Bongard Frederic , Darryl Sue. Diagnosis and Treatment Critical Care
Подождите немного. Документ загружается.

BLEEDING & HEMOSTASIS
427
Differential Diagnosis
The finding of a normal platelet count in the presence of pal-
pable or nonpalpable purpuric skin lesions (eg, ecchymoses
or petechiae) should suggest the possibility of increased
intravascular pressure, abnormalities or injury to blood ves-
sels (eg, vasculitis), thromboembolic events (including septic
thromboemboli), decreased integrity of the microcirculation
and its supporting structures, or primary cutaneous diseases.
Severe coagulation disorders may result in mucocutaneous
bleeding but are easily distinguished from thrombocytopenia
by the presence of markedly prolonged coagulation times
and quantitatively normal platelets. Impaired platelet func-
tion and vWD should be considered when easy bruising and
mucosal bleeding are present and can be identified in most
cases by prolongation of the bleeding time in the absence of
thrombocytopenia. Abnormalities of coagulation or platelet
function rarely result in petechial skin lesions.
Treatment
The most important aspect of treatment is to reverse the
underlying disease process. Platelet transfusions should be
given only when the risk of bleeding and the probability of
efficacy are sufficient to warrant the potential risks of blood
component therapy. Prophylactic platelet transfusions are
indicated for patients with decreased production of platelets
with counts under 10,000/μL to prevent fatal CNS hemor-
rhage. When life-threatening bleeding is present or invasive pro-
cedures are required, platelet transfusions may be useful if the
platelet count is less than 50,000/μL and no alternative therapies
are available. When decreased platelet survival is present,
platelet transfusions are not likely to effect a sustained rise in
platelet count and should only be given if life-threatening bleed-
ing is present or if an urgent invasive procedure is required.
Platelet transfusions may be harmful in patients with throm-
botic thrombocytopenic purpura–hemolytic uremic syndrome
and heparin-associated thrombocytopenia and should be
avoided unless active, life-threatening hemorrhage is present.
Medications such as aspirin that impair platelet function
should be avoided when thrombocytopenia is present—with
the exception of thrombotic thrombocytopenic purpura, in
which antiplatelet agents may be indicated as part of therapy.
Correction of severe anemia may decrease clinical bleeding in
thrombocytopenic patients. Antifibrinolytic agents such as
aminocaproic acid or tranexamic acid may decrease mucosal
bleeding in chronically thrombocytopenic patients but should
not be used if there is evidence of thrombosis (eg, in DIC) or
hematuria. Alternatives to platelet transfusion for patients
with bleeding owing to thrombocytopenia are outlined in
Table 3–2.
Current Controversies and Unresolved Issues
Intravenous administration of human immunoglobulin
(IVIG) may rapidly increase the platelet count in patients with
immune-mediated thrombocytopenia. Its effect is postulated
to be due to competitive blockade of macrophage receptors
for immunoglobulin, although other mechanisms may be
important. Although it may rapidly increase the platelet
count, its usefulness as therapy for immune thrombocytope-
nia is limited by only transient efficacy combined with its
high cost in the face of a low risk of life-threatening bleeding
in patients with autoimmune idiopathic thrombocytopenia.
It is appropriate for use in patients with serious bleeding
with this disorder as a temporizing measure while other ther-
apies are being administered. Anti-D antibodies (Rh
o
D
immune globulin) also may be useful in the management of
immune-mediated thrombocytopenia in patients who are
Rh-positive, presumably by a similar mechanism (antibody-
coated red blood cells block macrophage receptors). IVIG
has not been proven to be useful in treatment of thrombocy-
topenia owing to platelet alloimmunization.
Alloimmunization to platelet-specific antigen or HLA
antigens is a common sequela of repeated platelet transfu-
sions and limits the response to subsequent platelet transfu-
sions. For patients requiring long-term platelet support,
prevention of alloimmunization is desirable in order to pre-
serve the efficacy of transfusion therapy. Methods to mini-
mize platelet alloimmunization include reducing the total
number of all blood product transfusions, adhering to strict
criteria for transfusing platelets, removing the source of the
HLA sensitization (ie, white blood cells) before transfusion
of any blood product (by filtration or irradiation), and using
HLA-identical donors.
The optimal management of several conditions associ-
ated with thrombocytopenia is under active study. Treatment
of these conditions should be undertaken in consultation
with a hematologist to ensure accurate diagnosis and up-to-
date treatment.
APPROACH TO THE BLEEDING PATIENT
When approaching a patient with a suspected bleeding disor-
der, it is important to assess quickly the prior personal and fam-
ily history of bleeding, the pattern of bleeding, and the pattern
of laboratory abnormalities. If the personal or family history is
suggestive of an inherited bleeding disorder, the apparent
inheritance pattern, bleeding manifestations, and laboratory
abnormalities should suggest the most likely diagnoses. If the
personal and family histories are negative, defective hemostasis
is most likely acquired, although some of the milder inherited
disorders of hemostasis may not be manifested until significant
trauma or surgery occurs later in life. The pattern of bleeding
may suggest the nature of the hemostatic defect (Table 17–10),
and the pattern of laboratory abnormalities (Table 17–11) will
help to localize defects in the hemostatic system.
CURRENT CONTROVERSIES & UNRESOLVED
ISSUES
Gastrointestinal Hemorrhage
For more than a century, critically ill patients have been
observed to have a high rate of GI hemorrhage. Preventive
strategies emerged to decrease this complication, including
CHAPTER 17
428
the use of antacids, histamine-2(H2) receptor blockade, pro-
ton pump inhibitors (PPIs), and mucosal protective agents.
However, trials of these therapeutics have yielded conflict-
ing results, with some studies showing a benefit in selected
high-risk patients (eg, patients with mechanical ventila-
tion, hemostatic defects, or prior GI hemorrhage), others
showing an increase in hospital-acquired pneumonia and
complications related to drug interactions, and others
demonstrating no difference in any clinical outcome. Until
this issue is resolved, preventive therapy with mucosal protec-
tant agents (eg, sucralfate), H
2
blockade, or PPIs can be con-
sidered for patients identified to be at high risk for bleeding.
Underlying hemostatic defects should be treated according to
the general principles discussed earlier. The use of nons-
teroidal anti-inflammatory drugs (NSAIDs) and low-dose
aspirin increases the risk of GI bleeding independent of their
effects on platelet function. When Helicobacter pylori–associ-
ated ulcer is identified, long-term treatment with omeprazole
or eradication with antibiotics can decrease the risk of recur-
rent bleeding associated with aspirin or NSAIDs.
