Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.


HEPATOBILIARY DISEASE
717
Treatment
Initial management is based on restoring blood volume
through intravenous hydration and transfusion. Coagulopathy
should be corrected as appropriate with fresh-frozen plasma.
In patients with significant bleeding or with altered senso-
rium, endotracheal intubation for protection of the airway
may be necessary.
A. Medical Treatment—Vasoactive drugs may be started as
soon as the diagnosis is suspected. These drugs have proven
value in nonesophageal sites of bleeding, such as portal
hypertensive gastropathy and gastric varices. Vasoconstrictors
decrease portal flow and pressure by decreasing splanchnic
arterial flow. Vasodilators decrease hepatic vascular resist-
ance and cause peripheral vasodilation, resulting in reflex
splanchnic vasoconstriction.
Vasopressin’s main role is as a temporizing measure. An
infusion of 0.2–0.4 units/min successfully controls acute
variceal bleeding in half of patients. It is often used for 48–72
hours. However, recurrent episodes of bleeding are common
(approximately 50%). By decreasing the rate of bleeding, it
facilitates initial resuscitation and the performance of
endoscopy for local definitive therapy of the bleeding varices.
Secondary effects are significant vasoconstriction with
hypertension, bradycardia, and risk of myocardial infarction.
Vasopressin generally is used in conjunction with nitroglyc-
erin. The combination, which reduces cardiac ischemia, is
superior to vasopressin alone in controlling acute variceal
bleeding. Nitroglycerin is also delivered via continuous infu-
sion at a dose of 0.2 μg/kg per minute.
Somatostatin is an effective hormone in the control of
acute variceal bleeding. An intravenous infusion of 25–50 μg/h
has been found to be as effective as vasopressin, balloon tam-
ponade, and sclerotherapy in prospective randomized trials.
Octreotide, the longer-acting form of somatostatin, is the
agent of choice in the initial management of acute variceal
bleeding because it is at least as effective as vasopressin but
has fewer side effects. Octreotide has been found to be as
effective as balloon tamponade of the esophagus in a clinical
trial.
B. Endoscopic Management—Sclerotherapy is the treat-
ment of choice in the management of acute variceal bleeding.
Sclerotherapy is successful in 60–90% of patients during ini-
tial management and is superior to vasopressin and balloon
tamponade. Complications of sclerotherapy are esophageal
ulceration, bleeding, perforation, bacteremia, and mediastini-
tis. Complications can occur in 10–30% of patients.
Variceal ligation is an alternative to sclerotherapy. The
efficacy is high and comparable with that of sclerotherapy.
There seems to be a trend toward fewer complications with
ligation.
Balloon tamponade is used less frequently as a temporiz-
ing measure, showing an efficacy of approximately 60–70%,
comparable with that of vasopressin and sclerotherapy but
associated with a high complication rate. In a Sengstaken-
Blakemore tube, the gastric balloon is passed into the stomach
and inflated; the esophageal balloon is inflated only if bleed-
ing is not controlled by the gastric balloon to 35–50 mm Hg
of pressure using manometer control. The lowest pressure
possible that will control hemorrhage should be used.
Serious complications, with a 5% mortality, include aspira-
tion pneumonia, esophageal rupture, and mucosal ulcera-
tion. The risk of complications increases with prolonged use.
However, use of a Blakemore-Sengstaken tube or a Linton
tube can be a lifesaving maneuver if medical and endoscopic
measures fail to stop bleeding.
C. Nonsurgical Shunts (Transjugular Portosystemic
Shunt [TIPS])—This technique is used widely in the setting
of acute variceal bleeding because it offers a rapid decom-
pressive shunt that does not require laparotomy. It is used as
primary treatment for patients with bleeding gastric varices
and for patients with hypertensive portal gastropathy—
mainly because of the difficulty and poor results with endo-
scopic management.
D. Surgical Treatment—Surgery plays a role in the manage-
ment of patients who fail medical, endoscopic, and TIPS
management of acute variceal bleeding. The surgical options
are either shunt or nonshunt operations.
Shunt procedures are either total (eg, portacaval shunt,
mesocaval shunt, and central splenorenal shunt) or selective
(eg, distal splenorenal shunt). These procedures are so
named because they theoretically divert all (total) or only
part (selective) of the portal blood flow. The most commonly
used operation in the emergency setting is a portacaval
shunt. It is very effective in controlling acute bleeding and
preventing rebleeding, but the mortality rate is as high as
50%. All surgical procedures have some undesirable side
effects, including encephalopathy and/or ascites. The selec-
tive procedures loose their selective nature over time and also
have undesirable side effects.
Gastroesophageal devascularization (Sugiura operation)
is a nonshunt operation. It has fallen into disfavor because
the recurrence rate of bleeding is high, but it is indicated in a
selected subset of patients with portal vein thrombosis or
segmental portal hypertension with an acute bleeding
episode.
Ascites
ESSENTIALS OF DIAGNOSIS
History: abdominal distention.
Physical examination: fluid wave, shifting dullness, and
dullness to percussion.
Abdominal ultrasound may detect up to 100 mL of
ascitic fluid; ultrasound is useful in the diagnosis of
patients with minimal ascites.
Paracentesis; serum ascites albumin gradient >1.1 g/dL.
CHAPTER 34
718
General Considerations
A typical circulatory dysfunction characterized by arterial
vasodilation and high cardiac output coupled with increased
sinusoidal pressure and hepatic insufficiency is the cause of
ascites in cirrhotic patients. In addition, there is renal sodium
and water retention caused by stimulation of the renin-
angiotensin-aldosterone axis and activation of antidiuretic
hormone (ADH) secretion by the relative underfilling of the
arterial vascular compartment.
Over 50% of patients with cirrhosis will develop ascites. It
is therefore one of the most common complications of cir-
rhosis. Once ascites develops, the median survival is approx-
imately 1 year.
