maximum in the range of 300–550 nm,
which arises from the absorption of the B-ring (see
Table 7). Since the absorption spectrum depends on
the chemical substitution and the conjugated system,
the different groups of flavonoids (flavanons/iso-
flavanons, catechins, flavons/flavonols, etc.) have
distinct absorption bands. The anthocyanins show
absorption above 500 nm, whereas the catechins
have only a small maximum at 280 and no absorption
at higher wavelengths.
0024The combination of liquid chromatography with
mass spectroscopy for the detection of polyphenols
has been increasing in recent years. Mass-selective
detection is especially useful in identifying new struc-
tures and verifying known substances. With a quad-
rupole mainly the unfragmented molecule can be
detected. Using tandem mass spectrometry, the mol-
ecules can be fragmented in a selective way. Thus,
information on the chemical structure of the poly-
phenols can be obtained.
Intake and Bioavailability of Polyphenols
0025In the Western diet, the phenolic acids, catechins,
proanthocyanidins, and anthocyanins dominate
uptake. Especially for epidemiologic studies, it is
necessary to estimate accurately the amount of
tbl0007Table 7 Detections wavelengths of flavonoids
Structure Wavelengthused
foranalysis (nm)
Flavons, flavonols, flavonol glycosides 270
Anthocyanins 500–525
Isoflavones 236, 260, 280
Flavanones and glycosides 280, 290
Catechins 210, 280
tbl0006 Table 6 Content of hydroxycinnamic acid derivatives in
selected fruits (mg per 100 g (ml) fresh weight)
Food Chlorogenic acidisomers
Apple juice 6–20
Beer 0.2–2
Coffee beans, green 6–9 g per 100 g
Coffee beans, roasted 0.2–3 g per 100 g
Hydroxycinnamic acids
Strawberries 1–3
Blackcurrants 5–7
Gooseberries 2–7
Raspberries 2–3
Redcurrants 1–2
PHENOLIC COMPOUNDS 4511

polyphenols consumed. In general, fruits are richer in
polyphenols compared to vegetables. Beverages con-
tribute significantly to the uptake of polyphenols. Red
wine, coffee, and black tea contain high amounts of
polyphenols. Green tea, which also contains large
amounts of these substances, is consumed in East Asia
in high amounts. The uptake of polyphenols is esti-
mated to be 50–200 mg per meal. The phenolic acids
are constituents of coffee, fruits, vegetables, and
cereals. Flavanols, which occur predominantly in
onions,apples,andotherfruitsandvegetables,contrib-
ute about 20 mg per day. Catechins are provided by tea
and various fruits and proanthocyanidines by various
fruits, legume seeds, chocolate, and red wine. Flava-
nones are found in citrus fruits. The total uptake of
flavonoids in the Western diet is estimated to be about
1000 mg per day. Examples of the distribution and
quantity of flavonoids consumed daily include: 44 mg
from cereals; 79 mg from potatoes, bulbs, and roots;
45 mg from peanuts and nuts; and 162 mg from vege-
tables and herbs. The largest portion of flavonoid
intake comes from cocoa, cola, coffee, tea, beer and
wine (420 mg per day), with an additional 290 mg per
day from fruits and juices.
0026 The uptake of 100 mg of polyphenols results in a
concentration of about 300 mM in the gut. Normally,
the plasma levels will not exceed 1 mM. Urinary ex-
cretion varies from less than 1% to about 25%
depending on their gut absorption, enterohepatic
cycling, and metabolism. In particular, the type of
polyphenol and the glycosylation influence bioavail-
ability. The uptake of proanthocyanidines is limited
to monomers and dimers. A higher degree of poly-
merization results in a sharp decrease in bioavailabil-
ity. The metabolism of polyphenols in the small
intestine is mainly via glucuronidation, sulfatation,
and methylation. The colon microflora contributes to
the metabolism of the polyphenols. This has been
investigated in detail for several types of polyphenols,
and especially for quercetin. Most flavonoids enter the
diet as glycosides, with quercetin and rutin being the
most common flavonoid glycosides consumed. The
hydrophilic nature and their relatively high molecular
weight preclude absorption in the small intestine. Fur-
thermore, flavonoid b-glycosides resist intestinal
hydrolases, and consequently, flavonoid glycosides
can pass unaltered into the large intestine. The resident
microflora of the bowel produces glycosidases capable
of releasing the aglycone from its sugar. In addition,
the resident microflora can cleave the pyrone ring (ring
C), producing phenyl acetic and phenyl propionic
acids and other derivatives. But since glycosidase ac-
tivity proceeds at a faster rate than ring cleavage, the
intact flavonoid aglycone can persist in the large intes-
tine with a clear potential for absorption.
