Caballero B. (ed.) Encyclopaedia of Food Science, Food Technology and Nutrition. Ten-Volume Set
Подождите немного. Документ загружается.


Renal Secondary Hyperparathyroidism
0028 When renal function becomes compromised to such an
extent that creatinine, other nitrogenous metabolic
products, and Pi are retained abnormally and exces-
sively by the body, then several pathophysiological
adaptations occur that have serious effects on health.
One of the important adverse effects of the retention of
Pi is the rapid and progressive loss of mineral mass. The
chronic elevation of serum Pi causes a decline in serum
Ca
2þ
, which triggers PTH secretion. The net effect is a
constantly elevated PTH concentration that continues
to act on bone tissue, i.e., resorption, to try to raise
[Ca
2þ
] to its homeostatic set level. Since Pi is also
released from bone along with Ca
2þ
during resorption,
the serum Pi concentration also increases. Because the
kidneys cannot eliminate Pi adequately, [Ca
2þ
] can
never be raised to its set level, and bone tissue continues
to be degraded as part of an unending vicious cycle.
0029 Various dietary manipulations have been tried to
control the loss of bone mass, but no one regimen has
been very successful. Reductions of both dietary pro-
tein, especially animal protein, and phosphorus have
been moderately successful in slowing the progress of
chronic renal disease, but the diets are not very palat-
able or satisfying.
Conclusions
0030 Pi metabolism is much more complex than that of
calcium because of the many intracellular pathways
that utilize Pi ions at one stage or another. The cyto-
solic utilization of Pi is closely linked with that of
glucose for the formation of glucose 6-phosphate
and for triglyceride synthesis through glycerol
3-phosphate formation, as well as with other mol-
ecules, during the postprandial period. Pi is utilized
by cells for many diverse molecules, including regula-
tory peptides and phospholipids. Extracellular regu-
lation of Pi is closely associated with that of calcium
through PTH and other calcium-regulating hor-
mones. Under typical dietary conditions of excessive
phosphorus intake compared with calcium, i.e., low
Ca:P ratio, nutritional secondary hyperparathyroid-
ism and the long-term development of osteopenia are
likely to result. Food fortification with calcium and
calcium supplementation are common ways in which
the low Ca:P ratio can be minimized, but individual
behaviors aimed at selecting a diet higher in calcium
will be needed to overcome the adverse ratio (0.5),
despite calcium fortification and/or supplementation.
Renal secondary hyperparathyroidism, a serious con-
sequence of renal functional impairment, produces
severe bone loss because of altered homeostatic regu-
lation of Pi.
See also: Aging – Nutritional Aspects; Bone; Calcium:
Physiology; Carbohydrates: Requirements and Dietary
Importance; Cells; Cholecalciferol: Physiology; Dietary
Requirements of Adults; Energy: Measurement of Food
Energy; Hormones: Thyroid Hormones; Steroid
Hormones; Osteoporosis
Further Reading
Akesson K, Lau K-H, Johnston P, Iperio E and Baylink DJ
(1998) Effects of short-term calcium depletion and
repletion on biochemical markers of bone turnover in
young adult women. Journal of Clinical Endocrinology
and Metabolism 83: 1921–1927.
Anderson JJB and Garner SC (eds) (1996) Calcium and
Phosphorus in Health and Disease. Boca Raton, FL:
CRC Press.
Anderson JJB, Sell ML, Garner SC and Calvo MS (2000)
Phosphorus. In: Russell R et al. Present Knowledge in
Nutrition, 7th edn. Washington, DC: International Life
Sciences Institute.
Barger-Lux J and Heaney RP (1993) Effects of calcium
restriction on metabolic characteristics of premeno-
pausal women. Journal of Clinical Endocrinology and
Metabolism 76: 103–107.
Bringhurst FR (1989) Calcium and phosphate distribution,
turnover, and metabolic actions. In: DeGroot LJ (ed.)
Endocrinology, 2nd edn, vol. 2. Philadelphia, PA: WB
Saunders.
Brot C, Jorgensen N, Jensen LB and Sorensen OH (1999)
Relationships between bone mineral density, serum
vitamin D metabolites and calcium:phosphorus intake
in healthy perimenopausal women. Journal of Internal
Medicine 245: 509–516.
Calvo MS, Kumar R and Heath H III (1990) Persistently
elevated parathyroid hormone secretion and action in
young women after four weeks of ingesting high phos-
phorus, low calcium diets. Journal of Clinical Endocrin-
ology and Metabolism, 70: 1340–1344.
Calvo MS and Park YM (1996) Changing phosphorus
content of the U.S. diet: Potential for adverse effects on
bone. Journal of Nutrition 126: 1168S–1180S.
Harnack L, Stang J and Story M (1999) Soft drink
consumption among US children and adolescents: Nu-
tritional consequences. Journal of the American Dietetic
Association 99: 436–441.
Institute of Medicine, Food and Nutrition Board (1997)
Dietary Reference Intakes: Calcium, Phosphorus, Mag-
nesium, Vitamin D and Fluoride. Washington, DC:
National Academy Press.
Karkkainen M and Lamberg-Allardt C (1996) An acute
intake of phosphate increases parathyroid hormone se-
cretion and inhibits bone formation in young women.
Journal of Bone and Mineral Research 11: 1905–1912.
McKane WR, Khosla S, Egan KS et al. (1996) Role of
calcium intake in modulating age-related increases
in parathyroid function and bone resorption. Journal
of Clinical Endocrinology and Metabolism 81:
1699–1703.
PHOSPHORUS/Physiology 4545
National Research Council (1989) Recommended Dietary
Allowances, 10th edn, pp. 184–187. Washington, DC:
National Research Council, National Academy Press.
Slatopolsky E, Dusso A and Brown A (1999) The role of
phosphorus in the development of secondary hyperpar-
athyroidism and parathyroid cell proliferation in chronic
renal failure. American Journal of Medical Sciences 317:
370–376.
USDA (1978) Nutritive Value of Foods. Home and Garden
Bulletin No. 72. Washington, DC: US Department of
Agriculture.
Phylloquinone See Vitamin K: Properties and Determination; Physiology
Physical Properties of Food See Rheological Properties of Food Materials
PHYTIC ACID
Contents
Properties and Determination
Nutritional Impact
Properties and Determination
U Konietzny, Karlsruhe, Dettenheim-Liedolsheim,
Germany
R Greiner, Centre for Molecular Biology, Federal
Research Centre for Nutrition, Karlsruhe, Germany
Copyright 2003, Elsevier Science Ltd. All Rights Reserved.
Introduction
0001 The proper chemical designation for phytic acid
is myo-inositol(1,2,3,4,5,6)hexakisphosphoric acid.
