Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.

breaking down polyphosphates and even utilizing
phospholipids.
Occurrence
0008 Phosphorus is the 11th element in order of abundance
in the earth’s crust and occurs to the extent of 1120
p.p.m. Elemental phosphorus is not found free in
nature but is combined with other elements in the
form of inorganic minerals or as components of or-
ganic compounds. It occurs in various orthophosphate
minerals, notably fluoroapatite [3Ca
3
(PO
4
)
2
CaF
2
]
and also as hydroxyapatite [Ca
3
(PO
4
)
2
Ca(OH)
2
],
chloroapatite [Ca
3
(PO
4
)
2
CaCl
2
). About 90% of
phosphate rock is used directly to make fertilizers,
and the remainder is used to make phosphorus and
phosphorus acids. World production of phosphorus is
about 1.2 million tonnes per year but is declining. It is
obtained by the reduction of calcium phosphate with
C in an electric furnace at 1400–1500
C. Sand is
added to remove the slag (calcium silicate) and to
drive off the P
4
O
10
, which is then reduced to P by C:
2Ca
3
ðPO
4
Þ
2
þ 6SiO
2
! 6CaSiO
3
þ P
4
O
10
P
4
O
10
þ 10C ! P
4
þ 10CO:
0009 However, in the human body, it is the sixth most
abundant element, after O, H, C, N, and Ca. It is an
essential constituent of every known tissue and cell in
the body and accounts for about 1% of the body
weight. Eighty-five percent of it remains in hard tissue
and the rest in muscles and blood, as illustrated in
Figure 6. Ordinarily, about 70% of phosphorus
ingested in foods is absorbed by the body. As phos-
phate is a major constituent of all plant and animal
cells, it is present in all natural foods, mostly in the
organic form. Heavy concentrations of phosphorus
are found in the meristematic regions of actively
growing plants, where it is involved in the synthesis
of nucleoproteins. Phospholipids, along with pro-
teins, are significant constituents of cell membranes.
Generally, phosphorus is more concentrated in seeds.
In plant tissues and juices, it exists as phosphoric acid
and the phosphate anion. In some citrus fruits, it
occurs up to 2.7% as dihydrogen phosphate anion
ðH
2
PO
4
Þ.
0010Diets rich in protein and calories also contain P in
adequate amounts, regardless of the source of pro-
tein, carbohydrates, and fat. Milk and dairy products
are the richest source of P in the diet, but P is widely
available in other foods. The phosphorus contents in
various foods are listed in Table 3. Semirefined brown
sugar contains a high level of phosphorus, but refined
sugar is completely stripped of the element. In the
refinement of cereals, 50–60% of phosphorus is lost.
The dietary intake of P can vary substantially with the
types of foods consumed. Not all the P in our diet is of
natural origin. Polyphosphates are popular additives
in many meat products. These enhance the water-
binding properties of meat proteins. Total food
supplies in the UK and USA provide 0.8–1.5 g of
phosphorus per person per day, of which about 10%
is added artificially. During pregnancy and the lacta-
tion period, however, the requirement for phosphorus
is increased. In muscle tissues, phosphorus is present
as phosphocreatine (PCr), which synthesizes ATP. Its
Total body phosphorus, 1% (500−700 g)
H
2
PO
4
80%
HPO
2−
4
20%
PO
3−
4
<0.01%
Free, 83% Protein bound, 17%
Inorganic, 30% Organic, 70%
Blood
1%
Soft tissue and muscles
14%
Bone
85%
−
fig0006Figure 6 Distribution of phosphorus in the human body.
tbl0003Table 3 Phosphorus contents in various foods
Food group Content (mg per 100 g)
Cereal grains 100–500
Pulses 300–600
Leafy vegetables 20–500
Fruits 10–500
Nuts and oilseeds 10–1000
Condiments and spices 10–500
Fish and seafood 200–2000
Meat and poultry 150–800
Milk and dairy products 100–1000
Fats and edible oils 10–200
Sugars 10–400
Beverages 10–100
Starch
OP
ONa
O
OH
fig0005 Figure 5
PHOSPHORUS/Properties and Determination 4535
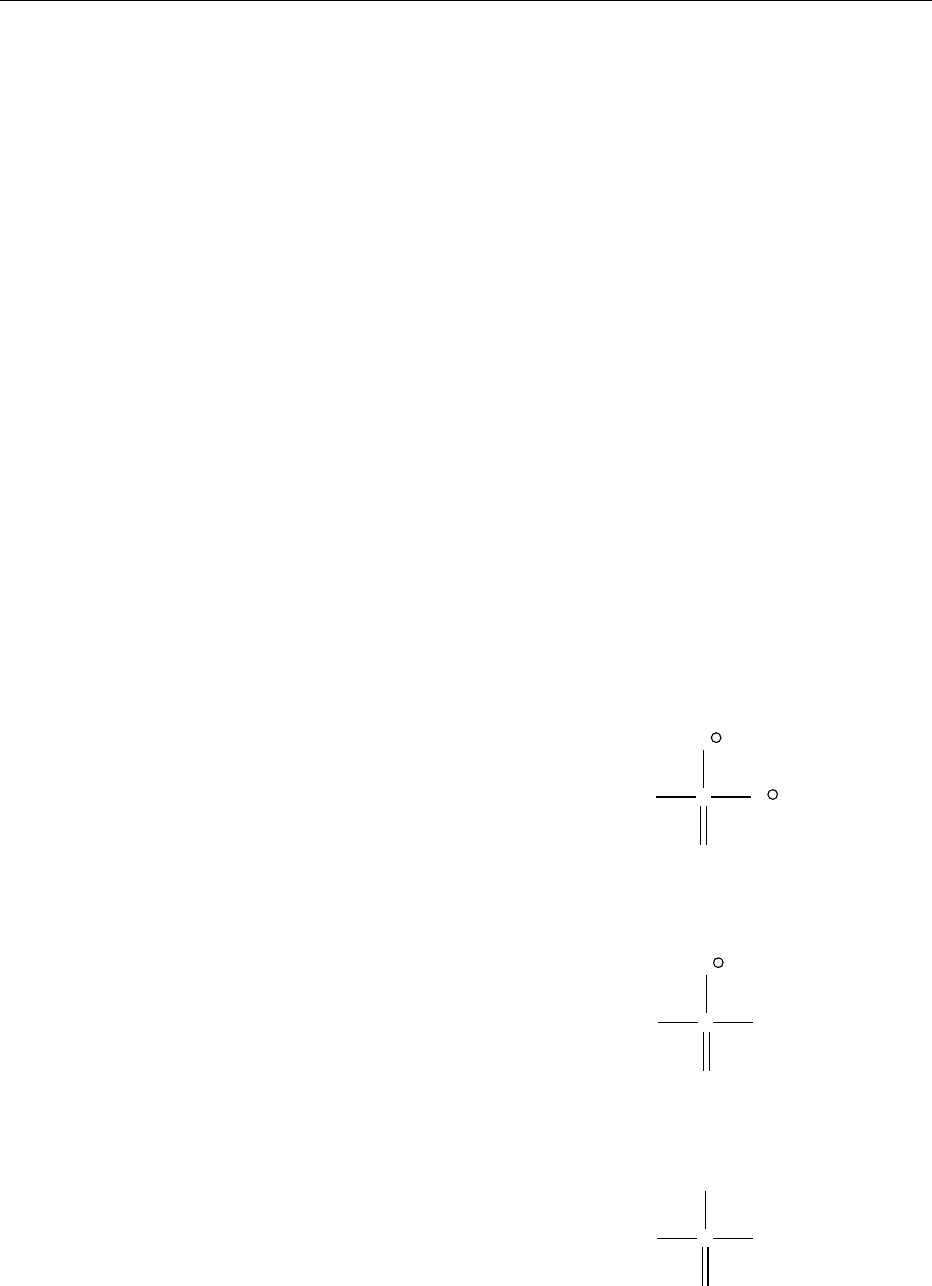
deficiency in adults may occur with excessive use of
alcohol, prolonged vomiting, liver disease, or hyper-
parathyroidism. The dietary content of P has been
shown to regulate physiologically the serum concen-
tration of PTH and thus indirectly the phosphate
homeostasis. Refer to individual food.