Gastroesophageal variceal hemorrhage is the most serious
form of GI hemorrhage, with 30% mortality observed with
first episodes. Varices occur in the setting of severe liver disease
so that—in addition to mechanical problems—coagulation
abnormalities, quantitative and qualitative platelet defects, and
excessive fibrinolysis contribute to bleeding. Management of
acute variceal bleeding requires GI specialists who can diag-
nose and treat varices endoscopically (eg, ligation, sclerosis,
or balloon tamponade) but also includes medical therapy
with vasoactive agents such as beta-blockers, nitrates, vaso-
pressin, and somatostatin analogues (eg, octreotide and
vapreotide). When these approaches fail, transjugular or sur-
gical shunting may be required to control bleeding.
Perioperative Bleeding
Preoperative evaluation of hemostasis should include
medical history with attention to adequacy of hemostasis—
spontaneous bleeding or bruising or unexpected bleeding
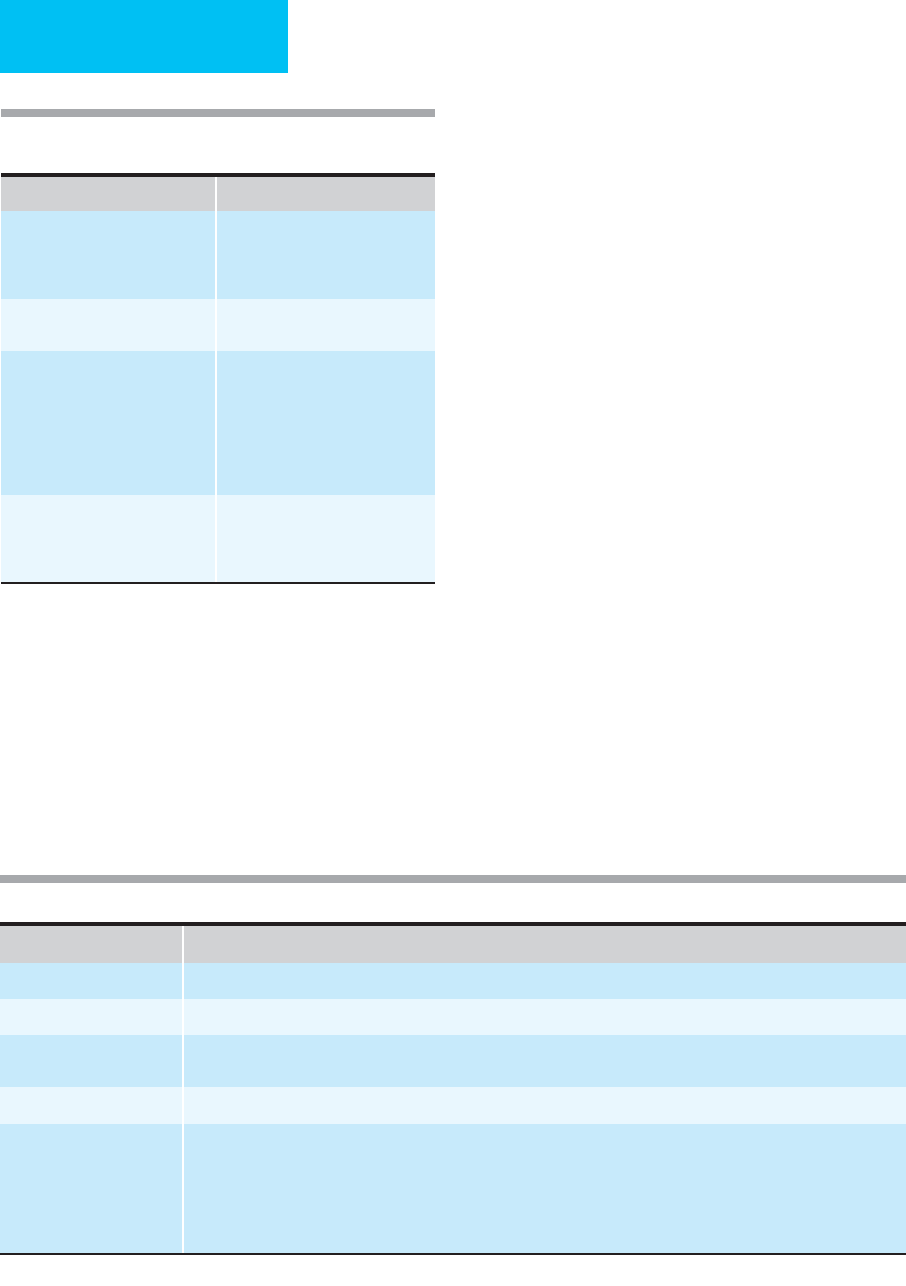
Pattern of Bleeding Possible Disorders
Ecchymoses and mucosal
bleeding; petechiae
Thrombocytopenia or platelet
dysfunction
von Willebrand disease
Severe coagulopathies
Spontaneous hemarthroses, soft
tissue hematomas
Hemophilia A and B, other severe
coagulopathies
Posttraumatic or surgical bleeding Thrombocytopenia or platelet
dysfunction
von Willebrand disease
Coagulopathies
Impaired vascular integrity
Inadequate surgical hemostasis
Massive injuries
Generalized oozing from mucosal,
venipuncture, surgical sites
Consumptive coagulopathies, DIC
Excessive fibrinolyis
Severe thrombocytopenia or
platelet dysfunction
Table 17–10. Approach to the bleeding patient by pat-
tern of bleeding.