Polyphenols as Antioxidants
0027It is well accepted that free-radical-mediated pro-
cesses can lead to chronic degenerating diseases. The
oxidative damage can be found on molecular level as
oxidized lipids, proteins, or DNA. These can be
detected, especially in patients with atherosclerosis,
certain cancers, neurodegenerative diseases, and lung
disorders. Many types of reactive oxygen species
(ROS) and reactive nitrogen species (RNS) have
been shown to induce a certain type of damage that
is associated with disease development. These include
the superoxide radical, hydrogen peroxide, hydroxyl
radical, lipid alkoxyl and peroxyl radicals. Protection
against damage induced by ROS/RNS can be
achieved in several different ways:
.
0028suppression of free-radical formation by antioxi-
dants;
.
0029scavenging of radicals by antioxidants to inhibit
initiation of chain reactions and slow down chain
propagation;
.
0030repair mechanisms;
.
0031transition metal chelation.
The polyphenols have structural features that charac-
terize their antioxidative potential. This is the presence
of hydrogen-donating substituents and the ability to
delocalize the resulting free electron. Owing to the
delocalization of the electron, the resulting radical
does not have sufficient energy for further radical reac-
tions. The antioxidative properties of flavonoids and
other phenolics from natural sources are assessed by
determining their activity as scavengers of radicals that
arisefrom lipidoxidation or otherbiological processes.
For evaluation of the antioxidant properties of flavo-
noids, the chemical structure can be related to the
activity. Three structural groups are important deter-
minants for radical scavenging: (1) the catechol struc-
ture (3
0
,4
0
-dihydroxy) in the B ring, which is the
obvious radical target site; (2) the 2, 3 double bond in
conjugation to the 4-keto function, which is respon-
sible for electron delocalization; and (3) the presence of
both hydroxyl groups (the 3-OH and the 5-OH) for
maximal radical-scavenging potentials. If all of these
structural features are present, a maximum radical-
scavenging potential would be expected.
0032Depending on the method of measuring the anti-
oxidative potential, relative reactivities of structurally
related polyphenols change. This can be seen espe-
cially in low-density-lipoprotein (LDL) oxidation ex-
periments, in which quercetin and luteolin are strong
antioxidants, that protect LDL with high efficiency,
whereas kaempferol does not. In particular, if chela-
tion of metal ions is the protecting mechanism, quer-
cetin is a very strong antioxidant.
4512 PHENOLIC COMPOUNDS

0033 A simple chemical assay broadly applicable in de-
termining the hierarchy of antioxidant activities of
polyphenols measures their ability to reduce the
ABTS-radical cation (2,2
0
-azinobis (3-ethylben-
zothiazoline-6-sulfonate)). With this method, the
relative ability of antioxidant or radical-scavenging
substances to scavenge the ABTS cation radical in the
aqueous phase is compared to a standard amount of
the synthetic antioxidant Trolox, the vitamin E
analog. Incubation of ABTS
oþ
with peroxidase (met-
myoglobin) and hydrogen peroxide results in the pro-
duction of the radical cation (ABTS
oþ
). This species is
blue–green in color and can be detected at 734 nm.