Salts of this acid, designated as phytates, are found
in plants, animals and soil. Phytate has been con-
sidered as an antinutrient due to its inhibitory effect
on the bioavailability of essential dietary minerals.
During food processing and digestion, phytate can be
dephosphorylated to produce degradation products,
such as myo-inositol pentakis-, tetrakis-, tris-, bis-,
and monophosphates. Besides the adverse effects
of phytate and other highly phosphorylated myo-
inositol phosphates on mineral bioavailability, some
novel metabolic effects of phytate and some of its
degradation products have been recognized. Certain
myo-inositol phosphates have been suggested to
have positive effects on heart disease by controlling
hypercholesterolemia and atherosclerosis and to pre-
vent renal stone formation. The most extensively
studied positive aspect of myo-inositol phosphates is
their potential for reducing the risk of colon cancer.
Furthermore, much attention has been focused on
myo-inositol with fewer than six phosphate residues,
since some of these compounds have been shown to
play an important part as intracellular second mes-
sengers and some have shown important pharmaco-
logical effects, such as the prevention of diabetes
complications and antiinflammatory effects. The
position of the phosphate groups on the myo-
inositol ring is therefore of great significance for
their physiological function. Thus, it is important to
have reliable techniques available to determine quali-
tatively and quantitatively myo-inositol phosphates
not only by the number of phosphate groups, but
also by the position of the phosphate groups on the
myo-inositol ring. (See Plant Antinutritional Factors:
Characteristics.)
4546 PHYTIC ACID/Properties and Determination
Structure, Occurrence, and Biological
Significance
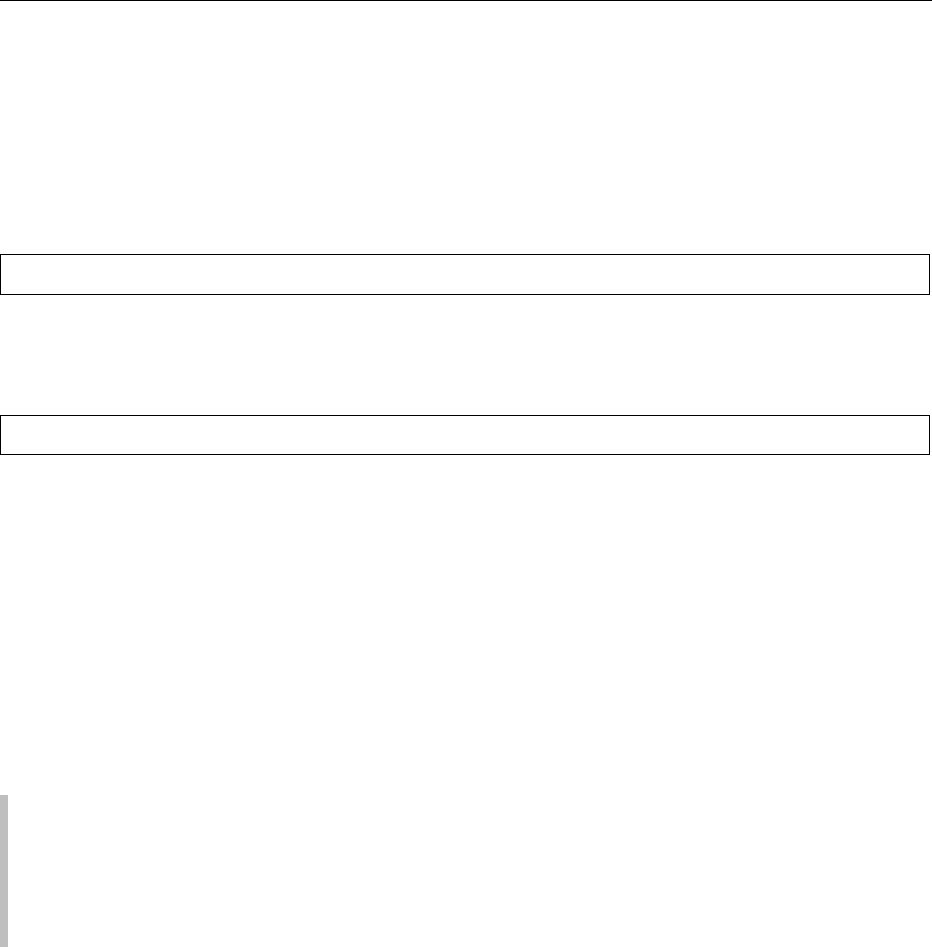
0002 Phytate is a meso compound and consequently pos-
sesses a plane of symmetry with either five equatorial
and one axial phosphate groups (5-eq/1-ax) or five
axial and one equatorial phosphate groups (5-ax/1-
eq: Figure 1). The carbon bearing the single axial or
equatorial phosphate group is numbered C2 and the
other ring carbons can be numbered C1–C6 from a
C1 atom either side of C2, proceeding around the ring
in a clockwise or counterclockwise fashion. Some less
phosphorylated myo-inositol derivatives are optically
active (Table 1). Their absolute configuration must be
clearly defined. According to convention, a counter-
clockwise numbering gives rise to myo-inositol phos-
phates with a d-prefix and a clockwise numbering to
myo-inositol phosphates with an l-prefix. The choice
of prefix is normally determined by giving preference
to that which results in the lowest numbering of
substituents (Figure 2). The predominant confor-
mation of the myo-inositol phosphates depends on
the specific myo-inositol phosphate, pH value, type
of cations present, and ionic strength. At pH values
above 9.5, phytate exists exclusively in the 5-ax/1-eq
conformation, whereas with myo-inositol pentakis-
phosphates a small amount of the 5-eq/1-ax con-
former is found in equilibrium with the predominant
5-ax/1-eq conformer. In myo-inositol phosphates
with fewer than five phosphate residues, the myo-
inositol ring appears to have a conformation in
which only the phosphate group at C2 is axially
oriented. Below pH 9.5 the 5-eq/1-ax conformer is
predominant with all myo-inositol phosphates. The
binding of some cations such as Cu
2þ
and Ca
2þ
is
proposed to occur mainly via phosphate groups at the
equatorial position of phytate; other cations such as
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
OPO
2−
3
OPO
2−
3
OPO
2−
3
OPO
2−
3
OPO
2−
3
OPO
2−
3
6(4)
5
1(3)
4(6)
3(1)
2
5-eq/1-ax
5-ax/1-eq
6(4)
5
1(3)
4(6)
2
3(1)
fig0001 Figure 1 Possible chair conformations of phytate.