Clinical Features
A. Symptoms and Signs—Patients with large-volume
ascites complain of increasing abdominal girth and abdomi-
nal pressure. Some patients complain of anorexia, early sati-
ety, and nausea or flank pain. Clinical findings of abdominal
distention and shifting dullness and demonstration of a fluid
wave support the diagnosis of ascites. Other stigmata of liver
disease that aid in diagnosis are jaundice, spider angiomas,
and large periumbilical collateral veins in the abdominal wall
(caput medusae).
B. Laboratory Findings—Diagnostic paracentesis is essen-
tial in the evaluation and management of patients with
ascites. Inspection of the fluid in patients with portal
hypertension reveals clear, straw-colored fluid. Laboratory
evaluation includes cell count, cytologic examination, albu-
min and protein concentrations, and bacteriologic analysis.
The serum-to-ascites albumin gradient, calculated by sub-
tracting the ascitic fluid albumin level from the serum albu-
min level, has been shown to be effective in differentiating
portal hypertensive from nonportal hypertensive ascites.
Patients with a gradient of more than 1.1 g/dL can be diag-
nosed as having portal hypertension with a reliability of
97%. A gradient of less than 1.1 g/dL suggests nonportal
hypertensive etiology. Further differentiation of the ascitic
fluid as a transudate or exudate provides insight into the
origin of ascites.
C. Imaging Studies—Ultrasonography may be helpful in
the detection of small volumes of ascitic fluid. Duplex
ultrasound of the portal and hepatic venous system is indi-
cated if portal vein thrombosis or hepatic vein thrombosis
is suspected. CT scanning is also helpful in the detection of
small volumes of ascites, usually as an incidental finding
when x-rays are requested for evaluation of intraabdominal
pathology.
Differential Diagnosis
Cirrhosis and chronic liver disease are the most common
causes of ascites (approximately 70–80% of patients). The
differential diagnosis of ascites is set forth in Table 34–4.
Spontaneous bacterial peritonitis is a frequent complica-
tion of cirrhotic patients with ascites. It has a 1-year mortal-
ity of 40% despite treatment with antibiotics. Because its
inception can be subclinical, bacteriologic analysis should be
done on every patient with new-onset ascites. Culture of
ascitic fluid in blood culture bottles is more reliable and is
successful in approximately 80% of patients.
Treatment
The treatment of ascites is directed at the underlying
pathogenesis.
A. Medical Treatment—Patients with mild ascites may be
managed with fluid (1.5 L/day) and sodium restriction (88
meq/day). Addition of an inhibitor of aldosterone (eg,
spironolactone) provides a slow sodium loss with preserva-
tion of potassium. Initial doses are 100 mg/day but may be
elevated progressively up to 400 mg/day. Monitoring of
weight and electrolytes is important so that adjustments can
be made to the initial therapy.
Patients with moderate ascites should, in addition, receive
loop diuretics (eg, furosemide). An initial dose of 40 mg/day
generally is well tolerated and may be increased up to
160 mg/day in adults. Careful monitoring of weight, elec-
trolytes, and serum creatinine may prevent complications
from diuretic therapy. Any rise in serum creatinine or urea
nitrogen warrants reduction of the diuretic dosage. An initial
daily weight loss of 500 g/day is acceptable in patients with
moderate ascites. If patients have peripheral edema, a weight
loss of approximately 1 kg/day is acceptable.
Patients with tense ascites in addition should be treated
with paracentesis. Up to 4–5 L may be removed safely.
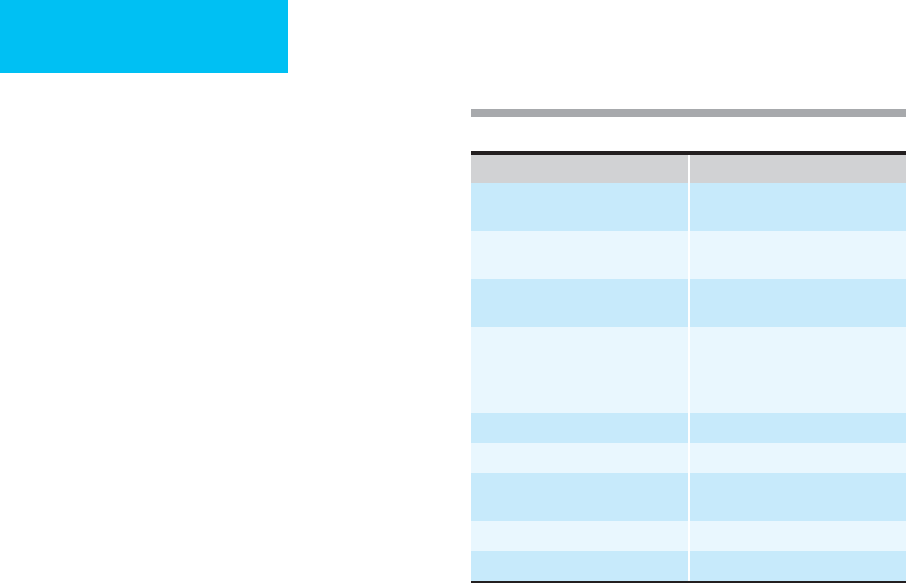
Organ System Cause
Hepatic Cirrhosis
Veno-occlusive disease
Cardiac Right ventricular failure
Constrictive pericarditis
Renal Nephrotic syndrome
Renal failure
Malignancy Ovarian
Gastric
Colorectal
Pancreatic
Immunologic Tuberculosis
Pancreas Pancreatitis
Lymphatic Congenital anomaly
Trauma
Digestive Malnutrition
Endocrine Myxedema
Table 34–4. Differential diagnosis of ascites.

HEPATOBILIARY DISEASE
719
Attention to salt and water restriction is also a critical com-
ponent of management. Patients who require large-volume
paracentesis may develop rapid contraction of the intravascu-
lar space after fluid shifting. Clinical trials have documented a
better outcome after large-volume paracentesis with the
simultaneous infusion of intravenous albumin. Intravenous
salt-poor albumin should be used routinely in patients under-
going large-volume paracentesis. As a rule of thumb, salt-poor
albumin (~10 g/L of ascitic fluid) is infused concomitantly
intravenously. Spontaneous bacterial peritonitis must be sus-
pected in patients with known liver disease who present with
fever, leukocytosis, and abdominal pain. Cell count of the
ascitic fluid is diagnostic if the polymorphonuclear neutrophil
count is 250 in the absence of a visceral source of infection.
