Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.


454 Marina Ruths and Jacob N. Israelachvili
smooth and chemically homogenous surfaces supported by perfectly elastic materi-
als. It can be responsiblefor such phenomena as rolling friction and elastoplastic ad-
hesive contacts [239,263–266] during loading–unloadingand adhesion–decohesion
cycles.
Adhesion hysteresis may be thought of as being due to mechanical effects such
as instabilities, or chemical effects such as interdiffusion, interdigitation, molecular
reorientations and exchange processes occurring at an interface after contact, as il-
lustrated in Fig. 9.12. Such processes induce roughness and chemical heterogeneity
even though initially (and after separation and re-equilibration) both surfaces are
perfectly smooth and chemically homogeneous. In general, if the energy change, or
work done, on separating two surfaces from adhesive contact is not fully recover-
able on bringing the two surfaces back into contact again, the adhesion hysteresis
Fig. 9.12. (a) Schematic representation of interpenetrating chains. (b)and(c): JKR plots
(contact radius r as a function of applied load L) showing small adhesion hysteresis for un-
crosslinked polystyrene and larger adhesion hysteresis after chain scission at the surfaces
after 18 h irradiation with ultraviolet light in an oxygen atmosphere. The adhesion hystere-
sis continues to increase with the irradiation time. (d) Rate-dependent adhesion of hexadecyl
trimethyl ammonium bromide (CTAB) surfactant monolayers. The solid curves [259] are fits
to experimental data on CTAB adhesion after different contact times [260] using an approxi-
mate analytical solution for a JKR model, including crack tip dissipation. Due to the limited
range of validity of the approximation, the fits rely on the part of the experimental data with
low effective adhesion energy only. From the fits one can determine the thermodynamic ad-
hesion energy, the characteristic dissipation velocity, and the intrinsic dissipation exponent
of the model. ((a) after [261]. Copyright 1993 American Chemical Society; (b)and(c)af-
ter [262]. Copyright 2002 American Association for the Advancement of Science; (d)af-
ter [259]. Copyright 2000 American Chemical Society)
9 Surface Forces and Nanorheology of Molecularly Thin Films 455
may be expressed as
W
R
> W
A
Receding Advancing
or
ΔW = (W
R
−W
A
) > 0 , (9.24)
where W
R
and W
A
are the adhesion or surfaceenergies for receding (separating)and
advancing (approaching) two solid surfaces, respectively.
Hysteresis effects are also commonly observed in wetting/dewetting phenom-
ena [267]. For example, when a liquid spreads and then retracts from a surface the
advancing contact angle θ
A
is generally larger than the receding angle θ
R
. Since the
contact angle, θ, is related to the liquid–vapor surface tension, γ
L
, and the solid–
liquid adhesion energy, W, by the Dupré equation,
(1+ cosθ)γ
L
= W , (9.25)
we see that wetting hysteresis or contact angle hysteresis (θ
A
>θ
R
) actually implies
adhesion hysteresis, W
R
> W
A
, as given by (9.24).
Energy-dissipatingprocessessuch as adhesion and contactangle hysteresis arise
becauseof practical constraintsof thefinite time ofmeasurements andthe finite elas-
ticity of materials. This prevents many loading–unloading or approach–separation
cycles from being thermodynamically reversible, even though they would be if car-
ried out infinitely slowly. By thermodynamically irreversible one simply means
that one cannot go through the approach–separation cycle via a continuous series
of equilibrium states, because some of these are connected via spontaneous – and
therefore thermodynamically irreversible – instabilities or transitions where energy
is liberated and therefore “lost” via heat or phonon release [268]. This is an area of
much current interest and activity, especially regarding the fundamental molecular
origins of adhesion and friction in polymer and surfactant systems, and the relation-
ships between them [239,259,260,262,264,269–272].