tbl0001 Table 1 myo-inositol phosphate isomers
No. of
phosphate
residues
No. of
isomers
No. of
enantiomeric
pairs
myo-inositolphosphate isomers
610 I(1,2,3,4,5,6)P
6
562
D
-I(1,2,3,4,5)P
5
/
L
-I(1,2,3,4,5)P
5
D
-I(1,2,4,5,6)P
5
/
L
-I(1,2,4,5,6)P
5
, I(1,2,3,4,6)P
5
, I(1,3,4,5,6)P
5
4156 D-I(1,2,3,4)P
4
/L-I(1,2,3,4)P
4
, D-I(1,2,4,5)P
4
/L-I(1,2,4,5)P
4
,
D
-I(1,2,5,6)P
4
/L-I(1,2,5,6)P
4
,
D-I(1,2,4,6)P
4
/L-I(1,2,5,6)P
4
,
D
-I(1,3,4,5)P
4
/L-I(1,3,4,5)P
4
,
D
-I(1,4,5,6)P
4
/
L
-I(1,4,5,6)P
4
, I(1,2,3,5)P
4
,
I(1,3,4,6)P
4
, I(2,4,5,6)P
4
3208 D-I(1,2,4)P
3
/L-I(1,2,4)P
3
, D-I(1,2,5)P
3
/L-I(1,2,5)P
3
,
D
-I(1,2,6)P
3
/L-I(1,2,6)P
3
,
D
-I(1,3,4)P
3
/L-I(1,3,4)P
3
,
D-I(1,3,6)P
3
/L-I(1,3,6)P
3
,
D
-I(1,4,5)P
3
/L-I(1,4,5)P
3
, D-I(1,4,6)P
3
/
L
-I(1,4,6)P
3
,
D
-I(2,4,5)P
3
/L-I(2,4,5)P
3
,
I(1,2,3)P
3
, I(1,3,5)P
3
, I(2,4,6)P
3
, I(4,5,6)P
3
2156 D-I(1,2)P
2
/L-I(1,2)P
2
,
D
-I(1,4)P
2
/
L
-I(1,4)P
2
, D-I(1,5)P
2
/L-I(1,5)P
2
, D-I(1,6)P
2
/
L
-I(1,6)P
2
,
D
-I(2,4)P
2
/
L
-I(2,4)P
2
,
D
-I(4,5)P
2
/L-I(4,5)P
2
, I(1,3)P
2
,I(2,5)P
2
, I(4,6)P
2
162
D
-I(1)PI
L
-I(1)P,
D
-I(4)P/L-I(4)P, I(2)P, I(5)P
Myo-inositol phosphate isomers found in nature are indicated in italics.
PHYTIC ACID/Properties and Determination 4547
Zn
2þ
,Mn
2þ
, and the alkali metal ions seem to have
preference for phosphate groups at the axial position.
A single-crystal X-ray analysis of the sodium salt
showed the 5-ax/1-eq conformer. In solution, the ino-
sitol ring also occurs over a wide pH range in a chair
conformation in which five phosphate residues are
arranged axially and only the phosphate at C2 is
equatorially-oriented. Raman data indicate that
alkali metal ions preferentially bind to, and thus sta-
bilize, the 5-ax/1-eq phytate conformer in the order
Li
þ
* Na
þ
>Cs
þ
. In contrast, the Raman spectrum
of solid Ca
6
-phytate is characterized by the 1-ax/5-eq
conformer.
Plants
0003 Phytate is ubiquitous among plant seeds and/or
grains, comprising 0.5–5% (w/w). It is primarily pre-
sent as a salt of the mono- and divalent cations K
þ
,
Mg
2þ
, and Ca
2þ
and accumulates in the seeds during
the ripening period. In dormant seeds phytate repre-
sents 60–90% of the total phosphate. Only a very
small part of the myo-inositol phosphates exists
as myo-inositol penta- and tetrakisphosphate of
unknown isomeric state. The function of these high
phytate concentrations in plant seeds is unclear. It has
been suggested that phytate may serve as a store of
phosphate, of cations, of the cell wall glucuronate
precursor, of high-energy phosphoryl groups and, by
chelating free iron, as a potent natural antioxidant. In
amoeba, two diphospho-myo-inositol pentakisphos-
phate isomers and one bis(diphospho)-myo-inositol
tetrakisphosphate isomer are present in concentra-
tions exceeding that of phytate and thus may in fact
represent a compact store of high-energy phosphate.
0004 Until now only little is known of the pathway of
phytate synthesis in either the plant or animal king-
dom. A study in the slime mould Dictyostelium dis-
coideum established that phytate synthesis from myo-
inositol proceeds via Ins(3)P, Ins(3,6)P
2
, Ins(3,4,6)P
3
,
Ins(1,3,4,6)P
4
, and Ins(1,3,4,5,6)P
5
. Early studies
of phytate synthesis in plants led to the proposal
that phytate synthesis from Ins(3)P was mediated by
phosphoinositol kinase(s) via a series of undefined
myo-inositol phosphates. Recently the first descrip-
tion of the synthetic sequence to phytate in the plant
kingdom was given. From the identities of myo-ino-
sitol phosphates found in duckweed (Spirodela poly-
rhiza L.), at a development stage associated with
massive accumulation of phytate, it was concluded,
that synthesis of phytate from myo-inositol proceeds
according to the sequence d-I(3)P, d-I(3,4)P
2
, d-
I(3,4,6)P
3
, d-I(3,4,5,6)P
4
, I(1,3,4,5,6)P
5
. An un-
answered question that relates to the pathway of phy-
tate synthesis in plants concerns the source of d-I(3)P.
Two enzyme activities that are capable of synthesizing
d-I(3)P have been identified. These are myo-inositol
phosphate synthase (EC 5.5.1.4), which converts glu-
cose-6-phosphate, the ultimate source of myo-inositol
in plants, to d-I(3)P, and myo-inositol kinase (EC
2.7.1.64), which converts myo-inositol to d-I(3)P.
The spatial and temporal distribution and the relative
contribution of these two enzymes to phytate synthe-
sis via d-I(3)P are unclear.