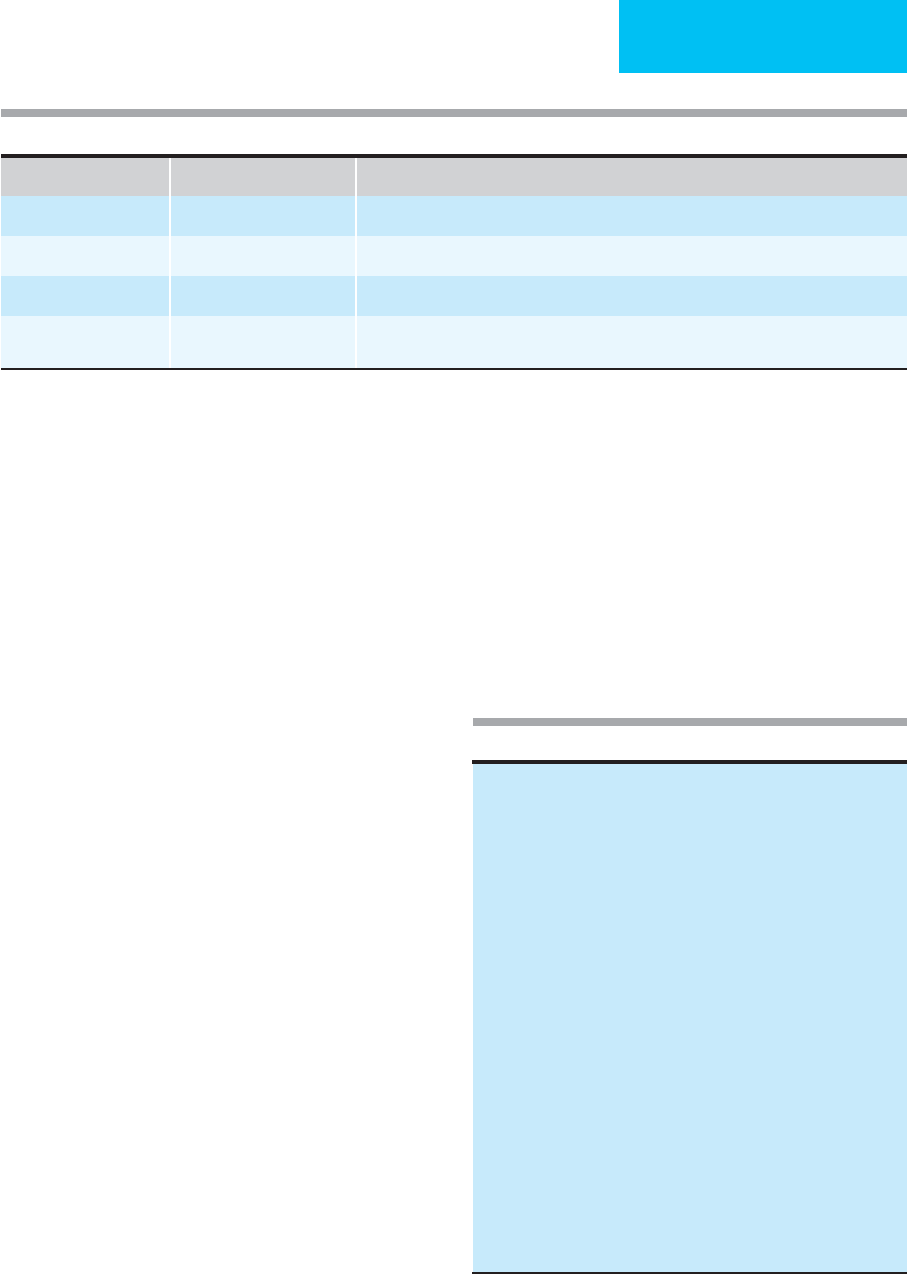
Table 17–11. Approach to the bleeding patient by pattern of screening laboratory abnormalities.
Abnormal Test Possible Defects
PT only Factor VII deficiency (inherited, vitamin K deficiency, warfarin effect) or inhibitor
aPTT only
∗
Factor XII, HMW kininogen, prekallikrein, XI, IX, or VIII deficiency or inhibitors to these factors; lupus anticoagulant
PT and aPTT Deficiency or inhibitor of factor X, V, II (prothrombin), fibrinogen
Multiple factor deficiencies (liver disease, DIC, consumptive coagulopathies, vitamin K deficiency, high dose warfarin effect)
Bleeding time prolonged Platelet dysfunction or thrombocytopenia, improper technique
All screening tests normal Other coagulation abnormalities:
Factor XIII deficiency
Excessive fibrinolyis
Paraproteinemia
Vascular defect (including inadequate surgical hemostasis)
Supporting tissue abnormality
∗
Deficiencies of factor XII, HMW kininogen, prekallikrein, and lupus-type inhibitors prolong the aPTT but do not cause bleeding.
BLEEDING & HEMOSTASIS
429
after dental extractions or prior surgical procedures suggests
the presence of an underlying hemostatic defect. Medical ill-
nesses such as liver or renal disease, myeloproliferative disor-
ders, and paraproteinemias predispose patients to excessive
postoperative bleeding. In the absence of symptoms of a
bleeding disorder or such medical illnesses, the value of rou-
tine laboratory screening has been questioned. Most postop-
erative bleeding results from surgical or technical problems
and not from hemostatic defects. The PT and aPTT are not
sensitive enough to detect mild defects and, when abnormal
in a patient with no previous bleeding history, may not accu-
rately predict the risk of bleeding. Whether any laboratory
screening should be done depends also on the type of
planned procedure (ie, minor versus major, use of cardiopul-
monary bypass, neurosurgical procedures, etc.). Table 17–12
outlines suggested laboratory screening prior to surgery. If
abnormalities in screening tests are detected, further evalua-
tion should be done to define the precise abnormality.
Intraoperative hemostasis with ligature, cautery, and top-
ical thrombogenic substances (eg, fibrin glue or topical
bovine thrombin) is essential to prevent postoperative bleed-
ing. In the postoperative period, excessive bleeding may be
due to a hemostatic defect that was not detected by the
screening tests. Mild coagulation factor deficiencies may not
prolong the aPTT, particularly if other factors are elevated
(eg, factor VIII increases in acute inflammatory states and
may mask mild deficiency of other factors). Preoperative use
of aspirin or other antiplatelet drugs may increase postoper-
ative bleeding; if bleeding is severe and the bleeding time is
prolonged, desmopressin or platelet transfusions may be
required for control of bleeding. Surgery on the prostate
gland or uterus may be associated with localized hyperfibri-
nolysis with excessive bleeding. Antifibrinolytic therapy with
aminocaproic acid can be considered for control of bleeding
(5-g loading dose followed by 1 g/h intravenously or orally
until bleeding has stopped), although there is some risk of
thrombi developing in the ureters (following prostate surgery)
that are resistant to lysis. Acute DIC may occur for many rea-
sons following surgery, or decompensation of previously
compensated DIC may develop as a result of tissue factor
release during surgery (Table 17–13).
Acute renal failure owing to hypotension, massive blood
loss, or medications can result in platelet dysfunction, which is
exacerbated by anemia. Posttransfusion purpura with throm-
bocytopenia may occur in previously sensitized individuals.
The use of intraoperative bovine thrombin as a hemostatic
agent may precipitate the development of antibodies to throm-
bin and factor V. There does not appear to be any significance
Bleeding History Type of Surgery Recommended Laboratory Tests
No suggestive history Minor (dental, skin biopsy) None
No suggestive history Major Platelet count, aPTT
Possible bleeding history Major, bypass pump PT, aPTT, platelet count, bleeding time; if normal, factor XIII assay, euglobulin clot lysis time
Positive bleeding history Minor or major Same as above; if negative: thrombin time, factor assays for VIII, IX, XI, alpha
2
-antiplasmin
assay, post-aspirin bleeding time
Table 17–12. Preoperative laboratory screening.