9.6 Introduction: Different Modes of Friction and the Limits
of Continuum Models
Most frictional processes occur with the sliding surfaces becoming damaged in one
form or another [263]. This may be referred to as “normal” friction. In the case of
brittle materials, the damaged surfaces slide past each other while separated by rela-
tively large, micron-sized wear particles. With more ductile surfaces, the damage re-
mains localized to nanometer-sized, plastically deformed asperities. Some features
of the friction between damaged surfaces will be described in Sect. 9.7.4.
There are also situations in which sliding can occur between two perfectly
smooth, undamaged surfaces. This may be referred to as “interfacial” sliding or
456 Marina Ruths and Jacob N. Israelachvili
“boundary” friction and is the focus of the following sections. The term “boundary
lubrication” is more commonly used to denote the friction of surfaces that contain
a thin protective lubricating layer such as a surfactant monolayer, but here we shall
use the term more broadly to include any molecularly thin solid, liquid, surfactant,
or polymer film.
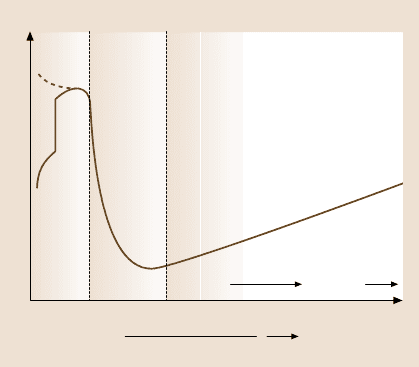
Experiments have shown that, as a liquid film becomes progressively thin-
ner, its physical properties change, at first quantitatively and then qualitatively
[44, 47, 273, 274, 276, 277]. The quantitative changes are manifested by an in-
creased viscosity, non-Newtonian flow behavior, and the replacement of nor-
mal melting by a glass transition, but the film remains recognizable as a liquid
(Fig. 9.13). In tribology, this regime is commonly known as the “mixed lubrica-
tion” regime, where the rheological properties of a film are intermediate between
the bulk and boundary properties. One may also refer to it as the “intermediate”
regime (Table 9.4).
For even thinner films, the changes in behavior are more dramatic, resulting in
a qualitative change in properties. Thus first-order phase transitions can now occur
to solid or liquid-crystalline phases [46,255, 261,275,278–281], whose properties
can no longer be characterized even qualitatively in terms of bulk or continuum
Velocity u Viscosity
Load
Bound-
ary
Inter-
mediate
(mixed)
Thick film (EHD)
Friction force
~1 nm 2–5 nm ~10 nm ~10 Pm
Fig. 9.13. Stribeck curve: an empirical curve giving the trend generally observed in the fric-
tion forces or friction coefficients as a function of sliding velocity, the bulk viscosity of the
lubricating fluid, and the applied load (normal force). The three friction/lubrication regimes
are known as the boundary lubrication regime (see Sect. 9.7), the intermediate or mixed lubri-
cation regime (Sect. 9.8.2), and thick film or elasto-hydrodynamic (EHD) lubrication regime
(Sect. 9.8.1). The film thicknesses believed to correspond to each of these regimes are also
shown. For thick films, the friction force is purely viscous, e.g., Couette flow at low shear
rates, but may become complicated at higher shear rates where EHD deformations of surfaces
can occur during sliding (after [1] with permission)

9 Surface Forces and Nanorheology of Molecularly Thin Films 457
Table 9.4. The three main tribological regimes characterizing the changing properties of li-
quids subjected to increasing confinement between two solid surfaces
a
Regime Conditionsforgetting
into this regime
Static/equilibrium
properties
b
Dynamic properties
c
Bulk
• Thick films (> 10
molecular diameters,
R
g
for polymers)
• Low or zero loads
• High shear rates
Bulk (continuum)
properties:
• Bulk liquid density
• No long-range order
Bulk (continuum)
properties:
• Newtonian viscosity
• Fast relaxation times
• No glass temperature
• No yield point
• Elastohydrodynamic
lubrication
Intermediate
mixed
• Intermediately thick
films(4–10
molecular diameters,
∼ R
g
for polymers)
• Low loads or
pressure
Modified fluid