0005During germination, phytate is rapidly hydrolyzed
in a stepwise manner by phytate-specific phospho-
hydrolases (phytases, EC 3.1.3.8, EC 3.1.3.26) or a
concerted action of phytases and other phosphatases
to supply the nutritional needs of the plant without an
accumulation of less phosphorylated myo-inositol
intermediates. Neither the isomer structure of these
intermediates nor the final product of phytate degra-
dation is known to date. From in vitro investigations
on the stereospecificity of phytate hydrolysis by puri-
fied phytases from cereals it was established that these
enzymes dephosphorylate phytate in a stereospecific
way by sequential removal of phosphate groups via
d-I(1,2,3,5,6)P
5
, d-I(1,2,5,6)P
4
, d-I(1,2,6)P
3
, and d-
I(2,6)P
2
to finally I(2)P. Moreover, the phytases from
bacteria and fungi investigated for phytate degra-
dation release five of the six phosphate groups, and
the end product was identified as I(2)P. Thus, the
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
OPO
2−
3
OPO
2−
3
HO
HO
OH
OH
OH
OH
6(4)
5
1(3)
4(6)
2
3(1)
D-I (1,4,5)P
3
(L-I (3,5,6)P
3
)
(
D-I (3,5,6)P
3
)
L-I (1,4,5)P
3
6(4)
5
1(3)
4(6)
2
3(1)
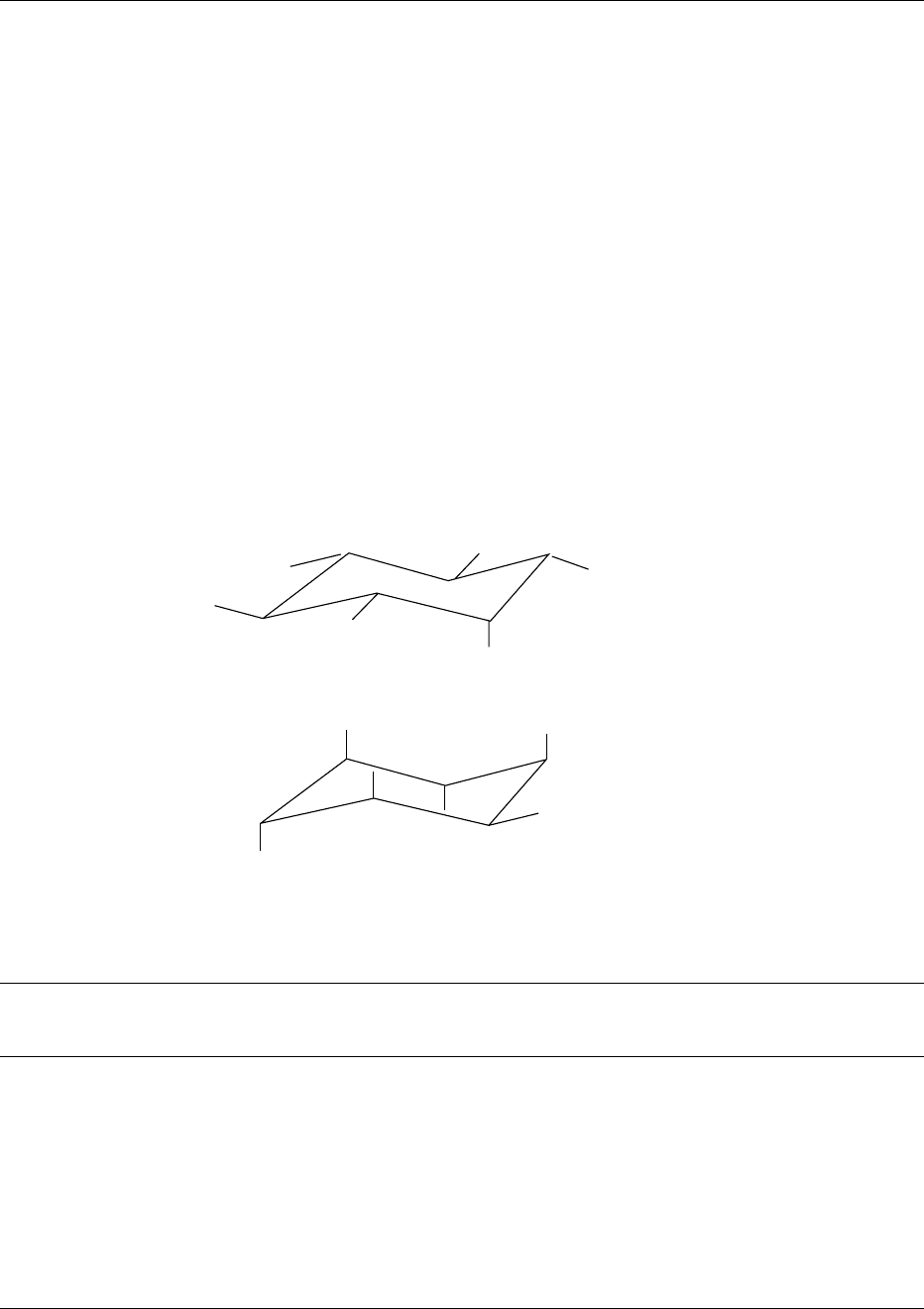
fig0002 Figure 2 Absolute configuration of D- and L-myo-inositol(1,4,5)trisphosphate.
4548 PHYTIC ACID/Properties and Determination
phosphate at C2 seems to be particularly resistant to
enzymatic cleavage. (See Enzymes: Functions and
Characteristics.)
Soil
0006 In the soil, phytate as well as less phosphorylated
myo-inositols are found. Their biological sources are
unknown.
Animals
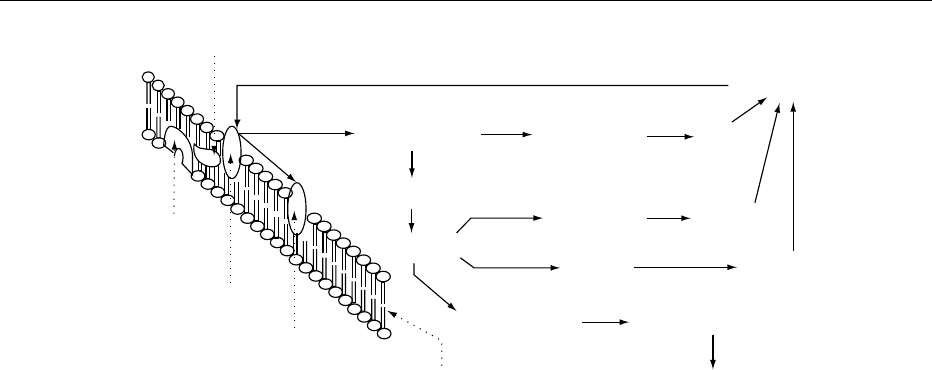
0007 In animal tissue, a considerable number of myo-inosi-
tol phosphates containing one to six phosphate resi-
dues have been found. In most tissues stimulation of
the phosphatidylinositol pathway (Figure 3) causes
release of d-myo-inositol(1,4,5)trisphosphate, which
is subsequently metabolized to a wide range of myo-
inositol phosphate isomers. For d-I(1,3,4)P
3
, meta-
bolism is complex, involving both dephosphorylation
via d-I(1,4)P
2
to d-I(4)P and phosphorylation to d-I
(1,3,4,5)P
4
, this latter compound being eventually de-
graded to d-I(1)P or d-I(3)P. The full significance of
this complex metabolism is not clear, but there is now
evidence that certain products of the phosphoinositide
metabolism play second messenger roles in most cells.
d-I(1,4,5)P
3
and d-I(1,3,4,5)P
4
bind to specific recep-
tors and regulate Ca
2þ
release from or movement
between intracellular Ca
2þ
stores. d-I(1,3,4,5)P
4
is
also the starting point for metabolic pathways gener-
ating other myo-inositol tetrakisphosphate isomers as
well as higher phosphorylated myo-inositols. There
are no known functions for these higher phosphoryl-
ated myo-inositols; these metabolites comprise the
bulk of myo-inositol phosphate content in mamma-
lian cells, but evidence for their association with cell
signaling was recently suggested.