Treatment should be initiated empirically on diagnosis. A
third-generation cephalosporin such as ceftriaxone or cefo-
taxime generally is the first-line therapy until a specific organ-
ism has been selected on the basis of ascitic fluid culture. A
5-day course generally is therapeutic. Most common organ-
isms are Escherichia coli, Klebsiella, and Streptococcus pneumo-
niae. Other organisms are Enterococcus, Bacteroides, and
Enterobacter. Antibiotics with a nephrotoxic profile, such as
aminoglycosides, should be avoided if at all possible.
B. Surgical Treatment—Ten percent of patients develop
intractable ascites. Refractory ascites may be treated by the
placement of a transjugular intrahepatic portosystemic
shunt. Transjugular intrahepatic portosystemic shunt inser-
tion lowers the rate of ascites recurrence and the risk of
developing hepatorenal syndrome compared with paracente-
sis plus albumin administration in patients with refractory
ascites. However, a recent meta-analysis documented
increased encephalopathy and absence of improvement in
survival. Transjugular intrahepatic portosystemic shunt
insertion is also recommended in the treatment of patients
with severe ascites and imminent rupture of umbilical/ven-
tral hernia or hepatic hydrothorax.
Operative therapy with placement of a peritoneovenous
shunt may be considered in patients with mild, stable liver
dysfunction who are otherwise refractory to less invasive
therapies. Although shunting is a simple surgical procedure,
the incidence of postoperative complications is high, related
to infection, coagulopathy, congestive heart failure, and early
shunt occlusion.
Patients with severe or rapidly deteriorating liver dys-
function should be considered for liver transplantation.
Hepatorenal Syndrome
ESSENTIALS OF DIAGNOSIS
Chronic liver disease.
Renal failure.
Circulatory abnormalities (low systemic vascular resist-
ance and blood pressure).
General Considerations
Hepatorenal syndrome is a clinical condition that occurs in
patients with chronic liver disease, advanced hepatic failure,
and portal hypertension characterized by impaired renal
function. The prevalence of hepatorenal syndrome in patients
with end-stage cirrhosis ranges between 7% and 15%. It is
associated with marked abnormalities in the arterial circula-
tion and activity of the endogenous vasoactive system. Severe
renal vasoconstriction causes a low glomerular filtration rate
(GFR), whereas in the extrarenal circulation there is predom-
inance of arterial vasodilation, which results in reduction of
total systemic vascular resistance and arterial hypotension.
Hepatorenal syndrome is caused by severe vasoconstric-
tion of the renal circulation. A number of vasoactive media-
tors are implicated in the development of vasoconstriction,
such as angiotensin II, norepinephrine, neuropeptide Y,
endothelin, adenosine, and cysteine leukotrienes. The most
commonly accepted explanation is the arterial vasodilation
theory, which proposes that renal hypoperfusion represents
the extreme manifestation of underfilling of the arterial cir-
culation secondary to massive vasodilation of the splanchnic
circulation. Splanchnic vasodilation is caused by nitric oxide,
prostaglandins, and vasodilator peptides. In the early phase,
urine output and renal function are maintained by renal
vasodilator factors. Hepatorenal syndrome develops later,
when vasoconstriction ensues from relative hypovolemia.
Hepatorenal syndrome may be precipitated by concomi-
tant illnesses or may occur spontaneously. Spontaneous bac-
terial peritonitis is the most common precipitating factor of
hepatorenal syndrome in patients with cirrhosis.
Clinical Features
A. Symptoms and Signs—Hepatorenal syndrome occurs as
a complication of cirrhosis, more commonly in patients with
ascites. Two types are recognized. Type I is characterized by
rapid and progressive impairment of renal function.
Dominant features are marked renal failure, oliguria or
anuria, and high levels of urea and creatinine. Most patients
have hyperbilirubinemia, coagulopathy, and encephalopathy.
The median survival is only 2 weeks. Type II consists of mild
and stable reduction in renal function. These patients typi-
cally present with diuretic-resistant ascites.
Four major criteria must be present to establish the diag-
nosis of hepatorenal syndrome: (1) low GFR (creatinine
clearance <40 mL/min or serum creatinine >1.5 mg/dL),
(2) absence of shock with ongoing bacterial infection, fluid
loss, and current treatment with nephrotoxic drugs, (3) no
improvement in renal function after withdrawal of diuretics,
and (4) no evidence of obstructive uropathy and absence of
proteinuria (<500 mg/day).
B. Laboratory Findings—Laboratory findings in hepatorenal
syndrome include a creatinine clearance of less than 40 mL/min
or a serum creatinine concentration of more than 1.5 mg/dL,
a urine sodium concentration of less than 10 meq/L, a urine
CHAPTER 34
720
osmolality that is greater than plasma osmolarity, urine red
blood cells fewer than 50/hpf, and a serum sodium concen-
tration of less than 130 meq/L.
Differential Diagnosis
Other causes of renal failure that must be excluded prior to
making a diagnosis of hepatorenal syndrome include hypov-
olemia causing prerenal failure (eg, use of diuretics or bleed-
ing), acute tubular necrosis (eg, following hypotension or
sepsis), drug-induced nephrotoxicity (eg, NSAIDs or amino-
glycosides), and glomerulonephritis (commonly associated
with proteinuria) secondary to autoimmune disease.
Treatment
Initial management requires volume replacement and reach-
ing a euvolemic state. Once hepatorenal syndrome is sus-
pected, medical management is difficult and more often than
not unsuccessful. Basic management requires monitoring of
urine output, patient weight, blood pressure, evaluation and
replacement of electrolytes, and possibly dialysis.
Intravenous clonidine has been shown to lower renal vas-
cular resistance and increase the GFR by as much as 25%.
Dopamine has been proposed as a selective vasodilator of the
renal circulation in selected reports, but the literature sug-
gests that there is only a very mild effect in increasing GFR.