Sepsis
Hypotension
Acute hepatic necrosis
Release of bone marrow thromboplastins
Liver injury (drugs, hypotension, blunt or penetrating trauma)
Tissue factor release during surgery
Peritoneovenous shunting
Use of cell saver device with excessive suction
Release of tissue factor from placenta
Abruptio placentae
Amniotic fluid embolism
Septic abortion
Placenta previa
Retained dead fetus
Trauma
Fat embolism
Brain injury
Extensive soft tissue injury
Burns
Decompensation of chronic (compensated) DIC
Dissecting aortic aneurysm
Kasabach-Merritt syndrome
Cancer
Infusion of activated clotting factors
Prothrombin complex concentrates
Recombinant activated factor VII
Table 17–13. Causes of postoperative DIC.

CHAPTER 17
430
to the antithrombin inhibitor; however, factor V inhibitors
may cause bleeding. Factor VIII inhibitors also may develop
postoperatively; the mechanism for this has not been defined.
Hetastarch, a plasma volume expander, may cause persistent
coagulation abnormalities, particularly in elderly patients,
those with preexisting hemostatic defects, or with prolonged
use. If bleeding is severe, plasmapheresis to remove these large
molecules may be required to control bleeding.
When postoperative bleeding is encountered, immediate
evaluation with history (to uncover preexisting risks and med-
ication use) and laboratory testing (ie, PT, aPTT, and platelet
count) should be done. Medical treatment of hemostatic
defects will depend on the specific abnormalities identified. If
history and screening laboratory tests are unrevealing, consid-
eration should be given to reexploration of the surgical site to
identify adequacy of surgical hemostasis.
Perioperative management of patients with inherited
bleeding disorders requires preoperative collaboration
among the surgeon, anesthesiologist, hematologist, and
blood bank to ensure that optimal hemostasis is achieved
during and after surgery. The details of therapy will depend
on the nature of the specific disorder, the type of surgical
procedure, and whether any additional medical complica-
tions are present.
Posttraumatic Bleeding
In the setting of massive trauma, multiple pathophysiologic
processes contribute to severe bleeding, including multiple
vascular defects, massive transfusion with dilution of coagu-
lation factors and platelets, DIC with acceleration of fibrinol-
ysis, and hypotension. The presence of coagulopathy
following trauma increases the risk of mortality. Severe coag-
ulation disturbances associated with trauma may result in
adrenal hemorrhage, which can further exacerbate hypoten-
sion. Certain types of injuries are particularly associated with
DIC: fat embolism, brain injury, extensive soft tissue injury,
and extensive burns. Pelvic ring fractures are associated with
massive blood loss, often hidden owing to tracking of bleed-
ing up into the retroperitoneal space. Control of bleeding in
such circumstances may be difficult, even with aggressive
blood product support—with fresh-frozen plasma, platelets,
red blood cells, and cryoprecipitate. rFVIIa has been used to
treat massive hemorrhage in trauma patients with some suc-
cess, but evidence supporting its use is primarily anecdotal.
Further studies are necessary to determine which patients are
most likely to benefit from rFVIIa and at what dose it should
be administered.
REFERENCES
Bakhtiari K et al: Prospective validation of the International Society
of Thrombosis and Haemostasis scoring system for disseminated
intravascular coagulation. Crit Care Med 2004;32:2416–21.
[PMID: 15599145]
Caldwell SH, Chang C, Macik BG: Recombinant activated factor VII
(rFVIIa) as a hemostatic agent in liver disease: A break from con-
vention in need of controlled trials. Hepatology 2004;39:592–8.
[PMID: 14999675]
Finazzi G, Harrison C: Essential thrombocythemia. Semin Hematol
2005;42:230–8. [PMID: 16210036]
Hedner U: Recombinant factor VIIa: Its background, development
and clinical use. Curr Opin Hematol 2007;14:225–9. [PMID:
17414211]
Kamal AH et al: How to interpret and pursue an abnormal pro-
thrombin time, activated partial thromboplastin time, and
bleeding time in adults. Mayo Clin Proc 2007;82:864–73. [PMID:
17605969]
Kantorova I et al: Stress ulcer prophylaxis in critically ill patients: A
randomized, controlled trial. Hepatogastroenterology
2004;51:757–61. [PMID: 15143910]
Levi M, de Jonge E, van der Poll T: Plasma and plasma components
in the management of disseminated intravascular coagulation.
Best Pract Res Clin Haematol 2006;19:127–42. [PMID:
16377546]
Levi M, de Jonge E, van der Poll T: New treatment strategies for dis-
seminated intravascular coagulation based on current under-
standing of the pathophysiology. Ann Med 2004;36:41–9.
[PMID: 15000346]
MacLeod JB et al: Early coagulopathy predicts mortality in trauma.
J Trauma 2003;55:39–44. [PMID: 12855879]
Mannucci PM, Duga S, Peyvandi F: Recessively inherited coagula-
tion disorders. Blood 2004;104:1243–52. [PMID: 15138162]
O’Connell KA et al: Thromboembolic adverse events after use of
recombinant human coagulation factor VIIa. JAMA
2006;295:293–8. [PMID: 16418464]
Roberts HR, Monroe DM 3rd, Hoffman M: Safety profile of recom-
binant factor VIIa. Semin Hematol 2004;41:101–8S. [PMID:
14872430]
Rodeghiero F, Tosetto A, Castaman G: How to estimate bleeding
risk in mild bleeding disorders. J Thromb Haemost
2007;5:157–66. [PMID: 17635722]
Rodeghiero F, Castaman G: Treatment of von Willebrand disease.
Semin Hematol 2005;42:29–35. [PMID: 15662613]
Spirt MJ: Stress-related mucosal disease: Risk factors and prophy-
lactic therapy. Clin Ther 2004;26:197–213. [PMID: 15038943]
Stein DM, Dutton RP: Uses of recombinant factor VIIa in trauma.
Curr Opin Crit Care 2004;10:520–8. [PMID: 15616396]
Tarantino MD, Aledort LM: Advances in clotting factor treatment
for congenital hemorrhagic disorders. Clin Adv Hematol Oncol
2004;2:363–8. [PMID: 16163206]
431
18
Psychiatric Problems
Stuart J. Eisendrath, MD
John R. Chamberlain, MD
ICUs provide the most advanced technology available to
patients with the most serious medical and surgical illnesses.
Perhaps as a consequence, these facilities are often associated
with some of the most stressful psychiatric conditions within
the hospital. Indeed, some physicians have jokingly referred
to the ICU as the “intensive scare unit.” These conditions in
ICUs may affect both patient and staff. This chapter will
describe some of the significant problems.
Most patients entering the ICU will attempt to manage
the stress of their stay with their characteristic coping mech-
anisms. Some regression generally occurs, however, as most
patients experience stressors such as fear of death, enforced
dependency, and potential permanent loss of function.
Separation from family and the loss of autonomy that
accompanies medical treatment in the ICU frequently lead to
further psychologic regression. They may try to cope with
stress by suppressing their feelings, using humor to laugh at
stressful aspects of their situation, or trying to anticipate a
return to good health. If these techniques fail, the individual
may turn to more primitive mechanisms such as projection,
passive-aggressive maneuvers, acting-out behavior, and gross
denial of illness. All this takes place during a time when the
patient must deal with serious and usually multiple medical
problems.