Antioxidants or radical scavengers in the added
sample suppress this color production to a degree
that is proportional to their concentration. In this
method, the activity of tested substances is expressed
as an equivalent of the millimolar concentration of a
Trolox solution. Table 8 shows the antioxidant activ-
ities of a range of flavonoids in comparison with the
reduction potentials of the flavonoid radicals. It can
be seen that the measured reducing properties of the
phenolics, in terms of their antioxidant activities in
this particular assay system, are consistent with the
reduction potentials of their radicals.
0034 The range of reduction potentials of selected flavo-
noid radicals, relative to those of ascorbic acid and
a-tocopherol, is shown in Table 8, from the more
oxidizable quercetin to kaempferol. These values re-
flect the ability of hydrogen-donating antioxidants to
scavenge the ABTS
oþ
radical cation, absorbing in the
near-infrared region (650–820 nm) compared with
that of ascorbic acid (E
7
) and Trolox equivalent
antioxidant activity (TEAC). Antioxidants reduce
the absorbance to an extent dependent on the anti-
oxidant activity. Spectral studies have revealed that
the radical site is on the B ring and that the A ring has
no influence on the properties of the radicals from the
B ring in flavonoids with a saturated C ring, such as
catechin. In flavone radicals in which the B ring with
a3
0
,4
0
-dihydroxycatechol structure is conjugated
through the C ring 2, 3-double bond, evidence sug-
gests that the radical is on the catechol B ring and that
spectra resemble those of 3,4-dihydroxycinnamate
radicals. The antioxidant activity of flavonoids
depends critically on the part of the molecule with
more efficient electron-donating properties. In most
flavonoids, this is the B ring. Thus, the antioxidant
activity of flavonoids as electron or hydrogen donors
relates to the reduction potentials and reactivities of
the substituent hydroxyl groups. A number of chem-
ical studies on the reactivity of flavonoids with a
range of radicals and the stability of the resultant
antioxidant radicals have also emphasized specific-
ally the role of the catechol structure in the B ring
and unsaturation in the C ring.
Conclusion
0035Substantial epidemiological evidence links high anti-
oxidant status in human populations with a low risk
of degenerative disease. The consumption of large
amounts of fruit and vegetables has been shown un-
equivocally in a considerable number of studies to be
associated with a lowered risk of several kinds of
cancer in a number of different body sites. It remains
to be proven with certainty whether this effect is due
to the content in the fruits and vegetables of antioxi-
dant substances, which are the protective agents, but
substantial circumstantial evidence points to the like-
lihood that antioxidant nutrient and nonnutrient sub-
stances in fruit and vegetables are the most important
anticarcinogenic factors in these foods. Besides
the classical antioxidants vitamin C, vitamin E, and
b-carotene, many phenolic substances are also pre-
sent in foods, including the flavonoids, which may
also contribute to the total antioxidant potential of
the diet and thus may lower the risk of cancer. Similar
considerations apply to arteriosclerosis, with its car-
diovascular and cerebrovascular risk connotations; it
has been clearly shown that antioxidant nutrients can
lower the oxidation of low-density lipoproteins in the
vascular wall, which is thought to be a major early
factor in the development of arteriosclerosis. It is also
possible that antioxidants can play an important role
in delaying the thrombotic events associated with the
onset of heart attack or stroke, and there is thus a
possibility that antioxidants have a dual effect in
lowering the risk of vascular accidents. If it is proven
that antioxidants can lower the risk of these degen-
erative diseases, it is vital that any enhancement of the
diet with antioxidants is safe and free from the risk of
any undesirable side-effects.
See also: Antioxidants: Natural Antioxidants; Role of
Antioxidant Nutrients in Defense Systems;
Chromatography: High-performance Liquid
Chromatography; Soy (Soya) Beans: Properties and
Analysis; Soy (Soya) Cheeses; Soy (Soya) Milk; Soy
(Soya) Bean Oil
tbl0008 Table 8 Reduction potentials (E
7
) of flavonoid radicals and the
TEAC
E
7
TEAC
Quercetin 0.33 4.7
Epigallocatechin gallate 0.43 4.8
Epigallocatechin 0.42 3.8
Taxifolin 0.5 1.9
Catechin 0.57 2.4
Rutin 0.6 2.4
Kaempferol 0.75 1.3
PHENOLIC COMPOUNDS 4513

Further Reading
Eisenbrand G, Dayan AD, Elias PS, Grunow W and
Schlatter J (2000) Carcinogenic and Anticarcinogenic
Factors in Food. Weinheim: Wiley-VCH.