0011 Plants lacking phosphorus may develop necrotic
areas on the leaves, petioles, or fruits; they may
have a general overall stunted appearance, and the
leaves may have a characteristic dark to blue–green
coloration.
Properties of Phosphates
0012 All phosphates are salts of oxyacids that contain a
P ¼0 group and at least one P–OH group that ionizes.
Some species also have P–H group where the hydro-
gen atom is not ionizable. Phosphates of metal ions
and other cations, mixed metal phosphates, and
condensed phosphates are well known because of
their commercial and technical importance. Many
phosphates, especially long-chain polyphosphates,
are known for their toxicity as they adversely affect
the osmotic pressure of body fluids and prevent
absorption of mineral nutrients. Phosphates are
capable of interacting with many of the constituents
of food systems, and inactivate metal ions, and are
thus important in food processing.
0013 Monosodium phosphate (NaH
2
PO
4
) is water-
soluble and is used as a phosphatizing agent on steel
surfaces. Its acidic property isused in effervescent laxa-
tive tablets and as a leavening agent in baking powder.
Monopotassium phosphate (KH
2
PO
4
) crystals show a
piezoelectric effect and are used in submarine sonar
systems. Disodium and dipotassium phosphates are
used as buffering agents to maintain pH. This property
is used for stabilizing meat. These salts are also used as
sequestering agents in the food industry. Sodium ortho-
phosphate (Na
3
PO
4
) is highly alkaline and finds use in
industrial hard surface cleaners. Its aqueous solution is
a valuable constituent of scouring products, paint
strippers, and grease saponifiers. Its complex with
sodium hypochlorite [(Na
3
PO
4
11H
2
O)
4
NaOCl] re-
leases activechlorine when wetted; this combination of
scouring, bleaching and bacterial actions makes the
adduct valuable in automatic dish washing powder
formulations. Potassium orthophosphate (K
3
PO
4
)is
used to regulate the rate of polymerization of styrene–-
butadiene rubber. Mono- and diammonium phos-
phates are used as fertilizers and nutrients. An
important property of ammonium phosphate is a
flame-retarding agent for cellulose materials. The
action depends on its dissociation to ammonia and
orthophosphoric acid when heated. Acid so generated
catalyzes the decomposition of cellulose to char and
smother the flame. Urea phosphate is generally used
to flameproof cotton fabrics. A dilute solution of
diammonium phosphate, with an initial pH of 7.85,
upon boiling evolves ammonia, and the pH drops to
5.78 in 2 h. This property is used for the precipitation
of colloidal dyes on wool fabrics. Dicalcium phos-
phates are used in pharmaceutical tablets as supple-
ments. Natural phosphate minerals are all
orthophosphates, the major ones being fluoroapatite;
partly carbonated hydroxyapatite makes up the min-
eral part of teeth. These are important constituents of
bones. Calcium orthophosphates are particularly im-
portant in fertilizer technology. (See Leavening
Agents; Stabilizers: Types and Function.)
0014Many phosphate complexes of transition metal ions
are known. Ce
4þ
,Th
4þ
,Zr
4þ
, U, and Pu form insol-
uble phosphates from fairly strong acid solution (3–6
M nitric acid). Condensed phosphates contain more
than one phosphorus atom and P–O–P bonds with
three main building units – the end unit (Figure 7),
middle unit (Figure 8), and branching unit (Figure
9).These units can be readily distinguished by reactiv-
ity with water and
31
P NMR. These can also be
incorporated into linear or cyclic polyphosphates.