properties include:
• Modified positional
and orientational order
a
• Medium- to long-
range molecular
correlations
• Highly entangled
states
Modified rheological
properties include:
• Non-Newtonian flow
• Glassy states
• Long relaxation times
• Mixed lubrication
Boundary
• Molecularly thin
films (< 4 molecular
diameters)
• High loads or
pressure
• Low shear rates
• Smooth surfaces or
asperities
Onset of non-fluidlike
properties:
• Liquid-like to solid-
like phase transitions
• Appearance of new
liquid-crystalline
states
• Epitaxially induced
long-range ordering
Onset of tribological
properties:
• No flow until yield
point or critical shear
stress reached
• Solid-like film behav-
ior characterized
by defect diffusion,
dislocation motion,
shear melting
• Boundary lubrication
BasedonworkbyGranick [273], Hu and Granick [274], and others [38,261, 275] on the
dynamic properties of short chain molecules such as alkanes and polymer melts confined
between surfaces
a
Confinement can lead to an increased or decreased order in afilm, depending both on the
surface lattice structure and the geometry of the confining cavity
b
In each regime both the static and dynamic properties change. The static properties include
the film density, the density distribution function, the potential of mean force, and various
positional and orientational order parameters
c
Dynamic properties include viscosity, viscoelastic constants, and tribological yield points
such as the friction coefficient and critical shear stress
458 Marina Ruths and Jacob N. Israelachvili
liquid properties such as viscosity. These films now exhibit yield points (character-
istic of fracture in solids) and their molecular diffusion and relaxation times can be
ten orders of magnitude longer than in the bulk liquid or even in films that are just
slightly thicker. The three friction regimes are summarized in Table 9.4.
9.7 Relationship Between Adhesion and Friction Between Dry
(Unlubricated and Solid Boundary Lubricated) Surfaces
9.7.1 Amontons’ Law and Deviations from It Due to Adhesion:
The Cobblestone Model
Early theories and mechanisms for the dependence of friction on the applied normal
force or load, L, were developed by da Vinci, Amontons, Coulomb and Euler [282].
For the macroscopic objects investigated, the friction was found to be directly pro-
portional to the load, with no dependence on the contact area. This is described by
the so-called Amontons’ law:
F = μL , (9.26)
where F is the shear or friction force and μ is a constant defined as the coefficient of
friction.This friction law has a broad range of applicability and is still the principal
means of quantitatively describing the friction between surfaces. However, particu-
larly in the case of adhering surfaces, Amontons’ law does not adequately describe
the friction behavior with load, because of the finite friction force measured at zero
and even negative applied loads.
When a lateral force, or shear stress, is applied to two surfaces in adhesive con-
tact, the surfaces initially remain “pinned” to each other until some critical shear
force is reached. At this point, the surfaces begin to slide past each other either
smoothly or in jerks. The frictional force needed to initiate sliding from rest is
known as the static friction force, denoted by F
s
, while the force needed to main-
tain smooth sliding is referred to as the kinetic or dynamic friction force, denoted
by F
k
. In general, F
s
> F
k
. Two sliding surfaces may also move in regular jerks,
known as stick–slip sliding, which is discussed in more detail in Sect. 9.8.3. Such
friction forces cannot be described by models used for thick films that are viscous
(see Sect. 9.8.1) and, therefore, shear as soon as the smallest shear force is applied.
In Sects. 9.7 and 9.8 we will be concerned mainly with single-asperity contacts.