Liver transplantation causes a dramatic improvement in
renal function. Recovery of renal function typically is seen
within 48–72 hours of transplantation. Long-term survival
after transplantation is very good—approximately 60% after
3 years. The perioperative phase may be more complicated
owing to a 30% requirement of temporary hemodialysis and
the higher incidence of morbidity and mortality than in
patients transplanted without hepatorenal syndrome.
Preoperative Assessment & Perioperative
Management of Patients With Cirrhosis
There is high perioperative morbidity and a high mortality
risk in patients with cirrhosis undergoing abdominal surgery
for any indication. Liver function may deteriorate from general
anesthesia. Anesthesia reduces cardiac output, induces
splanchnic vasodilation, and causes a 30–50% reduction in
hepatic blood flow.
The 30-day mortality for patients with cirrhosis undergo-
ing celiotomy is 30%. A 60% major complication rate also
was reported. The risk depends on a number of factors.
A. Physiologic Status—The Child-Turcotte-Pugh classifi-
cation of surgical risk is summarized in Table 34–5. This clas-
sification was first proposed (by Child) as a means of
predicting the operative mortality associated with portacaval
shunt surgery. The presence of ascites, encephalopathy, and
coagulopathy predicts mortality. There is a 10% mortality
rate for patients with Child class A cirrhosis, 30% for Child
class B, and 75% for Child class C.
Another model predictive of outcomes in patients with cir-
rhosis is the Model for End-Stage Liver Disease (MELD),
which is a numerical scale based on the etiology of cirrhosis,
bilirubin, creatinine, and international normalization ratio
(INR). Scores range from 6 (less ill) to 40 (gravely ill), and the
model is used for liver transplant candidates aged 12 and older.
B. Type of Surgery—The overall hospital mortality rate was
estimated at 21% for biliary surgery, 35% for peptic ulcer
disease, and 55% for colectomy. Newer techniques such as
laparoscopy and better patient selection have contributed to
a reduced mortality rate in recent reports of laparoscopic
cholecystectomy and appendectomy.
C. Other Factors—Active infection, a higher number of
blood transfusions, pulmonary complications, GI bleeding,
and the need for emergency surgery have a negative impact
on the outcome of surgery in patients with cirrhosis.
Liver Resection in Patients
with Cirrhosis
Hepatectomy is a major operation that induces a severe cata-
bolic response and immunosuppression. Cirrhotic patients
already suffer from underlying catabolism and immunosup-
pression. Liver resection in patients with underlying liver
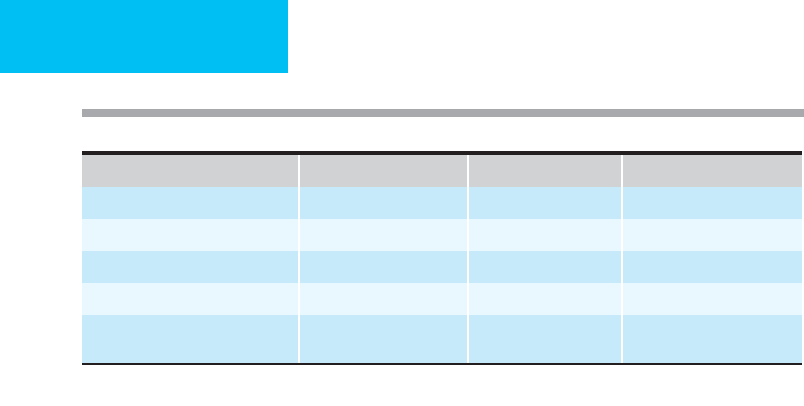
Clinical Variable 1 Point 2 Points 3 Points
Encephalopathy None Stage 1–2 Stage 3–4
Ascites Absent Slight Moderate
Bilirubin (mg/dL) <2 2–3 >3
Albumin (g/dL) 3.5 2.8–3.5 <2.8
Prothrombin time
(seconds prolonged or INR)
<4 s or
INR <1.7
4–6 s or
INR 1.7–2.3
>6 s or
INR >2.3
Interpretation: Child class A = 5–6 points, Child class B = 7–9 points, Child class C = 10–15 points.
Table 34–5. Child-Turcotte-Pugh estimate of surgical risk.

HEPATOBILIARY DISEASE
721
insufficiency carries the risk of postoperative liver failure. In
experienced hands, the mortality rate after hepatectomy
ranges from 5–50%.
Other postoperative complications are common, such as
the development of ascites (5%), encephalopathy (20%),
renal failure (15%), and upper GI bleeding (5%). Factors
that contribute to this great variability are the extent of resec-
tion and the patient’s underlying physiologic status.
Preoperative patient selection and perioperative manage-
ment are critical to successful outcome after liver resection in
patients with cirrhosis.
Preoperative Evaluation
Prior to operation, attention is directed to identification of
patients in whom the complication rate or mortality risk
is prohibitive. Numerous methods of identification have been
proposed. The clinical classification of patients according to
their Child score is a time-tested approach and is used most
commonly. Patients with Child A liver function have a hospi-
tal mortality risk for major hepatectomy of 3–15%. Patients
with Child B or C liver function tolerate liver resection poorly,
and that procedure should be withheld except in highly
selected individuals who require minimal surgery. Others have
proposed the use of the indocyanine green clearance test, the
lidocaine clearance test, and measuring the degree of fibrosis
in the unaffected liver as predictors of morbidity and mortal-
ity. Another factor that must be considered in selection of
patients is the volume of remaining liver estimated by CT scan.
As before any other major surgery, a good clinical history
and examination should alert the clinician to the presence of
any significant pulmonary, cardiac, hematologic, or renal
disorders. Screening of hematologic, biochemical, renal, pul-
monary, and cardiac function is essential.
Operative Management
Metabolic and hematologic derangements must be corrected
prior to surgery. Intraoperative monitoring of blood loss,
hemodynamics, and urine output is crucial. Patients with
hepatic dysfunction suffer from peripheral vasodilation and
are less responsive to catecholamines. It is therefore impor-
tant to maintain the circulatory volume. Maintaining liver
perfusion during surgery is critical to the prevention of any
further impairment of hepatic function. Therefore, anes-
thetic agents that do not impair hepatic perfusion and oxy-
genation should be selected.