Linear polyphosphates are salts with the general for-
mula [P
n
O
3n þ 1
]
(n þ 2)
(n ¼ 2–10) such as M
1
4
P
2
O
7
−O
P
O
−
O
−
O
fig0007Figure 7
−O
P
O
−
O−
O
fig0008Figure 8
−O
P
O−
O−
O
fig0009Figure 9
4536 PHOSPHORUS/Properties and Determination
and M
1
5
P
3
O
10
. Many polyphosphates with different
chain lengths have been known. Disodium dihydro-
gen pyrophosphate, Na
1
2
H
2
P
2
O
7
is mixed with
NaHCO
3
and used in bread making to leaven the
bread, that is to make it rise. They react and evolve
CO
2
when heated together. Sodium pyrophosphate,
Na
4
P
2
O
7
, is mixed with starch and a flavoring agent
to make instant pudding mixture. At one time, it was
also added to soap powders and solutions as a water
softener. Ca
2
P
2
O
7
is used as the abrasive/polishing
agent in fluoride paste. Cyclic polyphosphates or
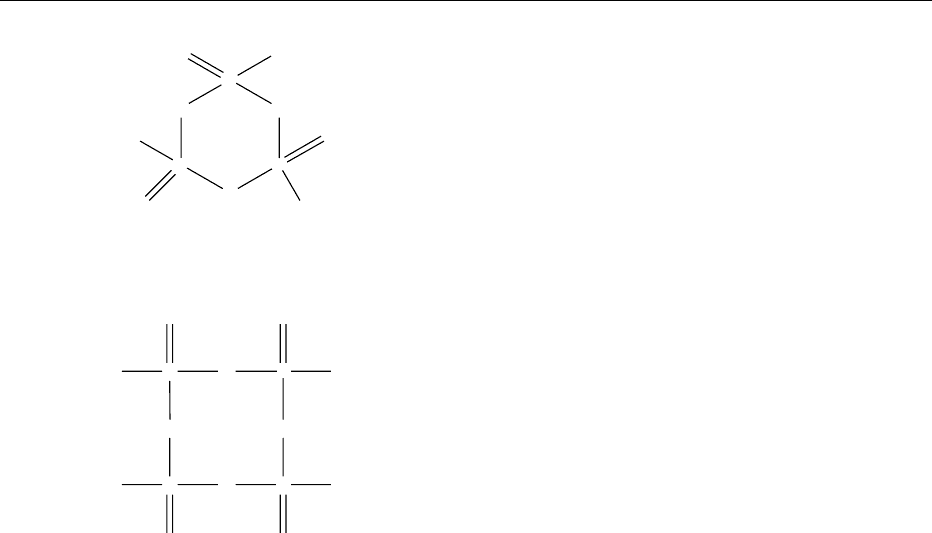
metaphosphates are the salts with the general formula
[P
n
O
3n
]
n
with n ¼3–7 such as M
3
P
3
O
9
(Figure 10)
and M
4
P
4
O
12
. The eight-membered ring of the P
4
O
4
12
(Figure 11) is puckered with equal bond lengths.
Condensed phosphates form soluble complexes
with many metals. These are usually prepared by
dehydration of orthophosphates under various condi-
tions of temperature (300–1200
C) and also by
appropriate hydration of dehydrated species. Chain
phosphates are used as water softeners in industry.
Polyphosphates aid in controlling the microbiological
population on the surface of poultry meat. Some
metaphosphates having an infinite chain length are
also known, e.g., KPO
3
.
0015 Organic phosphates contain phosphate groups
linked through OH groups of organic compounds
(–C–O–P linkage) such as sugars. Large numbers of
phosphate esters, RO–PO(OH)
2
are known. These
occur in the form of mono-, di-, and triphosphoesters
Each form has specific chemical properties leading to
different biological functions. These are constituents
of numerous highly active intracellular compounds.
Release of free energy by hydrolysis of ATP provides
the main source of energy for various metabolic pro-
cesses and for muscle contraction. Intracellular phos-
phate is a regulator of enzymes in the glycolytic
pathways. Some of the alkyl phosphorus compounds
are of industrial importance, particularly for solvent
extraction of metal ions from aqueous solutions.
They extract metal ions by cation exchange and/or
by solvation. Among these, di(2-ethylhexyl) phos-
phoric acid (DEHPA), tri-n-butyl phosphate (TBP),
and tri-n-octylphosphine oxide (TOPO) have been
used most extensively. Organic derivatives of fluoro-
phosphoric acid [FP ¼O(OH)
2
] have promising
properties as insecticides.
ANALYSIS
0016Phosphorus is generally detected on the basis of the
reaction between orthophosphoric acid and the
molybdate ion ðMoO
2
4
Þ, which gives a yellow-
colored precipitate in a strongly acid solution.
Total Phosphorus
0017Any solution containing phosphorus is fumed with
aqua regia almost to dryness, followed by heating
with 1 M nitric acid, whence lower oxidation states
are oxidized to the orthophosphate (PO
3
4
) form. The
resultant solution can be used for the estimation of
the total phosphorus by gravimetric, titrimetric, or
spectrophotometric methods. Some of these have
been recommended by the Association of Official
Analytical Chemists for the analysis of total phos-
phorus in vegetables, fruits, cereals, and other foods.
These are dried in a silica/platinum crucible and
heated over a low Bunsen flame to volatilize organic
matter. A 10% sodium bicarbonate solution is added
and the contents evaporated to dryness. Oil may be
burnt off at a lower temperature without smoking,
and finally, ashing is carried out in a muffle furnace at
500
C. The contents are dissolved in concentrated
nitric acid and heated to dryness, and then dilute
hydrochloric acid is added.
0018Gravimetric methods include the formation and
weighing of phosphorus as ammonium phospho-
molybdate, ammonium magnesium phosphate or
pyrophosphate, and quinoline molybdophosphate.
On addition of ammonium molybdate solution
(12.5 g dissolved in 75 ml of water are slowly added
to another solution containing 125 g of ammonium
nitrate in 125 ml of water and 175 ml of nitric acid
diluted to 500 ml) a yellow-colored precipitate is
O
P
O
P
O
P
O
O
−
O
O
−
O
−
O
fig0010 Figure 10
P
P
O
O
−
O
O
O
−
O
P
O
O
−
OP
O
−
O
O
fig0011 Figure 11
PHOSPHORUS/Properties and Determination 4537

obtained, which is filtered, dried at 105
C, and
weighed as (NH
4
)
3
PO
4
12 MoO
3
H
2
O. On further
heating at 450
C, a dark greenish blue complex,
P
2
O
5
24MoO
3
, is obtained, which may be weighed.
In another method, the ammonium molybdate is
replaced by a reagent containing sodium molybdate
and quinoline so that quinoline molybdophosphate,
(C
9
H
7
N)
3
[PO
4
12MoO
3
], is precipitated. Alterna-
tively, magnesia reagent (magnesium chloride and
ammonium chloride in ammoniacal solution) may
be used as the precipitating reagent. The precipitate
may be weighed as Mg(NH
4
)PO
4
6H
2
O, or after
heating in a muffle furnace at 1000
CasMg
2
P
2
O
7
.
0019 The most common titrimetric method consists
of precipitation as Mg(NH
4
)PO
4
.6H
2
O, filtration,
washing, and dissolution of the precipitate in excess
of dilute hydrochloric acid. An excess of standard
ethylene diamine tetraacetic acid (EDTA) solution is
added, and its pH is adjusted to 10. Excess EDTA is
titrated with a standard solution of magnesium chlor-
ide or magnesium sulfate using Eriochrome black
T as the indicator. Alternatively, the precipitate of
ammonium molybdophosphate may be titrated with
standard sodium hydroxide solution using phenol-
phthalein indicator.