A number of clinical syndromes may develop as a result
of the stressors inherently associated with occupancy of a bed
in the ICU. This discussion will focus on several that may
adversely influence recovery. Delirium, the so-called ICU
psychosis, generally is not due to psychological stress alone
but develops in association with altered brain function.
Anxiety may occur at any time during the patient’s stay in the
ICU—entry, midpoint, or discharge. Depression also may
occur at any point during the patient’s stay but most often
occurs after the immediate threat to life has been dealt with
and the patient must contemplate the subacute prognosis
and ultimate outcome of the disease. Finally, we will discuss
how the ICU environment affects the staff working there.
Delirium
ESSENTIALS OF DIAGNOSIS
Waxing and waning of consciousness.
Disorganized thinking.
Perceptual disturbances such as visual hallucinations.
Disorientation.
General Considerations
Early reports from ICUs gave rise to the diagnosis of an ICU
syndrome characterized essentially as a delirious state with
psychotic features such as hallucinations. It was believed to
be due to the stress an ill individual felt in a foreboding envi-
ronment with little privacy, lack of sleep, and sensory over-
load. Whether or not this syndrome actually existed as a
specific entity, current research indicates that delirium
occurring in the ICU is most likely due to factors similar to
those patients experience on general medical and surgical
wards—with the difference that ICU patients may have more
severe reactions because of their more severe illnesses.
Stress may play some role in producing delirium, but it is
typically only a contributing one. Other factors that alter
brain function are usually the primary etiologic agents. The
critical care physician should not automatically attribute
delirium to stress. Remembering that delirium is an organic
dysfunction of the CNS will facilitate the search for likely
causes and the provision of appropriate treatment. This
aggressive approach is justified given the serious impact of
delirium on morbidity and mortality in hospitalized
patients. Studies have shown that patients suffering an
episode of delirium have an increased risk of death, a longer
hospital stay, and a greater need for subsequent nursing
home placement. There are at least three broad hypotheses
Copyright © 2008 by The McGraw-Hill Companies, Inc. Click here for terms of use.

CHAPTER 18
432
relating to the production of delirium. Any one or any com-
bination of these factors may cause delirium in a particular
patient.
A. Increased Central Noradrenergic Production—Drug
or alcohol withdrawal states are the most common condition
producing delirium in the hospital and probably also the
most common condition responsible for increased nora-
drenergic discharge. Locus ceruleus–controlled production
of brain catecholamines is believed to play a central role in
this type of delirium.
B. Dopamine and Cholinergic Systems—Imbalance of
brain dopamine and cholinergic systems can be recognized
in central anticholinergic syndromes produced by atropinic
agents. These may produce a relative excess of dopaminergic
activity, giving rise to an agitated delirium. This hypothesis
also provides a framework for understanding how
dopamine-blocking agents such as haloperidol may act by
restoring the relative balance between dopaminergic and
cholinergic systems that is disturbed in delirium. Delirium-
producing agents such as the amphetamines, which cause a
shifting of the balance toward the dopaminergic side, also
can be better understood in this framework.
C. Toxic Causes of Delirium—A third framework for
understanding delirium involves the environment brain cells
operate in. Side effects of medications or endotoxins associ-
ated with bacterial infections can affect neuronal function-
ing. Similarly, conditions such as hyperthermia or hypoxia
can disturb functioning throughout the CNS. Many of the
preceding abnormalities are believed to affect the midbrain
particularly; this may, in turn, lead to fluctuations in the
reticular activating system with alterations in level of conscious-
ness and the ability to attend to stimuli in the environment—a
key feature of many cases of delirium.
D. Multifactorial Features—In the ICU there are typically
numerous factors that play a role in producing delirium. A
patient with asthma and pneumonia provides an example—
with fever and hypoxia as well as high levels of circulating
corticosteroids and catecholamines. Although single causes
should be sought diligently, it is unusual to be able to iden-
tify and correct just one causative factor of delirium.
Clinical Features
The clinical criteria for delirium in the ICU setting consists
of a number of signs and symptoms. Typically, the patient
has a waxing and waning of consciousness—may be somno-
lent at one moment and highly alert and agitated a short time
later. The patient’s ability to attend to external stimuli fluctu-
ates over time. Delirious patients usually have disorganized
thinking manifested by rambling or incoherent speech.
Patients frequently have perceptual disturbances such as mis-
perceptions of real objects (illusions) or frank hallucinations.
Patients usually have disorders in normal sleep-wake cycles and
altered psychomotor behavior (either agitation or retardation).
Patients also typically have disorientation to time, place, and
situation. Memory impairment, when present, is generally
global for both recent and remote events. Delirium usually
develops within hours to days. The presence of delirium sig-
nifies primarily organic brain dysfunction and is not a man-
ifestation of psychological distress alone.
The signs and symptoms listed in the preceding para-
graph are useful in making a clear-cut diagnosis.
Unfortunately for that purpose, however, many ICU patients
present only some of the criteria and may require ongoing
vigilance. Indeed, one of the keys to diagnosis in these
patients is repeated observations over time. The waxing and
waning of consciousness so common in delirious patients
requires serial monitoring because a patient may appear
quite lucid at one moment and quite confused a half hour
later. Nursing staff often have the best data on mental status
because of their continuing contact with the patients.
The physician should examine specifically for the presence
of delirium. This involves performing some type of specific
cognitive assessment such as the Folstein Mini Mental Status
Exam. However, intubated patients can be difficult to assess
with this instrument. Newer diagnostic instruments such as
the Cognitive Test for Delirium (CTD) can be very useful in
this setting. This instrument was developed for use in critical
care settings and reliably distinguishes patients with delirium,
dementia, and acute psychiatric illness. This type of examina-
tion tests the patient’s ability to attend to stimuli, concentrate
on a task, comply with simple memory requests, demonstrate
language capabilities through auditory comprehension, and
show orientation to time and situation. More important, this
type of test allows the examiner to detect cognitive deficits
that otherwise would go unrecognized. For example, psychi-
atric consultation may be sought for a patient who is poorly
compliant with the medical regimen. Many of these patients
prove to be suffering from delirium and have significant
memory deficits that make compliance impossible—they
cannot remember instructions for 30 seconds.
The patient may appear quietly confused and in a daze.
This type of delirium may be easily missed because the patient
presents no behavioral problem. Some delirious patients, on
the other hand, present behavioral problems ranging from
pulling out catheters and lines to biting or striking members of
the staff. These patients appear agitated, fearful, and restless.
They may be hallucinating and intensely involved in their psy-
chotic states. One physician described his state of mind during
his stay in the ICU as follows: “I saw a vulture flying over me.