Liver transection must be performed with two issues in
mind, minimizing blood loss and having adequate hemostasis
and securing biliary radicles to avoid postoperative biliary leak-
age, which is a cause of postoperative morbidity and sepsis.
Postoperative Care
Postoperative monitoring should include hemodynamics,
oxygen saturation, vital signs, fluid balance, electrolytes, and
blood glucose. Postoperative pain must be controlled to
avoid cardiopulmonary complications. Careful fluid man-
agement is a critical component of postoperative care.
Maintaining euvolemia is a priority. Owing to alterations in
the renin-angiotensin-aldosterone axis, cirrhotic patients
have a propensity for salt retention and third spacing of
extracellular water. This, in turn, manifests as ascites. When
salt restriction is required, 0.25% saline solution should be
used instead of 0.5%.
Parenteral nutritional support is provided in the form of
branched-chain amino acids because they reduce the cata-
bolic response and promote hepatic protein synthesis and
liver regeneration in cirrhosis. Adequate calories and fatty
acids are also provided. Perioperative nutritional support
reduces overall postoperative morbidity by decreasing septic
complications, the incidence of ascites, and deterioration of
liver function.
Potassium phosphate is used in the parenteral solution to
avoid hypophosphatemia, which occurs commonly after
hepatectomy. Phosphate is necessary for production of ATP
in the liver.
At least one study supports the use of salt-poor albumin
in septic cirrhotic patients as a means of reducing renal com-
plications and mortality.
Hyperbilirubinemia occurs commonly and generally is
transient, but progressive hyperbilirubinemia is an ominous
sign. Decreased sensorium, hypoglycemia, and acidosis are
present in severe hepatic failure and portend a poor prognosis.
Hepatic failure is complicated by a noncorrectable coagulopa-
thy and sepsis. Use of the bioartificial liver is potentially a life-
saving measure, but this resource is not yet widely available.
Liver transplantation is the only option (see Chapter 35).
REFERENCES
Azoulay D et al: Neoadjuvant transjugular intrahepatic portosys-
temic shunt: A solution for extrahepatic operation in cirrhotic
patients with severe portal hypertension. J Am Coll Surg
2001;193:46–51.
Belghiti J et al: Herniorrhaphy and concomitant peritoneovenous
shunting in cirrhotic patients with umbilical hernia. World J Surg
1990;14:242–6.
Block RS, Allaben RD, Walt AJ: Cholecystectomy in patients with cir-
rhosis: A surgical challenge. Arch Surg 1985;120:669–72.
Bolder U et al: Preoperative assessment of mortality risk in hepatic
resection by clinical variables: A multivariate analysis. Liver
Transpl Surg 1999;5:227–37.
Carbo J et al: Liver cirrhosis and mortality by abdominal surgery: A
study of risk factors. Rev Esp Enferm Dig 1998;90:105–12.
Conte D et al: Cholelithiasis in cirrhosis: Analysis of 500 cases. Am J
Gastroenterol 1991;86:1629–32.
Elcheroth J, Vons C, Franco D: Role of surgical therapy in manage-
ment of intractable ascites. World J Surg 1994;28:240–5.
Ercolani G et al: The lidocaine (MEGX) test as an index of hepatic
function: Its clinical usefulness in liver surgery. Surgery
2000;127:464–71.
Fan ST et al: Perioperative nutritional support in patients undergo-
ing hepatectomy for hepatocellular carcinoma. N Engl J Med
1994:331:1547–52.

CHAPTER 34
722
Farges O et al: Risk of major liver resection in patients with underly-
ing chronic liver disease: A reappraisal. Ann Surg 1999;229:210–5.
Garrison RN et al: Clarification of risk factors for abdominal opera-
tions in patients with hepatic cirrhosis. Ann Surg 1984; 199:
648–55.
Gopalswamy N, Mehta V, Barde CJ: Risks of intra-abdominal non-
shunt surgery in cirrhosis. Dig Dis 1998;16:225–31.
Grace ND: Diagnosis and treatment of gastrointestinal bleeding
secondary to portal hypertension. American College of
Gastroenterology Practice Parameters Committee. Am J
Gastroenterol 1997;92:1081–91.
Han MK, Hyzy R: Advances in critical care management of hepatic
failure and insufficiency. Crit Care Med 2006;34:S225–31.
Jakab F et al: Complications following major abdominal surgery in
cirrhotic patients. Hepatogastroenterology 1993;40:176–9.
Mansour A et al: Abdominal operations in patients with cirrhosis:
Still a major surgical challenge. Surgery 1997;122:730–5.
Metcalf AM et al: The surgical risk of colectomy in patients with
cirrhosis. Dis Colon Rectum 1987;30:529–31.
Sort P et al: Effect of intravenous albumin on renal impairment and
mortality in patients with cirrhosis and spontaneous bacterial
peritonitis. N Engl J Med 1999;341:403–9.
Sugiyama M et al: Treatment of choledocholithiasis in patients with
liver cirrhosis: Surgical treatment or endoscopic sphincterotomy?
Ann Surg 1993;218:68–73.
Wong R et al: Risk of nonshunt abdominal operation in the patient
with cirrhosis. J Am Coll Surg 1994;179:412–6.
Wu CC, Huwang CJ, Lui TJ: Definitive surgical treatment of
cholelithiasis in selective patients with liver cirrhosis. Int Surg
1993;78:127–30.
Ziser A et al: Morbidity and mortality in cirrhotic patients undergo-
ing anesthesia and surgery. Anesthesiology 1999;90:42–53.

723
0035
Burns
David W. Mozingo, MD
William G. Cioffi, Jr., MD
Basil A. Pruitt, Jr., MD
∗
Burns represent particularly challenging and difficult man-
agement problems. This chapter addresses the most common
causes, including thermal, electrical, and chemical burns.
Because of the far-reaching implications for care, additional
aspects of electrical injuries such as lightning strikes and
electrocution are discussed further with environmental
injuries in Chapter 37.