0008d-myo-inositol(1,3,4,5,6)pentakisphosphate was
also found in the erythrocytes of birds, turtles, and
frogs. The functional importance of this compound as
a key regulator of oxygen affinity becomes evident
with the discovery that erythrocytes of adult birds
contain virtually no 2,3-bisphosphogylcerate, the
potent allosteric regulator of hemoglobin in mamma-
lian erythrocytes.
Chemical Properties
0009Numerous studies have been made on the protona-
tion constants of phytate, but the results are often
conflicting. This could be due to the fact that the
protonation constants of phytate to a large extent
are dependent on the ionic strength of the medium.
Phytic acid contains six strong acid groups which are
completely dissociated in solution (pK
a
1.1–3.2),
three weak acid protons (pK
a
5.2–8.0), and three
very weak acid protons (pK
a
9.2–12). There unusually
high pK
a
values for the second protonization step
seem to be due to intramolecular hydrogen bonding
between the syn-axial phosphate residues at C1 and
C3 as well as C4 and C6. These pK
a
values imply that
phytic acid will be strongly negatively charged over a
wide pH range and have immense potential for bind-
ing positively charged species, such as cations or pro-
teins. Free phytic acid is an unstable compound and
decomposes to yield lower myo-inositol phosphates
and orthophosphate. It is generally isolated as a
sodium or calcium salt. In its free form, phytic acid
is a light-yellow to light-brown syrupy liquid, soluble
in polar solvents (water, methanol, ethanol, 2-propa-
nol, acetone tetrahydrofuran, dimethyl sulfoxide,
dimethyl formamide), but insoluble in nonpolar
solvents (benzene, toluene, hexane, chloroform). In
G-protein
Intracellular
Inositol
Receptor
PIP
2
DG
membrane Extracellular
D-I(1,4,5)P
3
D
-I(1,3,4,5)P
4
D
-I(1,3,4)P
3
D
-I(1,3,4,6)P
4
D
-I(1,3,4,5,6)P
5
I(1,2,3,4,5,6)P
6
D
-I(1,4,)P
2
D
-I(3,4)P
2
I(1,3)P
2
D
-I(4)P
D-I(3)P
D-I(1)P
fig0003 Figure 3 The phosphatidylinositol pathway. PIP
2
, phosphatidylinositol 4,5-bisphosphate.
PHYTIC ACID/Properties and Determination 4549

contrast to free phytic acid, their salts are very stable
compounds. The phosphate groups can be removed
hydrolytically by enzymes or acid/heat to yield a large
number of homologs and positional isomers ranging
from myo-inositol mono- to pentakisphosphates
(Table 1). In spite of the considerable number of
isomers identified in vivo, they still represent only a
small percentage of the number possible in theory.
Above pH 5, there is almost no decomposition of
phytate at 100
C within 10 h. The rate of acid hy-
drolysis is low – as in other orthophosphoric esters it
reaches a maximum at pH 4, e.g., 27% of phytate is
cleaved after 6 h at 100
C, and even the use of strong
acids leads to an only moderate increase in the rate
of hydrolysis. In 5 mol l
1
HCl 47% of phytate is
cleaved after 6 h at 100
C. Final products of acid
hydrolysis are myo-inositol and myo-inositol(2)mo-
nophosphate. As with enzymatic cleavage, the axial
phosphate at C2 seems to be particularly resistant to
hydrolysis. Complete decomposition of phytate was
achieved with 3 mol l
1
H
2
SO
4
at 165
C for 4 h.
Under acidic conditions and higher temperatures in
particular, monophosphorylated cis-diol groups of
the inositols, in competition with hydrolysis, exhibit
the phenomenon of phosphate migration. Thus, for
example, d-myo-inositol(1)phosphate can yield a mix-
ture of d-myo-inositol(1)phosphate, myo-inositol(2)-
phosphate and l-myo-inositol(1)phosphate. For this
migration, an intermediate formation of cyclic phos-
phodiesters is essential which is only sterically favored
in cis-diol groups.
Phytate–Cation Interaction
0010 Phytate forms complexes with numerous divalent and
trivalent cations. The stability and solubility of the
cation–phytate complexes depend on the specific
cation, pH value, phytate-to-cation molar ratio, and
the presence of other compounds in the solution.
Phytate has six reactive phosphates and meets the
criterion of a chelating agent. In fact, a cation can
complex not only within one phosphate group or
between two or more phosphate groups of one phy-
tate, but also between two or more phytate molecules
(Figure 4).
0011Studying the solubility and relative stability of vari-
ous phytate–metal complexes by potentiometric titra-
tion, the following order of stability at pH 7.4 was
found: Cu
2þ
>Zn
2þ
>Ni
2þ
>Co
2þ
>Mn
2þ
>Fe
3þ
>
Ca
2þ
. Most phytates tend to be more soluble at lower
than at higher pH values. The pH value below which
the solubilities increase is about 5.5–6.0 for calcium,
7.2–8.0 for magnesium, and 4.3–4.5 for zinc phytate.
In contrast, ferric phytate is insoluble at pH values in
the 1–3.5 range at equimolar Fe
3þ
-to-phytate molar
ratios. Solubility increases above pH 4, reaching 50%
at pH 10. When Fe
3þ
-to-phytate molar ratio is in-
creased to 3.5:1, there is increased solubility below
pH 2, reaching a maximum of 90% at pH 1.5 and
lower solubility at pH values above pH 4. By forming
a complex with Fe
3þ
that lacks iron-coordinated
water and thus is unable to catalyze the formation
of hydroxyl radicals in the Fenton reaction, phytate is
a good antioxidant.
0012Another important fact is the synergistic effect of
secondary cations, among which Ca
2þ
has been most
prominently mentioned. Two cations may, when pre-
sent simultaneously, act together to increase the quan-
tity of phytate precipitation. For example, Ca
2þ
enhanced the incorporation or adsorption of Zn
2þ
into phytate by formation of a Ca-Zn phytate. The
effect of Ca
2þ
on the amount of Zn
2þ
coprecipitated
with phytate is dependent on Zn
2þ
-to-phytate molar
ratios. For high Zn
2þ
-to-phytate molar ratios, Ca
2þ
displaces Zn
2þ
from phytate-binding sites and in-
creases its solubility. The amount of free Zn
2þ
is
directly proportional to the Ca
2þ
concentration. For
low Zn
2þ
-to-phytate molar ratios, Ca
2þ
potentiates
the precipitation of Zn
2þ
as phytate. The higher the
Ca
2þ
level, the more extensive the precipitation of the
−2O
3
PO
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
P
OO
O
O
P
OO
O
O
POO
O
O
−
−
−
−
−
−
P
OO
O
O
−
P
OO
O
O
−
−
−
Zn
Fe
+
+
+
+
+
+
OPO
2−
3
OPO
2−
3
Ca
fig0004 Figure 4 Phytate–cation interaction. DG, diacylglycerol.