I worried about any drop of blood spilling onto the sheets
because I knew that would bring the vulture down for the
attack.” This physician had been fearfully watching the ceiling
for several days but never told the doctors or nurses because
“they couldn’t do anything, and they’d think I was crazy.”
Many delirious patients resort to primitive psychological
coping mechanisms. They may exhibit massive denial of any
medical problem or externalize their problem as being the
fault of somebody else rather than of their illness. Delirious
patients often project anger about their helplessness onto

PSYCHIATRIC PROBLEMS
433
their caregivers. This gives rise to paranoid ideation about
caregivers trying to poison or otherwise harm them. Other
delirious patients—like the doctor in the preceding example—
experience hallucinations that are often threatening. These
hallucinations usually are visual and occur without any exter-
nal stimulus. Many patients will have visual illusions that
involve a misperception of actual external stimuli. For exam-
ple, one patient was convinced that the intravenous standard
in his room was actually a person poised to attack him.
Differential Diagnosis
In the critically ill patient—especially an intubated patient,
for whom communication is most difficult—delirium may
appear to be similar to other conditions.
A. Anxiety—Anxiety occurs quite frequently in the ICU. In
some instances, anxiety may occur in patients who have
delirium. Individuals with cognitive processing impairment
may be quite difficult to reassure. Thus many patients may be
anxious in the ICU, but only some will also have delirium—
although the majority of delirious patients will experience
anxiety and fear. Patients without delirium do not have the
significant cognitive impairment and deficits in reality test-
ing (such as hallucinations) so common in delirium.
B. Underlying Psychosis—ICU patients may have an
underlying psychosis (eg, schizophrenia) that can be confused
with delirium. Schizophrenic patients rarely have visual hal-
lucinations. Visual hallucinations usually are present owing to
an organic cause that requires investigation. Schizophrenic
patients who have paranoid delusions generally have well-
formed ones that are fixed over months and years. Delirious
patients, on the other hand, have rapidly developing delu-
sional beliefs that shift over hours to days. Most schizophrenic
patients have a history of psychiatric treatment and neurolep-
tic medications. Delirious patients generally do not have such
a history. Furthermore, schizophrenic patients typically have
onset of illness by the early to middle twenties, characterized
by a progressive decline in function. In contrast, delirium
often affects older patients and causes a precipitous decline in
mental status and functioning.
C. Neuroleptic Malignant Syndrome—Schizophrenic
patients—or any patient who has received a neuroleptic
medication—are at risk for development of neuroleptic malig-
nant syndrome, characterized by altered mental status, auto-
nomic instability, and extrapyramidal symptoms that are often
severe (eg, lead-pipe rigidity). This syndrome can develop par-
ticularly with high-potency neuroleptic medications when
the individual also suffers from complicating conditions such
as fever and dehydration. Diagnosis in these patients is often
assisted by elevated creatine phosphokinase (CPK) levels,
myoglobinuria, and decreased serum iron levels.
D. Depression—The subdued, quiet delirious patient may be
mistaken for a depressed one. The depressed patient, however,
usually has the depressive view of the world associated with
that disorder along with symptoms such as a sense of worth-
lessness, guilt, and global pessimism. In some instances, small
test doses of dextroamphetamine (eg, 2.5 mg twice daily) may
allow the clinician to differentiate depressive and delirious
patients. The depressed patient may become less depressed
after medication, whereas the delirious patient usually
becomes more agitated. A past psychiatric history of depres-
sive disorder also may help to differentiate the disorders.
E. Personality Disorder—It is not unusual for staff to apply
the pejorative label personality disorder to a patient who fails
to comply with their requests. Whenever such a diagnosis is
contemplated, one should seek confirmation from family or
friends. Personality disorders represent longstanding pat-
terns of maladaptive behavior. They do not develop acutely,
although they may be exacerbated by the stress of illness.
They may emerge in patients after the immediate threat to
life has passed. Psychiatric consultation is often helpful in
confirming this diagnosis.
F. Dementia—One way of conceptualizing the difference
between dementia and delirium is by applying the analogy of
acute and chronic renal failure. Delirium represents acute
cerebral insufficiency, whereas dementia is typically a
chronic condition. Dementia syndromes may represent a
variety of conditions (eg, multi-infarct dementia or
Alzheimer’s disease) characterized by generalized diminu-
tion of intellectual functioning. Most dementias—except for
the subcortical ones, such as in Parkinson’s disease—show
some evidence of cortical dysfunction such as aphasia or
apraxia. Dementia syndromes develop over months or years
rather than hours or days. The patient’s family should be
consulted to clarify baseline mental functioning.
A key finding is that delirious patients typically have a
waxing and waning of consciousness and the ability to attend
to stimuli; deficits in demented patients generally are fixed
throughout the day until nightfall, when “sundowning” may
occur. It is quite common to see dementia patients who have
preserved remote memory, whereas in delirium, all forms of
memory may be impaired. It is unusual for dementia
patients to suffer hallucinations, whereas delirious patients
frequently have hallucinations.
Electroencephalograms may aid in the differentiation of
delirium and dementia. The former often show generalized
slowing, whereas the latter do not.
Treatment
A. Establish the Cause—The key to treatment of delirium
is identification of its cause or causes whenever possible.
Since delirium is usually an acute process, a vigorous investi-
gation of reversible causes should be undertaken, much as
one would investigate the cause of acute renal failure and
attempt to correct it as rapidly as possible. Delirium should
be regarded as an example of acute cerebral failure that is asso-
ciated with significantly increased morbidity and mortality.
There are studies in which patients with delirium had a
CHAPTER 18
434
markedly higher mortality rate than other patients (8% ver-
sus 1%; likelihood ratio = 2.3), a higher rate of admission,
and a higher rate of institutionalization. Hospital stays often
are prolonged by an average of 7 days in delirious patients.
There are many causes of delirium. The most common
causes in the ICU are listed in Table 18–1. Withdrawal states
are among the most common causes of delirium in the gen-
eral hospital and always should be considered in assessing the
patient. In some instances, the patient may conceal or be
unable to provide information about drug or alcohol use.
Family and friends will be able to help to establish this his-
tory. In some instances, considerable probing may be neces-
sary. For example, one postoperative vascular surgery patient
denied the use of alcohol or drugs; persistent questioning,
however, revealed that she had increased her usual daily 0.5-mg
alprazolam dosage fourfold in the month prior to surgery
because of anxiety. When she entered the hospital, this med-
ication had been discontinued. Two days later, she had a
florid delirium that responded rapidly to alprazolam. The
benzodiazepines are the prototypical agents for the treat-
ment of alcohol and sedative withdrawal. They are safe in
large doses, prevent seizures, and are well tolerated. An
important feature for ICU use is that they are available in
oral, parenteral, and sublingual formulations.