I. THERMAL BURN INJURY
Approximately 1.25 million burn injuries occur each year
in the United States. House and structure fires account for
81% of the 3600 burn- and fire-related deaths that occur
each year. Flame injury following a house fire or ignition of
clothing is the most common burn injury in patients
admitted to burn centers. Scald burns, the most common
burn injury in children, are responsible for about 30% of
patients requiring hospitalization for burns. Most burn
injuries are treated adequately on an outpatient basis; how-
ever, approximately 60,000 patients per year require hospi-
tal care because of the extent of their injury, the presence of
comorbid factors, or extremes of age. Approximately 20,000
patients have injuries of such significance that care is best
undertaken in a designated burn care facility. The American
Burn Association has developed criteria to identify patients
who require treatment in a burn center. These criteria assess
the severity of injury and the need for specialized burn cen-
ter treatment based on the age of the patient; the extent,
depth, and location of the burn; the type of injury; and the
presence of preexisting comorbid factors or associated
injuries (Table 35–1).
Thermal burn injury initiates a deleterious pathophys-
iologic response in every organ system, with the extent
and duration of organ dysfunction proportionate to the
size of the burn. Direct cellular damage is manifested by
coagulation necrosis, with the magnitude of tissue destruc-
tion determined by the temperature to which the tissue is
exposed and the duration of contact.
Histopathologic Characteristics of Burned
Tissue
Following thermal injury, the region of the burn in which
protein coagulation and cell death has occurred has been
referred to as the zone of necrosis. In full-thickness injury, all
dermal elements are destroyed, whereas partial-thickness
burns are characterized by a variable and incomplete dermal
necrosis. Extending radially from the zone of necrosis are
areas of cellular damage referred to as the zones of stasis and
hyperemia. The zone of stasis is characterized by a decreased
microvascular blood flow, which may be restored to normal
with successful resuscitation or converted to necrosis follow-
ing inadequate perfusion, desiccation, or infection. Minimal
thermal injury induces a zone of hyperemia characterized by
an immediate inflammatory response and increased
microvascular blood flow. These early histopathologic
changes are depicted as concentric tissue zones about the
point of thermal contact. Coagulation necrosis of the skin
and skin appendages results in loss of normal skin functions;
the antimicrobial barrier is destroyed, control of water evap-
oration is lost, and regulation of body temperature is
impaired.
Mechanisms of Edema Formation
Following thermal injury, edema formation in the burn
wound and in unburned tissues is greatest in the first 6 hours
following injury and continues to a lesser extent for the first
24 hours postburn. Postcapillary venular constriction results
in a marked increase in capillary hydrostatic pressure and
the production of interstitial edema in the early postinjury
phase. In an animal model of burn injury, a strongly nega-
tive interstitial fluid hydrostatic pressure has been shown
to occur within 30 minutes of injury. The duration and
∗
The views of the authors do not purport to reflect the position of
the Department of the Army and the Department of Defense.
Copyright © 2008 by The McGraw-Hill Companies, Inc. Click here for terms of use.

CHAPTER 35
724
magnitude of the negative hydrostatic pressure change were
proportional to the size of the burn. An early increase in the
interstitial fluid colloid osmotic pressure following burn
injury resulting in a reversal of the transcapillary osmotic
pressure gradient also has been reported. Following initial
changes in the physical characteristics of burn tissue, subse-
quent edema formation generally has been attributed to an
increase in microvascular permeability owing to the effects of
humoral factors liberated from burned tissue and cytokines
produced by activated leukocytes. The plasma concentration
of histamine, a potent regulator of vascular permeability
present in abundance in mast cells, rises in proportion to
burn size immediately following injury. Many inflammatory
mediators, including activated proteases, prostaglandins,
leukotrienes, fibrin degradation products, and substance P,
have been reported to increase microvascular permeability
following burn injury. Specific antagonists to these agents
have been shown to decrease but not eliminate edema forma-
tion when administered prior to burn injury. The efficacy of
inflammatory mediator antagonists in decreasing microvas-
cular permeability when administered following burn injury
has not been demonstrated conclusively.
Leukocyte activation results in the production of
cytokines and other factors capable of increasing microvas-
cular permeability. Lysosomal enzymes, increased xanthine
oxidase activity, products of complement activation, and
oxygen radicals are generated following thermal injury and
are capable of increasing microvascular permeability and
burn wound edema. Interleukin 2 (IL-2)–activated human
killer lymphocytes have been shown to increase albumin flux
across monolayers of cultured endothelial cells in vitro. Even
though neutrophil depletion has been reported to protect
against postburn lung injury, it did not reduce burn wound
edema formation. The response of the leukocyte appears to
depend on the agent of injury, the proximity to the site of
injury, and exposure to humoral mediators.
Edema formation also occurs in unburned tissues follow-
ing a major burn. Conflicting reports exist concerning the
mechanism of this edema formation. In animal studies, an
increase in the ratio of lymph to plasma protein measured in
uninjured extremities following burn injury, as well as an
increase in extravasation of radiolabeled albumin into unin-
jured tissue, has been described. Others were unable to
demonstrate a change in ratios of lymph to serum protein
from an uninjured sheep extremity following burn injury,
implying that no change in vascular permeability in the
uninjured extremity had occurred. Edema accumulation
may be massive in unburned tissue following thermal injury,
and despite the conflicting reports, it is most likely due to
changes in oncotic pressure and the dilutional effects result-
ing from the infusion of large volumes of crystalloid fluid
required for burn resuscitation. Edema formation is charac-
terized by a shift of fluid and protein from the intravascular
into the extravascular compartment. Volume shifts occur in
proportion to the extent of burn, resulting in decreased
blood volume and decreased cardiac output, which, if
untreated, progress to hypovolemic shock.
Organ System Responses to Burn Injury
The magnitude and duration of the prototype organ
response to thermal injury of early hypofunction and later
hyperfunction depend on the extent of injury.