4550 PHYTIC ACID/Properties and Determination

ions. Mg
2þ
also has been shown in vitro to potentiate
the precipitation of Zn
2þ
in the presence of phytate;
however, Mg
2þ
has been found to exert a less pro-
nounced effect on Zn
2þ
solubility than Ca
2þ
.
0013 The knowledge about the interaction of the lower
myo-inositol phosphates with different cations is
limited. Recent studies have shown that myo-inositol
pentakis-, tetrakis-, and trisphosphates have a lower
capacity to bind cations (Ca
2þ
,Cu
2þ
,Zn
2þ
,Fe
2þ
,
Fe
3þ
) at pH values in the 5–7 range. The capacity to
bind cation was found to be a function of the number
of phosphate groups on the molecule. The cation-
myo-inositol phosphate complexes seem to become
more soluble as the number of phosphate groups
decreases. There is also some evidence for weaker
complexes when phosphate groups are removed from
phytate. Furthermore, the binding affinity of cations
to myo-inositol phosphates has been shown to be
affected by the orientation of the phosphate groups.
Phytate–Protein Interaction
0014 Phytate interactions with proteins are pH-dependent.
Phytate is known to form complexes with proteins at
both acidic and alkaline pH (Figure 5). At pH values
below the isoelectric point of the protein, the anionic
phosphate groups of phytate bind strongly to the
cationic groups of the protein to form insoluble com-
plexes that dissolve only below pH 3.5. The a-NH
2
terminal group, the e-NH
2
of lysine, the imidazole
group of histidine, and guanidyl group of arginine
have been implicated as protein-binding sites for phy-
tate at low pH values. These low-pH protein–phytate
complexes are disrupted by the competitive action of
multivalent cations.
0015 Above the isoelectric point of the protein, both
protein and phytate have a negative charge, but in
the presence of multivalent cations soluble protein–
cation–phytate complexes occur. The major protein-
binding site for the ternary complex appears to be the
unprotonated imidazole group of histidine. The ion-
ized carboxyl group of the protein are also suggested
sites. These complexes may be disrupted by high ionic
strength, high pH (> 10), and high concentrations of
the chelating agents.
0016Protein–phytate complexation may effect changes
in protein structure that can decrease enzymatic activ-
ity, solubility, and vulnerability to attack by proteo-
lytic enzymes. Phytate has been shown to reduce the
activity of lipase, a-amylase, pepsin, trypsin, and chy-
motrypsin in vitro. The inhibitory effect increases with
the number of phosphate groups per myo-inositol
molecule and the myo-inositol phosphate concentra-
tion. (See Protein: Interactions and Reactions Involved
in Food Processing.)
Application
0017Phytate has found industrial application, including
uses in the food industry (Table 2). The focus of
research on phytates includes occurrence and func-
tions in plant seeds, nutritional significance, preserva-
tive applications in food technology, and potential
medical and industrial uses. (See Preservatives: Food
Uses.)
Determination
0018The measurement of myo-inositol phosphates in any
material requires an initial extraction. The reagents
most commonly used to extract myo-inositol phos-
phates from foodstuff and biological samples include
3% trichloroacetic acid and 2.4% hydrochloric
acid. Since myo-inositol phosphates do not have a
POO
O
O
−
P
OO
−
O
O
−
P
OO
O
O
−
P
OO
−
O
O
−
−
−
OPO
2−
3
OPO
2−
3
OPO
2−
3
OPO
2−
3
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
−2
O
3
PO
+
NH
3
−CH
2
−protein
NH−protein
+
NH
2
C
H
2
N
pH<5
5<pH<10
O
C−CH
2
−protein
O
Ca
++
−
fig0005 Figure 5 Phytate–protein interaction.
PHYTIC ACID/Properties and Determination 4551
characteristic absorption spectrum, nor can they be
identified using specific colorimetric reagents, the
determination of these compounds has remained a
persistent problem.
Qualitative Separation Methods
0019 Qualitative separation and detection of myo-inositol
phosphates have been developed in the 1950s and
1960s. Paper chromatography has been shown to be
useful for separating myo-inositol phosphates by the
number of phosphate groups. Myo-inositol mono- to
hexakisphosphate could also be resolved relatively
rapidly by electrophoresis. Thin-layer chromato-
graphy, even if successfully applicable, has not been
widely adopted for the separation of myo-inositol
phosphates.
Quantitative Separation Methods
0020 Precipitative methods Quantitative methods for
determining phytate often employ the addition of a
controlled amount of Fe
3þ
to an acidic sample extract
to precipitate the phytate. Phytate is subsequently
estimated either by determining the phosphate, inosi-
tol, or iron content of the precipitate (direct method),
or by measuring the excess iron in the supernatant
(indirect method). The indirect methods are generally
more convenient and reproducible, because the stoi-
chiometric ratio of phosphate to iron in Fe
3þ
-myo-
inositol phosphate precipitates is affected by several
variables, including the way in which the precipitate
is washed. These methods are not specific for phytate
due to coprecipitation of less phosphorylated myo-
inositols and should therefore be limited to the analy-
sis of material which contains negligible amounts of
these myo-inositol phosphates.
0021 Nonprecipitative methods Nonprecipitative methods
for myo-inositol phosphate determination include
31
P-Fourier transform nuclear magnetic resonance
(
31
P-FT NMR) spectroscopy, near-infrared reflect-
ance spectroscopy, low-pressure anion-exchange
chromatography, several high-performance liquid
chromatographic (HPLC) separation systems, and
capillary electrophoresis. The main limitation of
31
P-FT NMR and near-infrared spectroscopy is that
these methods are specific for phytate only when
the sample contains negligible amounts of less phos-
phorylated myo-inositols. Additionally, sophisticated
instruments which are not available in most labora-
tories are required.