Experimentally, it has been found that during both smooth and stick–slip sliding at
small film thicknesses the local geometry of the contact zone remains largely un-
changed from the static geometry [45]. In an adhesive contact, the contact area as
a function of load is thus generally well described by the JKR equation, (9.22). The
friction force between two molecularly smooth surfaces sliding in adhesive contact
is not simply proportional to the applied load, L, as might be expected from Amon-
tons’ law. There is an additional adhesion contribution that is proportional to the
area of contact, A. Thus, in general, the interfacial friction force of dry, unlubricated

9 Surface Forces and Nanorheology of Molecularly Thin Films 459
surfaces sliding smoothly past each other in adhesive contact is given by
F = F
k
= S
c
A+ μL , (9.27)
where S
c
is the “critical shear stress” (assumed to be constant), A = πr
2
is the contact
area of radius r given by (9.22), and μ is the coefficient of friction. For low loads we
have:
F = S
c
A = S
c
πr
2
= S
c
π
R
K
L+ 6πRγ +
!
12πRγL+
(
6πRγ
)
2
2/3
;
(9.28)
whereas for high loads (or high μ), or when γ is very low [283–287], (9.27) reduces
to Amontons’ law: F = μL. Depending on whether the friction force in (9.27) is
dominated by the first or second term, one may refer to the friction as adhesion-
controlled or load-controlled, respectively.
The following friction model, first proposed by Tabor [288] and developed fur-
ther by Sutcliffe et al. [289], McClelland [290], and Homola et al. [45], has been
quite successful at explaining the interfacial and boundary friction of two solid
crystalline surfaces sliding past each other in the absence of wear. The surfaces
may be unlubricated, or they may be separated by a monolayer or more of some
boundarylubricant or liquid molecules. In this model, the values of the critical shear
stress S
c
, and the coefficient of friction μ, in (9.27) are calculated in terms of the
energy needed to overcomethe attractive intermolecularforces and compressive ex-
ternally applied load as one surface is raised and then slid across the molecular-sized
asperities of the other.
This model (variously referred to as the interlocking asperity model, Coulomb
friction,orthecobblestone model) is similar to pushing a cart over a road of cob-
blestones where the cartwheels (which represent the molecules of the upper surface
or film) must be made to roll over the cobblestones (representing the molecules of
the lower surface) before the cart can move. In the case of the cart, the downward
force of gravity replaces the attractive intermolecular forces between two material
surfaces. When at rest, the cartwheels find grooves between the cobblestones where
they sit in potential-energy minima, and so the cart is at some stable mechanical
equilibrium. A certain lateral force (the “push”) is required to raise the cartwheels
against the force of gravity in order to initiate motion. Motion will continue as long
as the cart is pushed, and rapidly stops once it is no longer pushed. Energy is dis-
sipated by the liberation of heat (phonons, acoustic waves, etc.) every time a wheel
hits the next cobblestone. The cobblestone model is not unlike the Coulomb and
interlocking asperity models of friction [282] except that it is being applied at the
molecular levelandfor a situation wherethe externalload is augmented by attractive
intermolecular forces.
There are thus two contributions to the force pulling two surfaces together:
the externally applied load or pressure, and the (internal) attractive intermolecular
forces that determine the adhesion between the two surfaces. Each of these contri-
butions affects the friction force in a different way, which we will discuss in more
detail below.

460 Marina Ruths and Jacob N. Israelachvili
9.7.2 Adhesion Force and Load Contribution to Interfacial Friction
Adhesion Force Contribution
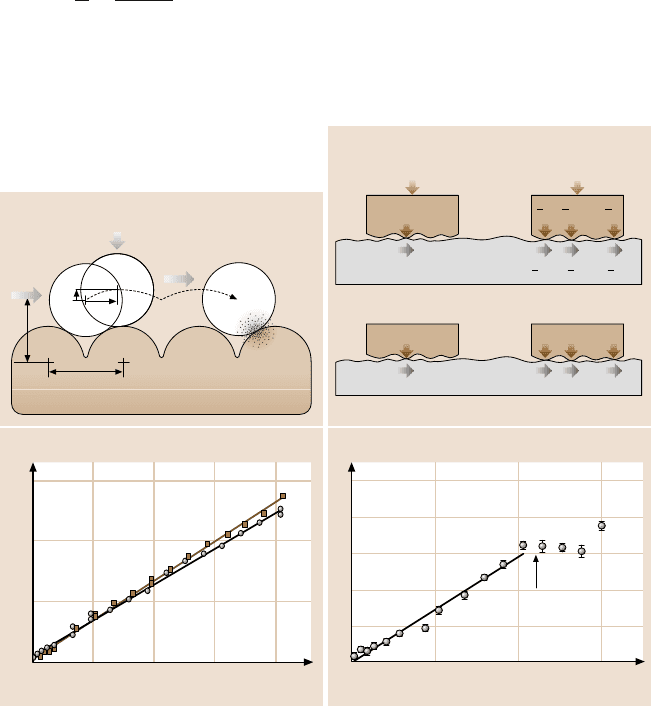
Consider the case of two surfaces sliding past each other, as shown in Fig. 9.14a.