Cardiovascular System
During the resuscitative phase, the initial cardiovascular
response to thermal injury is manifested by decreased cardiac
output and increased peripheral vascular resistance followed
by a progressive increase in cardiac output and decrease in
peripheral vascular resistance during the hypermetabolic
flow phase. The fall in cardiac output is proportional to the
size of the burn and attributable to the loss of fluid and pro-
tein from the intravascular into the extravascular compart-
ment. There is a corresponding reflex increase in peripheral
vascular resistance as a consequence of the neurohumoral
response to hypovolemia. A myocardial depressive factor has
been implicated as the cause of initial impaired myocardial
performance; however, this factor has not been identified.
Clinical studies have demonstrated that in the absence of
heart disease, the ventricular ejection fraction and velocity of
myocardial fiber shortening were increased following thermal
injury and that hypovolemia, as measured by decreased left
ventricular end-diastolic volume, was the cause of depressed
cardiac output. Fluid resuscitation following burn injury
improves cardiac performance as hypovolemia is corrected.
Table 35–1. American Burn Association burn center
referral guidelines.
Partial-thickness burns greater than 10% total body surface area
Burns that involve the face, hands, feet, genitalia, perineum, or major
joints
Third-degree burns in any age group
Electrical burns, including lightning injury
Chemical burns
Inhalation injury
Burn injury in patients with preexisting medical disorders that could
complicate management, prolong recovery, or affect mortality
Any patients with burns and concomitant trauma (such as fractures) in
which the burn injury poses the greatest risk of morbidity or
mortality. In such cases, if the trauma poses the greater immediate
risk, the patient may be initially stabilized in a trauma center before
being transferred to a burn unit. Physician judgment will be neces-
sary in such situations and should be in concert with the regional
medical control plan and triage protocols.
Burned children in hospitals without qualified personnel or equipment
for the care of children
Burn injury in patients who will require special social, emotional, or
long-term rehabilitative intervention
TBSA=total surface area.

BURNS
725
As microvascular permeability decreases, the plasma volume
deficit is replenished in the second 24 hours, and cardiac out-
put increases to supranormal levels. Peripheral vascular
resistance decreases below normal, and the postburn hyper-
metabolic state, which peaks in the second postburn week
and slowly recedes thereafter, is established. Studies have
demonstrated that the postresuscitation increase in cardiac
output is directed primarily toward the burn wound; that is,
the blood flow to a burned extremity is significantly
increased compared with an unburned extremity in the same
patient, and the increase is proportional to the extent of burn
on the involved extremity. As a consequence, a decrease in
cardiac output secondary to hypovolemia or pharmacologic
intervention may reduce the flow of oxygen and nutrients to
the wound and impair wound healing.
Lungs
Following thermal injury, even in the absence of associated
smoke inhalation, physiologic changes in pulmonary func-
tion occur. Immediately postburn, minute ventilation is
unchanged or slightly increased as a result of anxiety- and
pain-induced hyperventilation. With the initiation of fluid
resuscitation, respiratory rate and tidal volume increase pro-
gressively, resulting in a minute ventilation that may be two
to two and one-half times normal. The magnitude of this
increase is proportional to the extent of burn and is consid-
ered to reflect postinjury hypermetabolism. In patients with
circumferential burns of the thorax, the unyielding eschar
and underlying edema may restrict ventilation to the point of
requiring escharotomy incisions to relieve the restrictive ven-
tilatory defect.
Pulmonary vascular resistance increases immediately fol-
lowing thermal injury, and the increase is more prolonged
than the increase in peripheral vascular resistance. The
release of vasoactive amines and other mediators following
thermal injury may be responsible for the increased pul-
monary vascular resistance, and this process may exert a pro-
tective effect during fluid resuscitation by decreasing
pulmonary capillary hydrostatic pressure and thus prevent
pulmonary edema. Lung lymph flow studies have demon-
strated no change in pulmonary capillary permeability fol-
lowing cutaneous thermal injury. Complement activation
and generation of the chemotactic peptide C5A have been
shown to be temporally related to neutropenia, aggregation
of leukocytes in pulmonary capillaries, and intraalveolar
hemorrhages. In other laboratory studies, preburn depletion
of complement, neutrophils, and platelets was protective of
postinjury lung dysfunction. Preinjury treatment with cata-
lase and superoxide dismutase also improve postburn pul-
monary function, implicating toxic oxygen products
produced by activated neutrophils as mediators of the post-
burn pulmonary dysfunction.
Whether or not the infusion of large volumes of crystal-
loid solution associated with burn resuscitation causes post-
burn pulmonary changes remains controversial. The
accumulation of chest wall edema, exacerbated by infusion
of large volumes of resuscitation fluid, decreases total lung
compliance and promotes atelectasis and hypoxemia.
Furthermore, overzealous initial fluid resuscitation may
result in florid pulmonary edema as the edema fluid is
resorbed during the third to fifth postburn days. Consequently,
the smallest volume of resuscitation fluid that maintains ade-
quate organ perfusion should be administered to avoid
secondary pulmonary complications.
Kidneys
The renal response following thermal injury parallels the car-
diovascular response. In the immediate postburn period, renal
blood flow and the glomerular filtration rate are reduced in
proportion to the size of the burn and the magnitude of the
intravascular volume deficit. Delayed or inadequate fluid
resuscitation may cause inadequate renal perfusion and lead to
acute tubular necrosis and renal failure. Following a successful
resuscitation phase, cardiac output and renal blood flow are
increased as edema fluid is resorbed. A diuretic response is
observed during the period of edema resorption; however, this
response may be modified by a large evaporative loss of fluids
through the wound surface and slow rates of edema resorption
in patients with large-surface-area burns. Despite the
markedly increased cardiac output and renal plasma flow seen
in the flow phase of burn injury, the blood volume of patients
measured by
51
Cr red blood cell labeling was only 81% of pre-
dicted values. Plasma renin activity and antidiuretic hormone
levels are elevated, as predicted by the decreased blood vol-
ume, despite the findings of increased blood flow to the kid-
ney. This may explain in part the propensity for sodium
retention to occur during the course of treatment for thermal
injury. As in other organ systems, the duration of changes in
renal physiology is related to the timing of wound closure by
primary healing or autografting. Owing to the increased renal
blood flow, drugs excreted by the kidneys tend to have
markedly shortened half-lives, and appropriate dosing adjust-
ments of these drugs are necessary.