Medications given in the ICU frequently can produce sig-
nificant psychoactive effects. Table 18–2 lists a number of
drugs that have been established as having these effects.
Switching medications certainly is one of the easiest changes
to make in trying to manage a delirious patient.
Other causes such as hypoxia and hypoperfusion need to be
reversed before improvement from delirium can occur.
Delirium may be the first sign of an infectious process and
should suggest that possibility when no other cause is discov-
ered. For example, a postoperative patient who develops delir-
ium without any identifiable cause may be suffering from a
wound abscess that only becomes apparent several days later.
B. Nonpharmacologic Management—Once one or more
causes are identified, the primary goal of treatment is to
reverse the disorder. In a significant number of cases of delir-
ium, no specific cause can be identified; in others, the identi-
fied cause cannot be reversed easily (eg, an asthmatic on
high-dose corticosteroids). In these instances, symptomatic
treatment may be required. Family may be enlisted to spend
time at the bedside to provide an orienting stimulus. Other
environmental measures may include providing a clock, cal-
endar, and soft music. Staff should attempt to provide ade-
quate day-night orientation and allow the patient to sleep at
night as much as possible. Four-hour periods that allow for
all stages of sleep may be the most beneficial.
C. Pharmacologic Management—For many patients with
delirium, the aforementioned measures will not be enough.
Medications become indicated when behavioral control is
necessary or when distress is severe. For example, a quietly
delirious patient suffering from frightening hallucinations is
a candidate for pharmacotherapy.
1. Management of withdrawal syndromes—A number
of principles must be considered in initiating therapy. If a
withdrawal state is present, the appropriate agent to cover the
withdrawal should be given as soon as possible. Sufficient
medication must be given to abort the withdrawal process
and keep it from reemerging. Alcohol withdrawal is the most
common of these conditions. A frequent mistake is to under-
dose with a benzodiazepine early in the withdrawal process
and then try to catch up with a full-blown delirium later.
Early substantial doses of long-acting agents such as
Drug or alcohol withdrawal or intoxication
Medication effects (eg, corticosteroids, cimetidine, lidocaine)
Hypoxia
Hypoperfusion
Infections (eg, systemic or central nervous system, HIV)
Structural lesions (eg, subdural hematomas)
Metabolic disorders (eg, electrolyte abnormalities, hypercalcemia,
hyperglycemia)
Ictal or postictal states
Postoperative states
Fat emboli
Dehydration
Sleep deprivation
Environmental stress
Pulmonary embolism
Table 18–1. Common causes of delirium in the ICU.
Acyclovir Fluoroquinolone antibiotics
Amiodarone Ganciclovir
Amphetamines Histamine H
2
blockers
Amphotericin B Interferon alfa
Anticonvulsants Isoniazid
Antidepressants Ketamine
Antihistamines Ketonazole
Atropine and other Levodopa
anticholinergics Lidocaine
Barbiturates Methylphenidate
Benzodiazepines Metoclopramide
Beta-adrenergic blockers Metronidazole
Cimetidine Nonsteroidal anti-inflammatory drugs
Corticosteroids Opioids
Cycloserine Procaine derivatives
Cyclosporine Propafenone
Digitalis glycosides Quinidine
Dronabinol Trimethoprim-sulfamethoxazole
Epoetin alfa (erythropoietin)
Modified from: Drugs that cause psychiatric symptoms. Med Lett
Drugs Ther 1988;40:21–24.
Table 18–2. Drugs that may cause delirium in the ICU.

PSYCHIATRIC PROBLEMS
435
diazepam may provide timely control. However, lorazepam
may be preferred in patients with liver disease because its
metabolism (via glucuronide conjugation) is less affected in
such individuals.
2. Management of delirium of unknown cause—
a. Benzodiazepines—When the cause of delirium is not
known but withdrawal from sedatives or alcohol is suspected,
one might consider a pharmacologic probe by giving a ben-
zodiazepine such as 1–4 mg lorazepam. If the patient’s condi-
tion worsens, that essentially rules out alcohol and most
benzodiazepine withdrawals. A different course of pharma-
cotherapy then must be pursued. In addition, these agents are
preferred for the agitation present with anticholinergic over-
dose or when there is a need to raise the seizure threshold.
Relatively short-acting agents with no active metabolites, such
as lorazepam, are preferred for this application.
b. Haloperidol alone or with lorazepam—Haloperidol is
often administered intravenously even though the intravenous
form is not approved by the Food and Drug Administration
(FDA). Before initiating haloperidol, some cardiovascular con-
siderations must be addressed (discussed below). Initial dosage
depends on the age and size of the patient. A small elderly
woman may benefit from 0.2 to 0.5 mg haloperidol every
4 hours. A younger 70-kg man may start with 1–2 mg every
2 hours or even higher doses. A more agitated patient should
receive larger doses, and some studies have advocated an initial
infusion of 5–10 mg followed by a continuous infusion at a rate
of 5–10 mg/h. This medication has a mean distribution time of
11 minutes and a half-life of 14–17 hours. If the first dose fails
to produce significant improvement, the dose should be
repeated at 30-minute intervals to allow time for distribution
to occur. The second dose is usually twice the first dose. If
monotherapy fails to produce the desired effect, one may add
0.5–1 mg lorazepam. This combination has been found to
improve symptom control while decreasing the side effects of
treatment. If the patient remains agitated 30 minutes follow-
ing the addition of lorazepam, one may give doses of
haloperidol up to 5 mg and lorazepam 0.5–2 mg at 30-minute
intervals until control is established. In many instances, con-
trol of delirium can be achieved with 5–30 mg haloperidol
and 2–4 mg lorazepam. Some patients may require amounts
substantially above these levels. Doses up to 975 mg haloperi-
dol in a 24-hour period have been reported.
Once control is established, approximately half the first
24-hour total dosage can be given on the following day in
equally divided doses. Each subsequent day’s dosing is
reduced by half until further dosing is no longer necessary.
The goal, once symptom control is achieved, is to taper the
patient to the minimally required dose. Many cases of delir-
ium will clear within a few days, particularly when the cause
has been reversed.
When giving intravenous haloperidol, several points
need to be considered. Generally speaking, intravenous
haloperidol imposes a much lower risk of extrapyramidal
side effects than oral or intramuscular forms. The reason is
not completely clear. It may be that the intravenous route
allows brain receptors to bind with different forms of the drug.
It is possible also that most patients who receive intravenous
haloperidol have some form of central anticholinergic process
by virtue of their delirium that provides a protective effect
against extrapyramidal reactions. Nonetheless, when giving
haloperidol, the clinician should continue to monitor the
patient for adverse reactions, which include dystonias, akathisia,
and neuroleptic malignant syndrome.