When the two surfaces are initially in adhesive contact, the surface molecules will
adjust themselves to fit snugly together [291], in an analogous manner to the self-
positioning of the cartwheels on the cobblestone road. A small tangential force ap-
plied to one surface will therefore not result in the sliding of that surface relative
to the other. The attractive van der Waals forces between the surfaces must first be
overcome by having the surfaces separate by a small amount. To initiate motion, let
the separation between the two surfaces increase by a small amount ΔD, while the
lateral distance moved is Δσ. These two values will be related via the geometry of
the two surface lattices. The energy put into the system by the force F acting over
a lateral distance Δσ is
Input energy: F ×Δσ. (9.29)
Fig. 9.14. (a) Schematic illustration of how one molecularly smooth surface moves over an-
other when a lateral force F is applied (the “cobblestone model”). As the upper surface moves
laterally by some fraction of the lattice dimension Δσ, it must also move up by some frac-
tion of an atomic or molecular dimension ΔD before it can slide across the lower surface. On
impact, some fraction ε of the kinetic energy is “transmitted” to the lower surface, the rest be-
ing “reflected” back to the colliding molecule (upper surface) (after [292], with permission).
(b)Difference in the local distribution of the total applied external load or normal adhesive
force between load-controlled non-adhering surfaces and adhesion-controlled surfaces. In the
former case, the total friction force F is given eitherby F = μL for one contact point (leftside)
or by F =
1
3
μL+
1
3
μL+
1
3
μL = μL for three contact points (right side). Thus the load-controlled
friction is always proportional to the applied load, independently of the number of contacts
and of their geometry. In the case of adhering surfaces, the effective “internal” load is given by
kA,whereA is the real local contact area, which is proportional to the number of intermolec-
ular bonds being made and broken across each single contact point. The total friction force is
now given by F = μkA for one contact point (left side), and F = μ(kA
1
+ kA
2
+ kA
3
) = μkA
tot
for three contact points (right side). Thus, for adhesion-controlled friction, the friction is
proportional to the real contact area, at least when no additional external load is applied
to the system (after [287], with permission; copyright 2004 American Chemical Society).