Gastrointestinal Tract and Liver
GI and hepatic dysfunction are also related to the magnitude
of thermal injury. In patients with burns of more than 25% of
the total body surface, ileus, resulting from the combined
effects of hypovolemia and neurohumoral changes, is a
prominent feature. Nasogastric intubation for gastric decom-
pression is usually required. Following resuscitation, normal
GI motility commonly returns by the third to fifth postburn
day. Focal ischemic mucosal lesions of the stomach and duo-
denum may be observed as early as 3–5 hours following burn
injury, and in the absence of stress ulcer prophylaxis, these
early lesions may progress to frank ulceration. Intestinal bac-
terial translocation following thermal injury has been studied
extensively in the laboratory, and increased intestinal perme-
ability to low-molecular-weight sugars has been identified as

CHAPTER 35
726
a prodrome to the onset of infection in thermally injured
patients. However, the clinical significance and therapeutic
implications of these findings are yet to be fully elucidated.
As the magnitude of a burn increases, so does the likeli-
hood of early postburn hepatic dysfunction. An initial
increase in hepatic aminotransferase is common following
burns of more than 50% of the body surface area. This is most
likely due to the acute reduction in cardiac output, increased
blood viscosity, and associated splanchnic vasoconstriction
that occur immediately following thermal injury. Following
successful fluid resuscitation, the hepatic enzymes promptly
return to normal in most patients. The magnitude of initial
enzyme derangements has not been predictive of outcome;
however, the early onset of jaundice following thermal injury
is associated with a poor prognosis, probably indicating
preinjury hepatic dysfunction or severely compromised
hepatic perfusion during the resuscitative phase. The onset of
hepatic dysfunction later in the postburn period usually is
manifested by hyperbilirubinemia and elevation of liver
enzymes in a cholestatic pattern. These changes are most
often associated with sepsis or multiple-organ failure.
Nervous System
Nonspecific neurologic changes such as increased anxiety
and disorientation are observed commonly in patients with
extensive thermal injury and are most likely due to the neu-
rohumoral stress response and ICU isolation. Specific neuro-
logic changes are observed more commonly in patients with
high-voltage electrical injury or mechanical trauma.
Changing neurologic symptoms and signs, manifested by
increasing disorientation, obtundation, or seizures, may be
the earliest indications of hypoxemia, electrolyte or fluid
imbalance, sepsis, or the toxic effects of medications.
Changes in neurologic findings require prompt intervention
to identify and correct such abnormalities.
Endocrine System
The metabolic response to thermal injury is also proportion-
ate to the extent of burn and follows the typical biphasic
response documented in other organ systems. Immediately
following burn injury, during the period of hypovolemia, the
metabolic rate decreases; however, as resuscitation pro-
gresses, a catabolic or hypermetabolic hormonal pattern
emerges. Serum levels of catecholamines, glucagon, and
cortisol increase, whereas insulin and triiodothyronine lev-
els are decreased. There is an increase in net glucose flow,
with relative peripheral insulin resistance and a markedly
negative nitrogen balance. As the burn wounds heal or are
closed by autografting, the catabolic hormone response
dissipates, an anabolic state is eventually attained, and
restoration of lean body mass ensues. Septic complications
superimposed on thermal injury initially exaggerate the
hypermetabolic response, but if the septic state persists,
progressive deterioration and multisystem organ failure,
characterized by hypometabolism, may occur.
Hematopoietic System
Destruction of red blood cells following thermal injury
occurs to an extent proportional to the size and depth of
burn. In areas of full-thickness burn, red blood cells are
immediately coagulated in the involved microvasculature.
There is a continuing red blood cell loss in patients with
extensive burns of 8–12% of the red blood cell mass per day
caused by the continued lysis of cells damaged by heat,
microvascular thrombosis in zones of ischemia that subse-
quently become necrotic, and repeated blood sampling. In
the early postburn period, platelet number and fibrinogen
levels are depressed, with a corresponding rise in fibrin split
products. Following resuscitation, platelets and serum levels
of fibrinogen and factors V and VIII rapidly increase to
supranormal levels. Erythropoietin levels are increased coin-
cident with the anemia following thermal injury. Recent
studies have suggested that the rate of erythropoiesis may be
further increased by the administration of recombinant ery-
thropoietin and iron. However, a decrease in transfusion
requirements has yet to be demonstrated.
Immunologic Response
Infection remains the major cause of death among burn
patients. Following injury, dysfunction of the cellular and
humoral immune response occurs that is related to the
extent of injury. Destruction of the normal skin barrier
results in loss of mechanical protection from microbial pro-
liferation and allows microbial invasion into normal tissues.
Modern burn care—with emphasis on effective topical
antimicrobial agents, infection control policies, and timely
excision with autograft closure of burn wounds—has signif-
icantly decreased the incidence of burn wound infection.
Other infectious complications, principally pneumonia,
remain the major source of morbidity and mortality, and
treatment may be made difficult by the generalized immune
system dysfunction following thermal injury.
During the first postburn week, the total white blood cell
count is elevated, although peripheral blood lymphocyte
counts are reduced. Burn injury also causes apoptosis of lym-
phocytes in various solid organs following burn injury. This
process is glucocorticoid-mediated and can be blocked
experimentally by the administration of glucocorticoid
receptor antagonists. This process is not TNF-α– or Fas
ligand–dependent and may represent a counterregulatory
mechanism to reduce inflammatory stimuli. Delayed hyper-
sensitivity reactions and peripheral blood lymphocyte prolif-
eration in the mixed lymphocyte reaction are both inhibited
following thermal injury. Alterations in lymphocyte subpop-
ulations have been described that normalize over the second
postburn week in patients whose course is uncomplicated.
Further alterations occur prior to and during the onset of
septic complications. Alterations in IL-2 production and
IL-2 receptor expression by lymphocytes have been measured
following burn injury, and direct correlation has been estab-
lished between the extent of burn and the decrease in IL-2