0020 Several spectrophotometric methods such as the
molybdenum blue method and those using ammo-
nium vanadate and 1-amino-2-naphthol-4-sulfonic
acid have been used. A solution containing orthopho-
sphate and molybdate ions condenses in acid solution
to give phosphomolybdic acid, which upon reduction
with hydrazinium sulfate produces a blue color due to
molybdenum blue of uncertain composition. It ex-
hibits a maximum at 620–630 nm. When phosphate,
ammonium vanadate, and ammonium molybdate
react, a bright yellow-colored complex of phospho-
vanadomolybdate is formed with a l
max
of 460–
480 nm. In yet another method, molybdate reagent
and 1-amino-2-naphthol-4-sulfonic acid solution
(0.5 g with 30 g of sodium bisulfite and 6 g of sodium
sulfite made up to 250 ml) are added. After standing
for 10 min, the absorbance is measured at 650 nm.
Determination of P concentration in plasma and
other body fluids is the most common way to evaluate
physiological status. The urinary concentration deter-
mines mainly how the kidneys handle phosphate. In
such cases, the phosphate concentration is measured
by the Fiske–Subbarrow colorimetric method. Com-
monly, the results are reported in units of mass per
volume (mg per liter). In all these methods, a blank is
always taken, and a standard calibration curve is
constructed, from which the concentration of phos-
phorus is calculated. (See Spectroscopy: Overview.)
0021 Phosphate is also determined nephelometrically.
On adding molybdate strychnine reagent (in two
parts: solution A of molybdenum trioxide in 5 M
sulfuric acid, solution B of 1.6 g of strychnine sulfate
in 500 ml of water; both solutions are mixed before
use), a white-colored turbidity is obtained. The phos-
phorus content is determined from the calibration
graph.
0022In recent years, several instrumental methods, viz.
inductively coupled plasma (ICP) and/or direct cur-
rent plasma (DCP) atomic emission spectrome-
try(AES), spark source mass spectrometry (SSMS),
high-performance liquid chromatography (HPLC),
flow injection analysis with continuous microwave
oven digestion, an enzyme sensor system and X-ray
fluorescence (XRF) have been used. Since the advent
of body-imaging systems based on nuclear magnetic
resonance measurement, it is now possible to detect
phosphate in different tissues in vivo and determine
changes with time under different physiological con-
ditions. Thus,
31
P NMR can be used to detect levels of
ADP, ATP, PCr, etc., and the metabolic activity of the
cells can be determined. This method is used to study
mainly the functioning of human liver and heart.
Being most sensitive, it opens the most fascinating
horizons for understanding the role of phosphorus
in different metabolic pathways. With the availability
of nuclear reactors, more sensitive methods
employing radioactivity measurement have been
employed. On thermal neutron irradiation,
32
P(b
emitter, 1.71 MeV, half-life, 14.3 days) is formed by
the (n, g) reaction. A gas flow proportional counter
is used with an aluminum absorber (thickness
27 mg cm
2
). Only a small sample size (*50 mg) is
required. A derivative activation analysis method,
and those employing Cerenkov and Bremsstrahlung
counting have also been employed. Some radiotracer
methods employing
32
P have been used for investi-
gating the uptake of phosphate ions by plants through
roots. (See Chromatography: High-performance
Liquid Chromatography; Mass Spectrometry: Prin-
ciples and Instrumentation.)
Organic Phosphorus
0023Phosphorus-containing organic compounds are first
extracted into benzene or any other suitable solvent.
An aliquot is transferred to a beaker, and the solvent
is evaporated slowly on a water bath. The residue is
heated with concentrated nitric acid and then with a
small amount of potassium chlorate to dryness. After
cooling, concentrated hydrochloric acid is added
repeatedly until a clear solution is obtained. A 2.5%
ammonium molybdate and potassium iodide/sodium
carbonate solution is added. The flask is immersed in
a steam bath for 15–20 min. After cooling, 0.5%
sodium sulfite solution is added dropwise until the
4538 PHOSPHORUS/Properties and Determination

iodine color disappears. Finally, the absorbance is
measured at 650nm.
002 4 Organophosphorus compounds can also be com-
busted in a Schoninger oxygen flask to give orthopho-
sphate, which can be absorbed by either sulfuric or
nitric acid. Phosphorus can then be determined spec-
trophotometrically. Recently, malonyl dihydrazide
has been proposed for spectrophotometric extraction
of organophosphorus compounds in vegetables.
Chromatographic separations employing aluminum
oxide and thin-layer chromatography (TLC) have
also been used. Gas chromatography–mass spectrom-
etry (GC–MS) has been suggested for the confirm-
ation of organophosphorus pesticide residues in
foods. A TLC enzymatic method has been suggested
for the determination of organophosphorus com-
pounds down to a detection limit of 10
10
g.
(See Chromatography: Thin-layer Chromatography;
High-Performance Liquid Chromatograpy.)
Other Forms
0025 Phosphorus may also be found as phosphite PO
3
3
,
which may be determined as mercurous chloride or
ammonium magnesium phosphate. In the first case,
an acid solution of phosphite reduces mercury(II)
chloride solution (eqn 1), and mercury (I) chloride is
weighed, the latter being directly proportional to the
phosphite concentration.
2HgCl
2
þ H
3
PO
3
þ H
2
O !Hg
2
Cl
2
þ H
3
PO
4
þ2HCl ð1 Þ
In the other case, phosphite is oxidized by nitric acid
to phosphate, which is determined as
Mg(NH
4
)PO
4
6H
2
OorasMg
2
P
2
O
7
.
002 6 Yet another form of phosphorus is hypophosphite
ðPO
3
2
Þ. It is oxidized quantitatively by excess of ceric
(IV) sulfate in sulfuric acid solution in 30 min at
60
C; the excess Ce
4 þ
is titrated with ferrous ammo-
nium sulfate to a permanent red end point using
ferroin indicator (eqn 2).
4Ce
4þ
þ H
3
PO
2
þ 2H
2
O ! 4Ce
3þ
þ H
3
PO
4
þ 4H
þ
ð2 Þ
It is possible to determine both hypophosphite and
phosphite by affecting complete oxidation to phos-
phate. These different forms may also be differenti-
ated by ion chromatography.
Seealso: Chromatography:Thin-layerChromatography;
High-performanceLiquidChromatography;Gas
Chromatography; Leavening Agents; Mass
Spectrometry:PrinciplesandInstrumentation;
Phospholipids:PropertiesandOccurrence; Phytic
Acid:PropertiesandDetermination; Spectroscopy:
Overview;NuclearMagneticResonance; Stabilizers:
TypesandFunction; Starch:Structure,Properties,and
Determination
Further Reading
Baudler M (1987) Polyphosphorus compounds, new results
and new perspectives. Argewandte Chemie International
Edition in English 26: 419–441.