(c),(d) Friction force between benzyltrichlorosilane monolayers chemically bound to glass
or Si, measured in ethanol (γ<1mJ/m
2
). (c) SFA measurements where both glass sur-
faces were covered with a monolayer. Circles and squares show two different experiments:
one with R = 2.6cm, v = 0.15 µm/s, giving μ = 0.33 ±0.01; the other with R = 1.6cm,
v = 0.5 µm/s, giving μ = 0.30±0.01. (d) Friction force microscopy (FFM) measurements of
a monolayer-functionalized Si tip (R = 11 nm) sliding on a monolayer-covered glass surface
at v = 0.15 µm/s, giving μ = 0.30±0.01. Note the different scales in (c)and(d) (after [286],
with permission; copyright 2003 American Chemical Society)
9 Surface Forces and Nanorheology of Molecularly Thin Films 461
This energy may be equated with the change in interfacial or surface energy as-
sociated with separating the surfaces by ΔD, i.e., from the equilibrium separation
D = D
0
to D = (D
0
+ΔD). Since γ ∝ D
−2
for two flat surfaces (cf. Sect. 9.3.1 and
Table 9.2), the energy cost may be approximated by:
Surface energy change×area: 2γA
1−D
2
0
/(D
0
+ΔD)
2
≈ 4γA(ΔD/D
0
) , (9.30)
where γ is the surface energy, A the contact area, and D
0
the surface separation at
equilibrium. During steady-state sliding (kinetic friction), not all of this energy will
be “lost” or absorbed by the lattice every time the surface molecules move by one
lattice spacing: some fractionwill be reflected during each impactof the “cartwheel”
molecules [290]. Assuming that a fraction ε of the above surface energy is “lost”
every time the surfaces move across the characteristic length Δσ (Fig. 9.14a), we
obtain after equating (9.29) and (9.30)
S
c
=
F
A
=
4γεΔD
D
0
Δσ
. (9.31)
For a typical hydrocarbon or a van der Waals surface, γ ≈ 25mJm
−2
. Other typical
values would be: ΔD ≈ 0.05nm, D
0
≈ 0.2nm,Δσ ≈0.1nm,andε ≈ 0.1−0.5. Using
Impact
Upper layer (n + 1)
F
S
L
Lower layer (n)
D
F
k
Δσ
ΔD
σ
a)
b)
Load-controlled friction
L
PL
1
3
LL
1
3
L
1
3
L
1
3
PL
1
3
PL
1
3
PL
PkA
kA
1
kA
PkA
1
PkA
2
PkA
3
kA
2
kA
3
L
Adhesion-controlled friction
Single contact Multiple contacts
Friction force F (mN)
Load L (mN)
0
20
30
20
10
0
40 60 80
c)
SFA
Friction force F (nN)
Load L (nN)
0
5
5
4
3
2
1
0
10 15
d)
FFM
Monolayer
transition

462 Marina Ruths and Jacob N. Israelachvili
the aboveparameters,(9.31)predicts S
c
≈(2.5−12.5)×10
7
Nm
−2
for vander Waals
surfaces. This range of values compares very well with typical experimental values
of 2×10
7
Nm
−2
for hydrocarbon or mica surfaces sliding in air (see Fig. 9.16) or
separated by one molecular layer of cyclohexane [45].
The above model suggests that all interfaces, whether dry or lubricated, dilate
just before they shear or slip. This is a small but important effect: the dilation pro-
vides the crucial extra space needed for the molecules to slide across each other
or flow. This dilation is known to occur in macroscopic systems [293,294] and for
nanoscopic systems it has been computed by Thompson and Robbins [255] and Za-
loj et al. [295] and measured by Dhinojwala et al. [296].
This model may be extended, at least semiquantitatively, to lubricated sliding,
where a thin liquid film is present between the surfaces. With an increase in the
number of liquid layers between the surfaces, D
0
increases while ΔD decreases,
hence the friction force decreases. This is precisely what is observed,but with more
than one liquid layer between two surfaces the situation becomes too complex to
analyze analytically (actually, even with one or no interfacial layers, the calcula-
tion of the fraction of energy dissipated per molecular collision ε is not a simple
matter). Furthermore, even in systems as simple as linear alkanes, interdigitation
and interdiffusion have been found to contribute strongly to the properties of the
system [143,297]. Sophisticated modeling based on computer simulations is now
required, as discussed in the following section.
Relation Between Boundary Friction and Adhesion Energy Hysteresis
While the above equations suggest that there is a direct correlation between friction
and adhesion, this is not the case. The correlation is really between friction and ad-
hesion hysteresis, described in Sect. 9.5.4. In the case of friction, this subtle point is
hidden in the factor ε, which is a measure of the amount of energy absorbed (dissi-
pated, transferred, or “lost”) by the lower surface when it is impacted by a molecule
from the upper surface. If ε = 0, all the energy is reflected, and there will be no
kinetic friction force or any adhesion hysteresis, but the absolute magnitude of the
adhesion force or energy will remain finite and unchanged. This is illustrated in
Figs. 9.17 and 9.19.