Harborne JB, Mabry TJ and Mabry H (eds) (1975) The
Flavonoids. London: Chapman & Hall.
Harborne JB and Mabry TJ (1982) The Flavonoids:
Advances in Research. London: Chapman & Hall.
Harborne JB (ed.) (1994) The Flavonoids: Advances in
Research Since 1986. London: Chapman & Hall.
Lindsay DG and Clifford MN (eds) (2000) Nutritional
enhancement of plant-based food in European trade
(NEODIET). Journal of the Science of Food and Agri-
culture 80.
Macheix J-J, Fleuriet A and Billot J (1990) Fruit Phenolics.
Boca Raton, FL: CRC Press.
Packer L and Cadenas E (eds) (1996) Handbook of Anti-
oxidants. New York: Marcel Dekker.
Packer L, Hiramatsu M and Yoshikawa T (eds) (1999)
Antioxidant Food Supplements in Human Health. San
Diego, CA: Academic Press.
Rice-Evans C and Packer L (eds) (1998) Flavonoids in
Health and Disease. New York: Marcel Dekker.
Shahidi F and Naczk M (1995) Food Phenolics: Sources,
Chemistry, Effects, Applications. Lancaster, PA: Tech-
nomic.
Shahidi F and Ho C-T (eds) (2000) Phytochemicals and
Phytopharmaceuticals. Champaign, IL: AOCS Press.
Shahidi F and Wanasundara PKJ (1992) Phenolic antioxi-
dants. Critical Reviews in Food Science and Nutrition
32: 67–103.
PHOSPHOLIPIDS
Contents
Properties and Occurrence
Determination
Physiology
Properties and Occurrence
B F Szuhaj, Central Soya Company Inc., Fort Wayne,
IN, USA
This article is reproduced from Encyclopaedia of Food Science,
Food Technology and Nutrition, Copyright 1993, Academic Press.
Introduction
0001 Phospholipids have been scientifically studied since
the 1700s and became commercially available as leci-
thin in the 1930s. Their primary commercial source
today is the soya bean, but phospholipids can be
found in all living cells as part of the cellular mem-
branes. This article covers the properties and occur-
rence of phospholipids as commercial lecithins, their
chemistry, manufacture, composition, specifications,
and their potential use in food systems.
Occurrence
0002 The International Lecithin and Phospholipid Society
defines lecithin as ‘a complex mixture of glycero-
phospholipids obtained from animal, vegetable or
microbial sources, containing varying amounts of
substances such as triglycerides, fatty acids, glycoli-
pids, sterols, and sphingophospholipids.’ Lecithins
are natural surfactants primarily derived from soya
beans and eggs. They are found most abundantly
in seeds and nuts, eggs, brains, and cell walls, in a
concentration range of 0.5–2%. (See Eggs: Structure
and Composition; Fatty Acids: Properties; Soy (Soya)
Beans: Properties and Analysis; Triglycerides: Struc-
tures and Properties.)
Properties of Phospholipids (Lecithins)
0003There are three types of properties necessary to define
phospholipids and lecithins: (1) chemical, (2) phys-
ical, and (3) functional.
Chemical Properties
0004The chemical composition of deoiled and liquid
soya bean lecithin is shown in Table 1. There are
approximately 17 different compounds in commer-
cial lecithin, including carbohydrates, phytosterols,
and minor phytoglycolipids. The three major phos-
pholipids are phosphatidylcholine, phosphatidyl-
ethanolamine, and phosphatidylinositol.
4514 PHOSPHOLIPIDS/Properties and Occurrence