Berner YN (1997) Phosphorus, In: O’Dell BL and Sunde RA
(eds.) Handbook of Nutritionally Essential Elements,p.
63–92. New York: Marcell Dekker.
Berner YN and Shike M (1989) Consequences of phosphate
imbalance. Annual Review of Nutrition 8: 121–148.
Corbridge DEC (1985) Phosphorus. An Outline of its
Chemistry, Biochemistry and Technology, 3rd edn.
Oxford: Elsevier.
Ellinger RH (1975) Phosphates in food processing. In: Furia
TA (ed.) Handbook of Food Additives, pp. 617–780.
Cleveland, OH: CRC Press.
Goldwhite H (1981) Introduction to Phosphorus Chemis-
try. Cambridge: Cambridge University Press.
Kanazawa T (ed.) (1989) Inorganic Phosphate Materials.
Tokyo: Kodansha.
Tietz NW (1976) Electrolytes. In: Tietz NW, Carraway
WT,Freier EF, Kachmar JF and Rawausley HM (eds)
Fundamentals of Clinical Chemistry. Philadelphia, PA:
Saunders.
Toy ADF (1975) Chemistry of Phosphorus. New York:
Pergamon Press.
Weginwar RG, Samudralwar DL and Garg AN (1989) De-
termination of phosphorus in biological samples by ther-
mal neutron activation followed by b-counting. Journal
of Radioanalytical and Nuclear Chemistry Articles 133:
317–324.
Physiology
J J B Anderson, University of North Carolina, Chapel
Hill, NC, USA
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001Phosphorus, an essential nutrient, is required for
many different functions in body tissues, both intra-
cellularly and extracellularly. This element, which
exists in biological systems as phosphates, is used by
cells to make structural molecules and outside of cells
to make the crystals of bones and teeth; it serves as a
component of intracellular regulatory molecules; it
serves as a buffering component in both intra- and
extracellular fluids, and it is an important factor in
cellular energetics, especially in the mitochondria,
where it is used to make most of the high-energy
PHOSPHORUS/Physiology 4539

bonds needed for cellular activities. Lastly, the metab-
olism of inorganic phosphate is closely linked to that
of calcium, and this review will, therefore, also deal
to some extent with calcium and its homeostatic con-
trol. Adequate phosphorus and calcium intakes are
critical not only for skeletal growth, but also for
growth and development of soft tissues, especially in
neonates. (See Bone; Calcium: Physiology; Cells;
Energy: Measurement of Food Energy.)
0002 Phosphorus, in both the inorganic phosphate (Pi)
and organic phosphate forms (Po), is abundant in
nearly all foods traditionally consumed. This element
is especially rich in animal products, such as meats,
fish, poultry, eggs, milk, and other dairy products, but
it also exists in cereal grains and most vegetables in
good quantities. Because Pi ions are so readily
absorbed across the small intestine, with an efficiency
of roughly 65–75% in adults and with a somewhat
higher efficiency in children, the prompt rise in blood
Pi after a meal or snack influences calcium homeo-
stasis, when Pi ions enter the blood from the gut,
essentially unaccompanied by the more slowly
absorbed calcium ions. An elevation in the plasma
concentration of Pi tends to depress the serum
calcium concentration, mainly Ca
2þ
, through the for-
mation of a complex of the two ions, and this decline
in Ca
2þ
stimulates parathyroid hormone (PTH)
release. Although the specific mechanism through
which Pi exerts this effect on plasma Ca
2þ
has not
been fully established, many experimental data sup-
port the existence of this phenomenon.
0003 Dietary phosphorus deficiency, although highly
unlikely because of the abundance of this element in
foods, contributes to low serum Pi concentration and
thereby limits bone mineralization via osteoblasts and
the total amount of bone mineral mass deposited in the
skeleton. Furthermore, Pi deficiency increases bone
turnover, which, during infancy, can lead to rickets.
Dietary Sources of Phosphorus
0004 Although both organic and inorganic forms of phos-
phorus are widely distributed in foods, 75% or so of
the phosphorus in foods is converted to Pi following
the various digestive steps in the stomach and upper
small intestine. The organic phosphorus usually
remains associated with the fat-soluble dietary
molecules that are absorbed without digestion of the
phosphate groups from these molecules, as is the case
with phosphatidylcholine (lecithin).
0005 Phosphate additives in processed foods and in
beverages can also make an important contribution
to total phosphorus intakes. Concern has been raised
about the quantity of phosphate added to cola-type
soft drinks, which usually contain approximately
60 mg of phosphate per 12-oz. (336-g) container in
the United States, but in reality, much greater
amounts of phosphates are added to other processed
foods commonly consumed in developed nations.
Phosphate additives are used most by the food indus-
try in baked goods, meats, cheeses, and milk products
in the United States.
0006Mean phosphorus intakes of American women,
according to recent surveys, range between 900 and
1200 mg per day, depending on age and caloric intake;
older women consume less phosphorus than younger
women, and active women consume more than sed-
entary individuals. Men usually consume closer to an
average of 1500 mg per day, and their intakes also
decline with age and with declining activity and
caloric intake. The US Recommended Dietary Allow-
ance (RDA) for each sex over 19 years of age was
reduced in 1997 to 700 mg (Institute of Medicine)
from 800 mg (NRC), whereas recommended calcium
intakes were increased for adults until the end of life.
Adequate intakes of phosphorus are readily achieved
from foods in the USA, but those of calcium are not.
The ratio of Ca:P in typical diets of American adult
females is approximately 0.5:1, which raises concern
among nutritionists because of the potential adverse
effects of nutritional secondary hyperparathyroidism
(see below). (See Dietary Requirements of Adults.)
0007Table 1 gives representative values of these same
foods; the Ca:P ratio of these foods is also given as
reference for later discussion of this ratio under
Nutritional Secondary Hyperparathyroidism.
Intestinal Absorption of Phosphates
0008The net absorption of Pi is highly efficient; and the Pi
efficiency is more than twice that of calcium absorp-
tion, which is usually stated as being between 25 and
30% in adults. The efficiency of Pi absorption by
infants has been reported to be as high as 80–90%.