The following simple model shows how adhesion hysteresis and friction may
be quantitatively related. Let Δγ = γ
R
−γ
A
be the adhesion energy hysteresis per
unit area, as measured during a typical loading–unloading cycle (see Figs. 9.17a
and 9.19c,d). Now consider the same two surfaces sliding past each other and as-
sume that frictional energy dissipation occursthrough the same mechanism as adhe-
sion energy dissipation, and that both occur over the same characteristic molecular
length scale σ. Thus, when the two surfaces (of contact area A = πr
2
)moveadis-
tance σ, equating the frictional energy (F ×σ) to the dissipated adhesion energy
(A×Δγ), we obtain
Friction force: F=
A×Δγ
σ
=
πr
2
σ
(
γ
R
−γ
A
)
, (9.32)
or Critical shear stress: S
c
= F/A =Δγ/σ , (9.33)

9 Surface Forces and Nanorheology of Molecularly Thin Films 463
which is the desired expression and has been found to give order-of-magnitude
agreement between measured friction forces and adhesion energy hysteresis [261].
If we equate (9.33) with (9.31), since 4ΔD/(D
0
Δσ) ≈ 1/σ, we obtain the intuitive
relation
ε =
Δγ
γ
. (9.34)
External Load Contribution to Interfacial Friction
When there is no interfacial adhesion, S
c
is zero. Thus, in the absence of any adhe-
sive forces between two surfaces, the only “attractive” force that needs to be over-
come for sliding to occur is the externally applied load or pressure, as shown in
Fig. 9.14b.
For a preliminary discussion of this question, it is instructive to compare the
magnitudes of the externally applied pressure to the internal van der Waals pressure
between two smooth surfaces. The internal van der Waals pressure between two flat
surfaces is given (see Table 9.2) by P = A
H
/6πD
3
0
≈ 1GPa(10
4
atm), using a typical
Hamaker constant of A
H
= 10
−19
J, and assuming D
0
≈ 2nm for the equilibrium
interatomic spacing. This implies that we should not expect the externally applied
load to affect the interfacialfriction force F, as defined by (9.27),until the externally
applied pressure L/A begins to exceed ∼ 100MPa (10
3
atm). This is in agreement
with experimentaldata [298] where the effect of load became dominant at pressures
in excess of 10
3
atm.
For a more general semiquantitative analysis, again consider the cobblestone
model used to derive (9.31), but now include an additional contribution to the
surface-energy change of (9.30) due to the work done against the external load or
pressure, LΔD = P
ext
AΔD (this is equivalent to the work done against gravity in the
case of a cart being pushed over cobblestones). Thus:
S
c
=
F
A
=
4γεΔD
D
0
Δσ
+
P
ext
εΔD
Δσ
, (9.35)
which gives the more general relation
S
c
= F/A = C
1
+ C
2
P
ext
, (9.36)
where P
ext
= L/A and C
1
and C
2
are characteristic of the surfaces and sliding con-
ditions. The constant C
1
= 4γεΔD/(D
0
Δσ) depends on the mutual adhesion of the
two surfaces, while both C
1
and C
2
= εΔD/Δσ depend on the topography or atomic
bumpiness of the surface groups (Fig. 9.14a). The smoother the surface groups the
smaller the ratio ΔD/Δσ and hence the lower the value of C
2
. In addition, both C
1
and C
2
depend on ε (the fraction of energy dissipated per collision), which depends
on the relative masses of the shearing molecules, the sliding velocity, the temper-
ature, and the characteristic molecular relaxation processes of the surfaces. This is
by far the most difficult parameter to compute, and yet it is the most important since
it represents the energy-transfermechanism in any friction process, and since ε can