The incidence of cardiovascular side effects from
haloperidol is very low. Haloperidol has been found to pro-
long the QT interval and has been linked with rare episodes
of torsade de pointes, ventricular fibrillation, and sudden
death. These effects generally seem to occur with high doses
of the medication and have led to recommendations for
establishing a baseline ECG and subsequent monitoring of
the QT interval. Additional recommendations are to monitor
serum magnesium and potassium. Most cases of hypoten-
sion with haloperidol have been in association with hypov-
olemia. Administering the medication in a slow infusion over
5–10 minutes may be helpful in preventing hypotension, so
intravenous pushes should be avoided.
c. Newer agents—Newer agents such as the atypical
antipsychotics (including risperidone, quetiapine, and olan-
zapine) are receiving increased attention in the treatment of
delirium. They have fewer neurologic side effects (eg,
extrapyramidal symptoms) than the typical antipsychotics
(eg, haloperidol), particularly in elderly and seriously med-
ically ill individuals who are most at risk for delirium. In one
prospective, double-blind trial, haloperidol and risperidone
were equally efficacious in the treatment of delirious
patients. In a small open-label trial, low doses of risperidone
(average maintenance dose of 0.75 mg/day) improved cogni-
tive and behavioral symptoms of delirium. Olanzapine was
compared with haloperidol in a randomized, prospective
trial. While both groups demonstrated similar clinical
improvement and a lessened need for benzodiazepine seda-
tion, 6 of 45 patients treated with haloperidol were found to
have extrapyramidal symptoms, whereas no patients treated
with olanzapine developed extrapyramidal or other side
effects. Quetiapine has been found, in small studies, to be
effective for the treatment of delirium and well tolerated
when used at low doses. One drawback to atypical neurolep-
tic use is that only olanzapine and ziprasidone are currently
available in immediate-release parenteral formulations.
d. Other pharmacologic interventions—When the combi-
nation of haloperidol (or another antipsychotic) and
lorazepam fails to achieve control, additional interventions
may be necessary, including sedation (with opioids, propo-
fol, barbiturates, or benzodiazepines), pharmacologic paral-
ysis, and mechanical ventilation. These measures, however,
do not enhance the patient’s sense of control, and if sedation
is inadequate, they can be quite terrifying to the patient.
A newer agent for sedation and analgesia in the critical
care patient is dexmedetomidine, an α
2
-agonist. Reported
advantages of this medication are minimal interference with

CHAPTER 18
436
respiration and the ability for patients to be aroused easily. It
is currently approved for infusions up to 24 hours in adults.
D. Social and Psychologic Management—A key consider-
ation in managing delirium involves the psychological
aspects. The patient’s family often has major concerns about
the prognosis for mental recovery. The physician should
reassure both the family and the patient that the condition is
usually reversible and that return to baseline mental func-
tioning can be expected. Empathically telling the patient that
the physician understands the confusion the patient feels
may convey a real sense of hope. Encouraging the patient to
report any strange phenomena such as hallucinations may
make the patient feel more at ease. Similarly, informing the
family that accusations and delusional ideas brought forth
during an episode of delirium have no real meaning is obvi-
ously useful.
Current Controversies and Unresolved Issues
Delirium is such a heterogeneous entity that many issues
remain to be delineated by prospective studies. Molecular
mechanisms for delirium and the role of neurotransmitters
need to be established. We still do not know what the optimal
medications are for controlling delirium, the maximum daily
dosage of haloperidol and other agents, and whether patients
really benefit from large doses of medications or if the prac-
tice helps the staff more than the patient. The use of atypical
antipsychotic medications in the elderly also requires further
study because the available information suggests that use of
at least some of these agents in elderly patients with demen-
tia may be associated with an increased risk of cerebrovascu-
lar adverse events. Other questions relate to possible
preventive measures, whether psychological factors alone can
cause delirium in the absence of organic factors, and a possi-
ble final common pathway for all cases of delirium.
Depression
ESSENTIALS OF DIAGNOSIS
Depressed mood.
Feelings of worthlessness and inappropriate guilt.
Negative thinking.
Recurrent thoughts or wishes for death or suicide.
General Considerations
Depression occurs in 20–42% of the medically ill. Any
patient with a medical illness severe enough to require
admission to the ICU faces a number of psychological issues,
including real and potential losses. For example, the patient
with myocardial infarction must deal with the threat to life as
well as the potential loss of future health and normal function.
These losses are enough to produce depressive symptoms in
many patients and sad feelings in most patients. An individual
undergoing major surgery must deal with the loss of bodily
integrity and the threat of death. These actual and potential
losses may produce a sense of helplessness in many patients.
Patients are usually placed in unfamiliar surroundings
with little sense of autonomy. These conditions may generate
a depressive view of the world. Cognitive distortions can
develop so that the individual begins to make mistakes in
judgment such as forecasting catastrophe or interpreting
innocuous events negatively. Patients may begin to think of
themselves as worthless and feel they are a burden to family
and friends. Motivation to participate in medical care may be
impaired. In an interesting study, healthy volunteers were
admitted to an ICU to investigate the psychological changes
associated with that environment. Subjects developed
decreased vigor as well as increased confusion and fatigue
that were attributed solely to the ICU environment. The sub-
jects also were found to engage in introspection and to have
a negative view of the hospital environment. In another
study of patients undergoing coronary artery bypass graft-
ing, those with longer stays in the ICU were at risk for greater
levels of depressive symptoms postoperatively.
This depressive picture is often associated with biologic
changes. Neurotransmitter levels and receptor function
change significantly. Serotonin and noradrenergic systems
have substantial alterations. Endocrine dysfunction in both
the pituitary-adrenal and thyroid axes may become dis-
turbed. Even immune function may be altered.
Clinical Features
Patients in the ICU who have clinically significant depression
can by assessed by general criteria for depression. These cri-
teria, however, have only limited usefulness in the ICU
patient because of the context. For example, neurovegetative
symptoms are of limited value; many ICU patients have
appetite and sleep disturbances, but that does not mean they
are depressed.
Nonetheless, some signs and symptoms of depression in
ICU patients can be used to make a diagnosis of depression.
The presence of a depressed or lowered mood is a hallmark.
Irritability, particularly when out of character for an individ-
ual, is another marker of depression. Diminished interest in
activities such as interacting with family or viewing televi-
sion often indicates depression. Pronounced thoughts about
death or wishes for it in the form of suicidal ideation also
indicate depression.
Additional criteria can be used in assessing a patient for
depression. One group of factors involves the depressive view
of the world a depressed patient develops. For example, see-
ing oneself, one’s doctor, and one’s future in negative terms
correlates positively with a diagnosis of depression. Feelings
of guilt, worthlessness, and hopelessness are also common in
depression. Feeling helpless means the individual feels
unable to help himself or herself get better; feeling hopeless
means the individual does not believe anyone else can help