Typical meals that contain representative items from
all food groups, including dairy, have ratios of Ca:Pi
approaching 0.7:1.0. If the actual amount of calcium
in the meal is 350 mg, and the amount of phosphorus
is 500 mg, then 75% of the phosphorus, or 375 mg,
will be absorbed, most of it within the first postpran-
dial hour, but only 30% or 105 mg of calcium will be
maximally absorbed, and this process usually takes
several hours to be completed. The net effect is that
the rise in blood Pi concentration tends to depress the
serum Ca
2þ
, perhaps through a reciprocal adjustment
in the serum ion concentration product, i.e., [Ca
2þ
]
[Pi] ¼ constant, a mechanism proposed in the 1940s.
Parathyroid hormone (PTH) secretion from the para-
thyroid glands responds to the depressed serum Ca
2þ
concentration as long as absorbed Pi continues
4540 PHOSPHORUS/Physiology

to lower the Ca
2þ
concentration. (See Hormones:
Thyroid Hormones.)
0009 Pi absorption occurs by two major routes, transcel-
lular and paracellular, throughout the small intestine
and probably also the large intestine. The transcellular
route is considered the more significant route, but not
much is known about the paracellular pathway in
terms of location in the small intestine or the quanti-
tative contribution of this component. The transcel-
lular route involves at least two distinct mechanisms
tbl0001 Table 1 Phosphorus and calcium composition of representative foods in a serving and the Ca:P ratio of these foods
Fooditem Weight (g) Calcium (mg) Phosphorus (mg) Ca:Pratio
Dairy
Milk, whole 244 291 228 1.3:1
Milk, 2% fat 244 297 232 1.3
Milk, 1% fat 244 300 235 1.3
Milk, skim 245 302 247 1.2
Cheddar cheese 28 204 145 1.4
Egg, whole, poached 50 28 90 0.3
Meat, fish, and poultry
Salmon, pink canned 85 167 243 0.7
Shrimp, canned 85 98 224 0.9
Beef, ground, lean with 10% fat 85 10 196 0.05
Liver, beef, fried 85 9 405 0.02
Pork roast, cooked 85 9 218 0.04
Chicken breast, cooked, boneless 85 18 210 0.09
Fruits
Avocado, raw, skinless 251 23 30 0.8
Orange, whole, peeled 131 54 26 2.1
Pineapple, raw, dried 155 26 12 2.2
Raisins 14 9 14 0.6
Watermelon 926 30 43 0.7
Breads and cereals
White bread, soft 25 21 24 0.9
Whole-wheat bread, soft 28 14 24 0.6
Bran flakes cereal 35 19 125 0.2
Corn flakes cereal 25 1 9 0.1
Shredded wheat cereal 25 11 97 0.1
Macaroni, cooked 130 14 85 0.2
Noodles (egg), cooked 160 16 94 0.2
Rice, white, instant 165 5 31 0.2
Spaghetti, cooked 130 14 85 0.2
Legumes, nut, seeds
Almonds, shelled 130 304 655 0.5
Beans, Lima, cooked 190 55 293 0.2
Peas, black-eye, cooked 250 43 238 0.2
Peas, split, cooked 200 22 178 0.1
Vegetables
Asparagus, cooked 145 30 73 0.4
Beets, cooked 100 14 23 0.6
Beet greens, cooked 145 144 36 4.0
Broccoli, cooked 180 158 112 1.4
Cabbage, cooked 145 64 29 2.2
Carrots, cooked 155 51 48 1.1
Corn, sweet, cooked 140 2 69 0.03
Kale, cooked 110 206 64 3.2
Pepper, sweet, cooked 73 7 12 0.6
Potatoes, baked 156 14 101 0.1
Squash, summer, cooked 210 53 53 1.0
Sweet potatoes, baked 114 46 66 0.7
Tomatoes, raw 135 16 3 5.3
Miscellaneous
Tomato soup with milk 250 168 155 1.1
Tomato soup with water 245 15 34 0.4
Split-pea soup with water 245 29 149 0.2
Source: Home and Garden Bulletin No. 72, USDA.
PHOSPHORUS/Physiology 4541

of entry at the brush-border membrane (mucosa) and
probably as many as part of the exit step at the
basolateral membrane (serosa). Much of the Pi
absorption is considered passive because of cotran-
sport with Na
þ
or other cations. Because of the
cotransport mechanism with Na
þ
following a meal,
Pi ions may be much more rapidly absorbed than if a
separate and independent mechanism for Pi absorp-
tion per se were required. Understandings of the
mechanisms of Pi absorption remain limited. The
hormonal form of vitamin D, 1,25-dihydroxyvitamin
D, increases Pi absorption via the intracellular route,
but much less is known of this pathway than the
vitamin D-mediated Ca
2þ
absorption. (See Cholecal-
ciferol: Physiology.)
0010 Some secretions of Pi ions into the gastrointestinal
tract occur at every level, i.e., salivary glands, stom-
ach, intestine, pancreas, liver, and large intestine.
Thus, net Pi absorption represents the difference
between total Pi absorption and endogenous Pi
secretion. Pi ions not absorbed by the gut pass into
the stools.
Blood Concentrations of Phosphates
0011 The distribution in human blood serum is compared
with the distribution of calcium in Table 2. Po in the
lipid fraction of blood plasma generally represents a
fairly stable value in individuals on diets with consid-
erable amounts of phosphorus, i.e., typical Western
diets, but it will decline if the diet becomes deficient
or much lower in phosphorus. The reason for this
change is not clear, but it may result from a homeo-
static adaptation to the low-phosphorus intake
through which membrane-bound Po groups are
cleaved and released to the blood in order to keep
the plasma Pi concentration at or near its ‘set’ level.
The set level, which is determined genetically in a
species, is regulated by various homeostatic mechan-
isms; in the case of serum Pi, several hormones, espe-
cially PTH, are involved in maintaining serum Pi at or
near its set level.
0012 The hormonal form of vitamin D – 1,25-dihydroxy-
vitamin D – is thought to enhance the intestinal
absorption of Pi, as it does for Ca, but relatively little is
known about the cellular mechanism ofthis action. For
example, Pi-carrier proteins have been identified at the
brushbordermembrane,butnointracellularPi-binding
proteins have been found after vitamin D treatment in
animalmodels.
0013Pi ions in the blood or extracellular fluids are dis-
tributed to all tissues in the body to meet cellular
needs and to be taken up by the bone fluid compart-
ment for incorporation in hydroxyapatite crystals.
During tooth development, Pi ions are taken up by
cells (odontoblasts, ameloblasts) and transferred into
the extracellular compartment of the developing
hydroxyapatite crystals.