0022Low-pressure anion-exchange chromatography
Low-pressure anion-exchange chromatography is
widely used in the determination of myo-inositol
phosphates. The method currently accepted by the
Association of Official Analytical Chemists (AOAC)
for measuring phytate in foods and feeds is based
on a step gradient (0.7 mol l
1
NaCl) anion-exchange
method (Dowex AG1-X8). Unfortunately, the anion-
exchange resin also retains less phosphorylated myo-
inositols. The method should therefore be limited to
the analysis of material with negligible amounts of
these myo-inositol phosphates. Myo-inositol mono-to
hexakisphosphate and even some positional isomers
could be resolved using anion-exchange chromato-
graphy with a linear eluting gradient of hydrochloric
acid or a stepwise elution with either hydrochloric
acid or ammonium formate/formic acid solutions
of increasing concentrations. Unfortunately, these
methods require long elution times (up to 24 h) and
a large number of eluate fractions must be hydrolyzed
for quantitation as phosphate or inositol, since these
systems preclude the use of refractive index and con-
ductivity detection methods. Methods designed by
those studying calcium metabolism are dependent
on the use of radiolabeled myo-inositol phosphates
to facilitate detection and quantitation, but it is not
feasible to label existing myo-inositol phosphates in
dietary constituents.
tbl0002 Table 2 Application of phytate
Action Application
Metal chelation Prevention of color and quality changes in processed agricultural (chestnut, bean sprouts, pickles,
asparagus, etc.) and fishery (tuna, clams, shrimps, crabs, etc.) products
Removing metal ions from wine
Rust-proofing and dissolving-out prevention inside cans
Prevention of oxidation in oil/water emulsion-type food such as cream, dressings, butter, chesses, soups
Additive for etching solution for offset printing
Anticorrosion agent for paints, antifreezes, and metal surfaces (steel, tin, aluminum, iron)
Stabilizer for perfumes and cosmetics
Antioxidant for industrial oils and greases
pH control Prevention of quality changes by controlling pH value
Fermentation promoter Improvement of product yield and quality by promoting the growth of microorganisms such as lactic acid
bacteria and yeasts (fermented food, antibiotics, methanol, etc.)
4552 PHYTIC ACID/Properties and Determination
0023 High-performance liquid chromatoraphy More re-
cently, HPLC techniques have been introduced into
myo-inositol phosphate determination. Purification
of crude acid extracts of biological samples is usually
required prior to injection on to the analytical HPLC
system. The techniques used for detection and quan-
titation of the myo-inositol phosphates is heavily de-
pendent on the system employed for their separation.
The myo-inositol phosphates may be separated using
anion-exchange, reverse-phase, micellar and ion
chromatography, and detected/quantified by a variety
of techniques, including refractive index, conducti-
vity, indirect photometry, online postcolumn spectro-
photometric detection, and offline phosphate or
inositol assay. Among these, ion-pair reverse-phase
and anion-exchange chromatography are largely
used. (See Chromatography: High-performance
Liquid Chromatography.)
0024 Ion-pair reverse-phase chromatography Ion-pair re-
verse-phase chromatography with refractive index
detection has been successfully applied to analysis of
myo-inositol phosphates. The retention of myo-inosi-
tol phosphates on reverse-phase packings is markedly
increased through the use of ion-pair reagents, allo-
wing the simultaneous separation of myo-inositol
tris- to hexakisphosphates, but neither myo-inosi-
tol mono- or bisphosphates nor the individual pos-
itional isomers are resolved (Figure 6). However,
sample extracts must be passed through anion-ex-
change resin to remove orthophosphate and concen-
trate the myo-inositol phosphates. Acidic column
eluent is then evaporated to dryness to remove hydro-
chloric acid and reconstituted in water prior to injec-
tion on to a silica-based C18 reverse-phase HPLC
column. The mobile phase consisted of formic acid/
methanol and tetrabutylammonium hydroxide. The
affinity of myo-inositol phosphates for the stationary
phase increases with the increasing number of phos-
phate groups on the inositol ring and with increasing
pH. (See Chromatography: High-performance Liquid
Chromatography.)
0025 Anion-exchange chromatography To date, pub-
lished procedures involving anion-exchange HPLC
fall into two categories: isocratic and gradient ion-
chromatographic techniques. The capability of resol-
ving the different myo-inositol phosphates depends on
the stationary phase used and the chromatography
conditions. Myo-inositol mono- to hexakisphosphates
have been successfully resolved by isocratic elution
from low-capacity weak anion-exchange columns.
These single eluent systems are compatible with re-
fractive index, indirect photometric, thermospray
mass spectrometric, and conductivity detection.
However, in the case of conductivity detection, sensi-
tivity is low unless counterions in the eluent are
continously removed using a suppressor column or
membrane suppressor system.
0026In the last few years a number of isomer-specific
ion-exchange chromatography methods with gradi-
ent elution for separation and quantitation of myo-
inositol phosphates in the picomolar range have been
developed. Eluents with high ionic strength, such as
formate, acetate, citrate, phosphate, nitrate, sulfate,
sodium chloride, or hydrochloric acid, have been
used. The most commonly used detection method
with gradient elution is online postcolumn derivatiza-
tion or complexation reactions followed by spectro-
photometric detection. Three approaches have been
employed in the postcolumn detection and quantita-
tion of myo-inositol phosphates. The first is based on
the direct reaction of myo-inositol phosphates with a
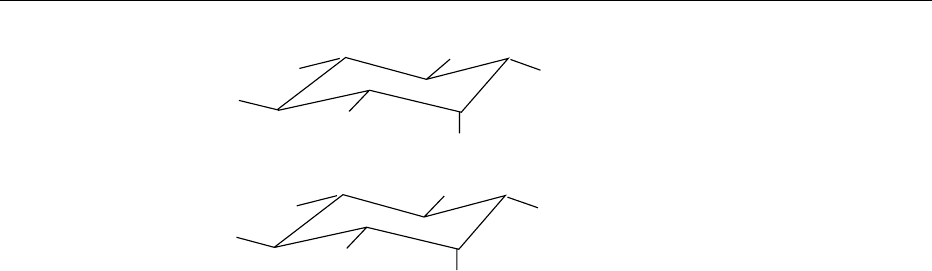
13.4−
26.7−
8.607
12
18.23
28.423
IP
3
IP
4
IP
5
IP
6
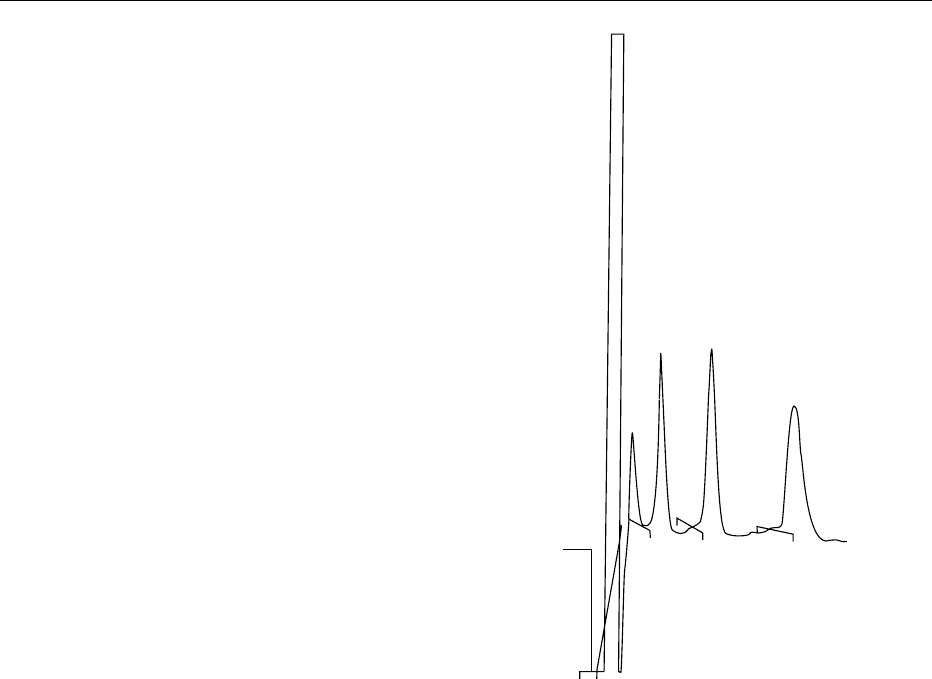
fig0006Figure 6 Chromatographic profile of a myo-inositol phosphate
standard by high-performance liquid chromatography (HPLC)
ion-pair chromatography on Ultrasep ES 100 RP18 (2
250 mm). The column was run at 45
C and 0.2 ml min
1
of an
eluent consisting of formic acid:methanol:water: tetrabutylammo-
nium hydroxide (TBAH: 44:56:5:1.5 v/v), pH 4.25. Myo-inositol
phosphates were detected by refractive index a standard.