Phosphate Homeostatic Mechanisms
0014The serum Pi concentration is not as rigorously
regulated at any time during the life cycle as that
of calcium. Several hormones are involved in Pi
homeostasis. PTH, calcitonin, and the hormonal
form of vitamin D are considered the major regula-
tors, but many other hormones also affect Pi homeo-
stasis, including insulin, glucagon, growth hormone,
estrogens, adrenaline, and adrenal corticosteroids.
(See Hormones: Steroid Hormones.)
0015An elevation of PTH acts to stimulate renal Ca
2þ
reabsorption while acting to block renal Pi reabsorp-
tion during this postprandial period, and at the same
time, PTH is thought to be relatively inactive on bone
cells because of the dominance of calcitonin. The net
effect of PTH actions on different tissues is to try to
conserve Ca
2þ
in the face of an elevated plasma Pi.
However, if PTH remains elevated after the blocking
effect of calcitonin decays, then an increased transfer
of Ca
2þ
from the bone fluid compartment to blood
and PTH-stimulated osteoclastic resorption together
can slowly deplete bone when the dietary phosphorus
intake greatly exceeds calcium on a long-term basis.
This potential mechanism, then, is considered a
highly likely way in which low-calcium diets contrib-
ute to osteopenia and subsequent fractures that
characterize osteoporosis. The name given to this
condition is nutritional ‘secondary hyperparathyroid-
ism’ (see below). (See Osteoporosis.)
0016Regulation of the plasma Pi concentration is not
well understood, but PTH and other hormones are
involved through their renal, intestinal, and skeletal
activities. High Pi intakes are handled readily and
promptly by healthy individuals, primarily through
increased renal excretion. The problem of a high-Pi
intake coupled with low-calcium consumption in a
typical dietary pattern, however, is handled at the
expense of bone mineral through a chronic elevation
of PTH and 1,25-dihydroxyvitamin D. Whether
tbl0002 Table 2 Serum fractions of phosphates and calcium
Phosphate form Percentage
of total
Calcium Percentage
of total
Free HPO
4
2
44 Free Ca
2þ
48
Free H
2
PO
4
10 Protein-bound 46
Protein-bound 12 Complexed 3
Cation-bound 34 Other 3
Adapted from Bringhurst FR (1989) Calcium and phosphate distribution,
turnover, and metabolic actions. In: DeGroot LJ (ed.) Endocrinology,2nd
edn, vol. 2. Philadelphia, PA: WB Saunders.
4542 PHOSPHORUS/Physiology

1,25-dihydroxyvitamin D actually exerts a major en-
hancing effect on intestinal Pi absorption is not clear,
but it does increase cellular transfer of Ca
2þ
from the
gut lumen to the blood.
0017 Other hormones that also have an influence on Pi
homeostasis are important metabolic regulators, such
as insulin following carbohydrate ingestion. Insulin
stimulates glucose uptake in many extrahepatic
tissues, and Pi moves into the cells along with glucose,
probably in a 1:1 molar ratio. However, in situations
when glucagon is elevated, glucagon enhances renal
excretion of Pi, probably through a mechanism that
blocks renal tubular Pi reabsorption. Calcitonin
is also phosphaturic. Growth hormone and other
anabolic hormones and local tissue factors generally
stimulate Pi incorporation into organic structures,
much like insulin, to meet the needs of growth, cell
division, or structural requirements for tissue
maintenance. (See Carbohydrates: Requirements and
Dietary Importance.)
0018 Another aspect of Pi homeostasis involves mem-
brane-bound organic (Po) molecules, such as the
phospholipids. Animal models fed low-phosphorus
intakes have increased degradation of these Po mem-
brane components, which release Pi to the blood and
help to maintain the blood Pi concentration at its set
level. These findings strongly suggest that membrane
phospholipids have a role in Pi homeostasis.
0019 Estrogen may also enhance Pi transfer into cells
because plasma Pi becomes somewhat elevated in
postmenopausal women and in women who have
had an oophorectomy. This rise may also reflect
bone turnover and Pi release to blood from the skel-
eton. Estrogen-replacement therapy in postmeno-
pausal women causes a slight decline in serum Pi.
0020 Excess Pi in cells can be stored in various organ-
elles, such as mitochondria and endoplasmic reticu-
lum, along with Ca
2þ
as calcium phosphates, which
can be solubilized in times of need to be retrieved for
cellular requirements.
Functional Roles of Phosphates
0021 Phosphorus as Pi and/or Po exists in all tissues, both
intracellularly and extracellularly. In extracellular
sites, phosphorus exists in the hydroxyapatite crystals
of bones and teeth and in phosphorylated proteins in
diverse extracellular matrices. The phosphorylation
of Type I collagen in bone may trigger the mineral-
ization process. Phosphates also serve as a buffer
system in blood and other extracellular fluids.
0022 Intracellularly, phosphates also serve as important
buffers, and Po is a component of numerous classes of
molecules, including membranes, high-energy mol-
ecules, regulatory proteins, regulatory phospholipids,
and nucleoproteins. In the phospholipids of nervous
tissue, Po is a critical component of many diverse
molecules. Phosphorylation of inositol to phosphati-
dylinositol and cleavage of inositol triphosphate rep-
resent an important regulatory mechanism in cells. In
addition, phosphorylation of certain enzymes by a
variety of protein kinases and the dephosphorylation
of these same enzymes (proteins) by phosphatases is
central to activation and inactivation of key regula-
tory enzymes controlling specific metabolic pathways
in cells. Pi uptake by cells for the synthesis of regula-
tory peptides and regulatory phospholipids is also
important in metabolically active cells. Thus, phos-
phorus is much more widely distributed within cells
than is calcium, and it serves many diverse roles.
0023Pi uptake by cells is enhanced by insulin, but other
hormones also increase the uptake of this anion,
including estrogens, adrenalin, calcitonin, and many
growth factors, such as insulin-like growth factor
(IGF-1). Once in the cytosol, Pi ions are used for
phosphorylating glucose and related intermediate
molecules derived from glucose in a meal. In addition,
Pi ions are shuttled across intracellular organelle
membranes for use or storage. For example, in
mitochondria, Pi ions are essential if oxidative phos-
phorylation is to be adequately coupled. Mitochon-
dria also store roughly 20% of the cells’ Pi as calcium
salts. Similarly, the endoplasmic reticulum (ER) uses
and stores Pi for phosphorylation of various proteins.
Also, the ER contains approximately 30% of total
cellular Pi for storage and use in phosphorylation of
proteins and other molecules. The nucleus, Golgi
complex, and lysosomes contain the remainder of
the total Pi.
Phosphate in Health and Disease
0024The balance of phosphorus is determined by the dif-
ference between input and output, and it is kept re-
markably constant in healthy individuals until late in
the life cycle when lean tissue loss accelerates. The
balance is clearly negative prior to death in ill patients
because of the death of numerous cells without re-
newal. Pi absorption declines slightly late in life, i.e.,
sometime after the fifties, because of a reduced effi-
ciency of absorption and because food energy intake
also declines in the elderly. Thus, the overall input of
Pi is lowered. Pi excretion also declines slightly in
healthy older individuals, but it declines more so if
renal function is seriously compromised. Normally,
urinary excretion of Pi approximates 67% of Pi
consumed in the diet, and this percentage holds
throughout life in healthy individuals. Unabsorbed
phosphorus makes up nearly all of the remainder of
fecal Pi elimination, although sweat and skin losses
PHOSPHORUS/Physiology 4543
do contribute a small percentage to the total excretion
of Pi.
0025 Several issues of Pi homeostasis need further ex-
planation because of their potential impact on health
and disease in populations of developed nations.
Aging and Renal Function
0026 Serum Pi concentration changes little in women with
a healthy renal function, but it does increase when the
renal function becomes compromised, though the
percent reduction in GFR needed for this increase in
serum Pi has not been established. Less is known of
changes of the serum Pi concentration in men with
increasing age, but the same relationship to renal
function probably holds in men as in women. Because
intestinal Pi absorption efficiency remains fairly con-
stant in aging women, whereas calcium absorption
efficiency declines within a decade or so following
the menopause, it is possible that PTH secretion is
increased in these women 10 or more years beyond
the menopause without any other perturbation.
Under these conditions, serum PTH concentrations
rise, but they typically remain within the upper limit
of normality. Severe reductions in renal function
clearly elevate serum Pi concentration. Refer to
Renal Secondary Hyperparathyroidism.
Nutritional Secondary Hyperparathyroidism
0027The major concern of nutritionally induced hyper-
parathyroidism, even if the PTH level remains within
the upper range of normal, is the reduction of both
bone mass and bone mineral density. A low Ca:P ratio
from a diet that provides too little calcium and plen-
tiful amounts of phosphorus may increase PTH in a
fairly persistent manner, which in turn increases bone
turnover. If bone formation cannot keep up with
PTH-governed bone resorption, bone loss will follow
(Figure 1). Although not fully established by research
evidence, this scenario of a persistently elevated PTH
in response to a low Ca:P ratio (0.5:1) is suspected
of contributing first to osteopenia and then to osteo-
porotic bone that is more fragile and at increased risk
of fracture (see Table 1 for examples of foods with
low Ca:P ratios). Recent reports, for example, have
shown that the adverse effects of a low Ca:P diet can
be improved and largely overcome by simple calcium
supplementation. Because the diets of so many ado-
lescents and adults in the USA and probably adults in
other technologically advanced nations with signifi-
cant use of phosphate additives and inadequate
calcium intakes have low Ca:P ratios, it is expected
that rates of osteoporotic fractures, especially of hip
fractures, will increase in the next several decades.
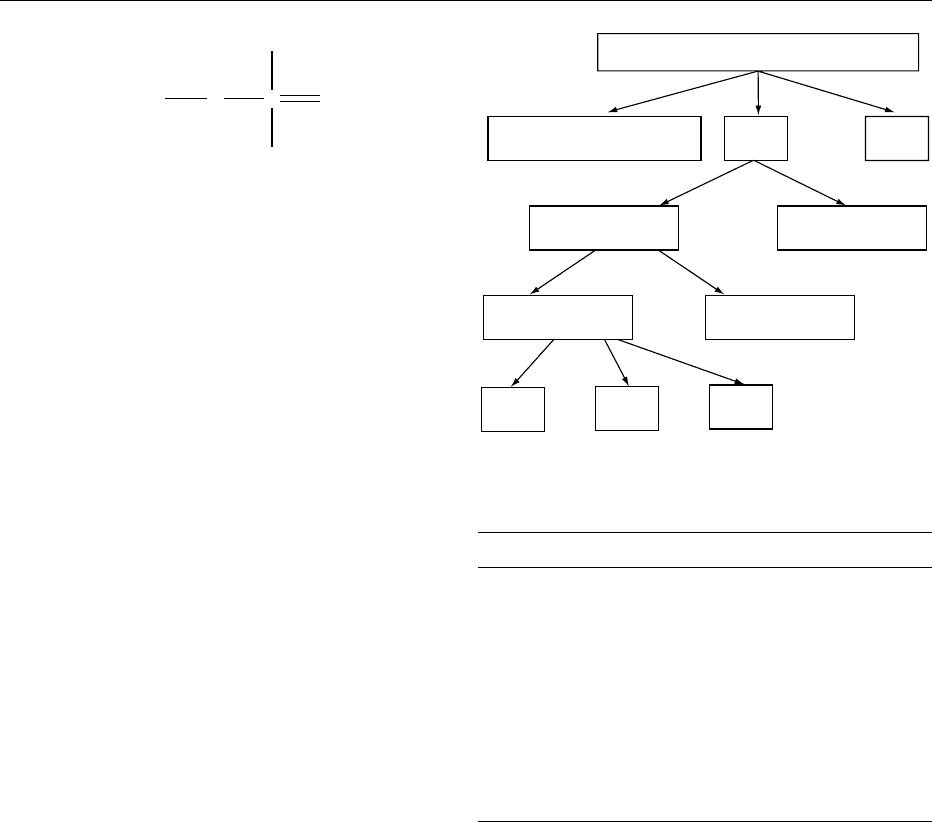
Chronic low-Ca, high-P diet
Serum Pi
Serum PTH
Bone
resorption
Serum Ca
2+
Serum Ca
2+
Serum Ca
2+
returns to low normal
until next meal
Renal excretion
of Pi
Serum Pi
Serum Pi
returns to high normal
until next meal
Renal
1,25-dihydroxyvitamin D
production
Serum Ca
2+
fig0001 Figure 1 Illustration of steps in development of long-term nutritional secondary hyperparathyroidism: chronic low-calcium, high-
phosphorus diet. Reproduced from Phosphorus, Encyclopaedia of Food Science, Food Technology and Nutrition, Macrae R, Robinson RK
and Sadler MJ (eds), 1993, Academic Press.
4544 PHOSPHORUS/Physiology