Peaks (8.607) IP
3
; (12) IP
4
; (18.23) IP
5
; (28.423) IP
6
.
PHYTIC ACID/Properties and Determination 4553

reagent to form a fluorescent complex or one which
has an absorbance in the ultraviolet or visible part of
the spectrum. For example, the eluate from the
column was mixed, online, with 0.1% Fe(NO
3
)
3
in
2% HClO
4
to form ultraviolet-absorbing phytate–
Fe
3þ
–ClO
4
complexes. The use of postcolumn deri-
vatization through ligand-exchange reaction between
the iron(III)-sulfosalicylate complex and eluted myo-
inositol phosphates has been described as an alterna-
tive. Furthermore, a complexometric technique based
on competition between myo-inositol phosphates and
the cation-specific reporter dye 4-[2-pyridylazo]resor-
cinol for the transition metal yttrium has been de-
scribed. The second approach is based on the online
enzymatic hydrolysis of myo-inositol phosphates
which is then mixed with a molybdate solution in
the reaction coil. The colored phosphomolybdate
complex may be quantified spectrophotometrically.
Finally, myo-inositol phosphates may be quantified
by online thermospray mass spectrometric techniques.
0027 A remaining problem, however, is to separate
isomers from the whole spectrum of myo-inositol
phosphates in the same run. Separation is generally
performed on HPLC columns with gradient elution
in two combined systems. Myo-inositol mono- to
trisphosphates have been acidic gradient-eluted, post-
column-derivatized, and ultraviolet-detected and
myo-inositol bis- to hexakisphosphates have been
alkali gradient-eluted and detected using chemically
suppressed conductivity detection. The sensitivity of
the analysis of myo-inositol mono- and bisphosphates
was improved 10–100 times by using sodium acetate
gradient elution in a sodium hydroxide environment
and pulsed amperometric detection.
0028 Capillary electrophoresis Recently capillary electro-
phoresis has been applied to the determination of
myo-inositol phosphates. Capillary electrophoresis is
attractive, since only a few nanoliters of sample are
used in each analysis, there is the potential for con-
current separation of mono- to hexakisphosphate
species in the same analysis, and run times are usually
short due to the intrinsically high efficiency of the
technique. Indirect ultraviolet detection was used to
allow the detection of the nonchromophoric myo-
inositol phosphates. Thus, no derivatization of the
compounds is needed. Separations of all six myo-
inositol phosphate groups in deionized water has
been achieved in about 13 min. The position isomers
myo-inositol(1)phosphate and myo-inositol(2)pho-
sphate are easily separated in a phthalate electrolyte
system, demonstrating the potential for separating
myo-inositol phosphate isomers. However, further
work is required on the development of capillary
electrophoretic methods for the separation and
quantiation of the different myo-inositol phosphate
isomers.
0029All in all, efficient analytical systems for separation
and quantitation of myo-inositol phosphate isomers
are available. One problem in developing methods to
determine myo-inositol phosphate isomers is their
availability as reference compounds. They may be
produced by chemical or enzymatic hydrolysis of
phytate. Then identification of these isomers is
needed. This requires sophisticated methods. The
earliest developed technique is chemical analysis by
oxidation with periodate, reduction, dephosphoryla-
tion, and subsequent identification of the polyols
found. Further information can be obtained from
the above-mentioned cis phosphate migration. In
the past few years, high-resolution nuclear magnetic
resonance (NMR) spectroscopy has evolved as a
much simpler technique in the identification of
the isomeric nature of myo-inositol phosphates.
Thirty-nine of the 63 theoretically possible myo-ino-
sitol phosphate isomers can be identified by NMR.
Only for the 24 enantiomeric pairs among these
isomers (Table 1) absolute configurations are
indistinguishable from NMR, but these enantiomers
are also not separated on the achiral columns in
use. Separation techniques using chiral columns
have to be developed to resolve the enantiomers.
The absolute configuration of such enantiomers may
be determined using high-affinity binding proteins or
enzymatic assays.
See also: Chromatography: High-performance Liquid
Chromatography; Electrophoresis: General Principles;
Enzymes: Functions and Characteristics; Phosphorus:
Properties and Determination; Physiology; Plant
Antinutritional Factors: Characteristics; Preservatives:
Food Uses
Further Reading
Buscher BAP, Irth H, Anderson E, Tjaden UR and van der
Greef J (1994) Determination of inositol phosphates in
fermentation broth using capillary zone electrophoresis
with indirect UV detection. Journal of Chromatography
A678: 145–150.
Cheryan M (1980) Phytic interactions in food systems.
CRC Critical Reviews in Food Science and Nutrition
13: 297–335.
Cosgrove DJ (ed.) (1980) Inositol Phosphates: Their
Chemistry, Biochemistry and Physiology. Amsterdam:
Elsevier.
Dean NM and Beaven MA (1989) Methods for the analysis
of inositol phosphates. Analytical Biochemistry 183:
199–209.
Graf E (ed.) (1986) Phytic Acid: Chemistry and Applica-
tion. Minneapolis: Pilatus Press.
4554 PHYTIC ACID/Properties and Determination
