Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

444 Marina Ruths and Jacob N. Israelachvili
It is questionable whether the hydration or hydrophobic force should be viewed
as an ordinary type of solvation or structural force that reflects the packing of water
molecules. The energy (or entropy) associated with the hydrogen-bondingnetwork,
which extends over a much larger region of space than the molecular correlations,
is probably at the root of the long-range interactions of water. The situation in wa-
ter appears to be governed by much more than the molecular packing effects that
dominate the interactions in simpler liquids.
9.4.5 Polymer-Mediated Forces
Polymers or macromolecules are chain-like molecules consisting of many identical
segments (monomers or repeating units) held together by covalent bonds. The size
of a polymer coil in solution or in the melt is determined by a balance between van
der Waals attraction (and hydrogen bonding,if present) between polymer segments,
andtheentropyofmixing,whichcausesthepolymercoiltoexpand.Inpolymermelts
abovetheglasstransitiontemperature,andatcertainconditionsinsolution,theattrac-
tionbetweenpolymersegmentsisexactlybalancedbytheentropyeffect.Thepolymer
solution will thenbehavevirtuallyideally,and thedensitydistributionof segmentsin
the coil is Gaussian. This is called the theta (θ) condition, and it occurs at the theta
or Flory temperature for a particular combination of polymer and solvent or solvent
mixture. At lower temperatures (in a poor or bad solvent), the polymer–polymerin-
teractionsdominateovertheentropic,andthecoilwillshrinkorprecipitate.Athigher
temperatures (good solvent conditions), the polymer coil will be expanded.
High-molecular-weightpolymers form large coils, which significantly affect the
properties of a solution even when the total mass of polymer is very low. The radius
of the polymer coil is proportional to the segment length, a, and the number of seg-
ments, n. At thetaconditions,the hydrodynamicradiusof the polymer coil (theroot-
mean-square separation of the ends of one polymer chain) is theoretically given by
R
h
= an
1/2
, and the unperturbed radius of gyration (the average root-mean-square
distance of a segment from the center of mass of the molecule) is R
g
= a (n/6)
1/2
.
In a good solvent the perturbed size of the polymer coil, the Flory radius R
F
,is
proportional to n
3/5
.
Polymers interact with surfaces mainly through van der Waals and electrostatic
interactions. The physisorption of polymers containing only one type of segment
is reversible and highly dynamic, but the rate of exchange of adsorbed chains with
free chains in the solution is low, since the polymer remains bound to the surface
as long as one segment along the chain is adsorbed. The adsorption energy per seg-
ment is on the order of k
B
T. In a good solvent, the conformation of a polymer on
a surface is very different from the coil conformation in bulk solution. Polymers ad-
sorb in “trains”, separated by “loops” extending into solution and dangling “tails”
(the ends of the chain). Compared to adsorption at lower temperatures, good sol-
vent conditions favor more of the polymer chain being in the solvent, where it can
attain its optimum conformation. As a result, the extension of the polymer is longer,
even though the total amount of adsorbed polymer is lower. In a good solvent, the
9 Surface Forces and Nanorheology of Molecularly Thin Films 445
polymer chains can also be effectively repelled from a surface, if the loss in confor-
mational entropy close to the surface is not compensated for by a gain in enthalpy
from adsorption of segments. In this case, there will be a layer of solution (thick-
ness ≈R
g
) close to the surfaces that is depleted of polymer.
The interaction forces between two surfaces across a polymer solution will de-
pend on whetherthe polymer adsorbsonto the surfacesor is repelled fromthem, and
also on whether the interaction occurs at “true” or “restricted” thermodynamicequi-
librium. At true orfull equilibrium,the polymerbetweenthe surfacescan equilibrate
(exchange) with polymer in the bulk solution at all surface separations. Some theo-
ries [198,199]predict that, at full equilibrium,the polymer chains would movefrom
the confined gap into the bulk solution where they could attain entropically more fa-
vorable conformations, and that a monotonic attraction at all distances would result
from bridging and depletion interactions (which will be discussed below). Other
theories suggest that the interaction at small separations would be ultimately re-
pulsive, since some polymer chains would remain in the gap due to their attractive
interactions with many sites on the surface (enthalpic) – more sites would be avail-
able to the remaining polymer chains if some others desorbed and diffused out from
the gap [73,200–202].
At restricted equilibrium, the polymer is kinetically trapped, and the adsorbed
amount is thus constant as the surfaces are brought toward each other,but the chains
can still rearrange on the surfaces and in the gap. Experimentally, the true equilib-
rium situation is very difficult to attain, and most experiments are done at restricted
equilibriumconditions. Even the equilibrationof conformationsassumed in theoret-
ical models for restricted equilibrium conditions can be so slow that this condition
is difficult to reach experimentally.
In systems of adsorbing polymer, bridging of chains from one surface to the
other can give rise to a long-range attraction, since the bridging chains would gain
conformational entropy if the surfaces were closer together. In poor solvents, both
bridging and intersegment interactions contributeto an attraction [26]. However, re-
gardlessof solventand equilibrium conditions,a strong repulsiondue to the osmotic
interactions is seen at small surface separations in systems of adsorbingpolymers at
restricted equilibrium.
In systems containing high concentrations of non-adsorbing polymer, the dif-
ference in solute concentration in the bulk and between the surfaces at separations
smaller than the approximate polymer coil diameter (2R
g
, i.e., when the polymer
has been squeezed out from the gap between the surfaces) may give rise to an at-
tractive osmotic force (the “depletion attraction”) [207–212]. In addition, if the
polymer coils become initially compressed as the surfaces approach each other, this
can give rise to a repulsion (“depletion stabilization”)at large separations[210]. For
a system of two cylindrical surfaces or radius R, the maximumdepletion force, F
dep
,
is expected to occur when the surfaces are in contact and is given by multiplying the
446 Marina Ruths and Jacob N. Israelachvili
depletion (osmotic) pressure, P
dep
= ρk
B
T, by the contact area πr
2
,wherer is given
by the chord theorem: r
2
= (2R−R
g
)R
g
≈ 2RR
g
[3]:
F
dep
/R = −2πR
g
ρk
B
T , (9.19)
where ρ is the number density of the polymer in the bulk solution.
If a part of the polymer (typically an end group) is different from the rest of
the chain, this part may preferentially adsorb to the surface. End-adsorbed poly-
mer is attached to the surface at only one point, and the extension of the chain is
dependent on the grafting density, i.e., the average distance, s, between adsorbed
end groups on the surface (Fig. 9.8). One distinguishes between different regions of
increasing overlap of the chains (stretching) called pancake, mushroom, and brush
Force/Radius F/R (mN/m)
Distance D (nm)
0
150
10
2
10
1
10
–1
10
–2
50 100
Force/Radius F/R (mN/m)
Distance D (nm)
0
200
10
2
10
1
10
–1
10
–2
50 100 150
0.10
0.05
0
–0.05
050100 150 200 250 300
F/R
Time MW= 1.1 × 10
6
3h 8h > 24h
Distance D (nm)
}
R
F
MW = 140,000
MW = 26,000
2L
2L
MW = 1.1 × 10
6
MW = 1.6× 10
5
R
g
R
g
In Out In
a) b)
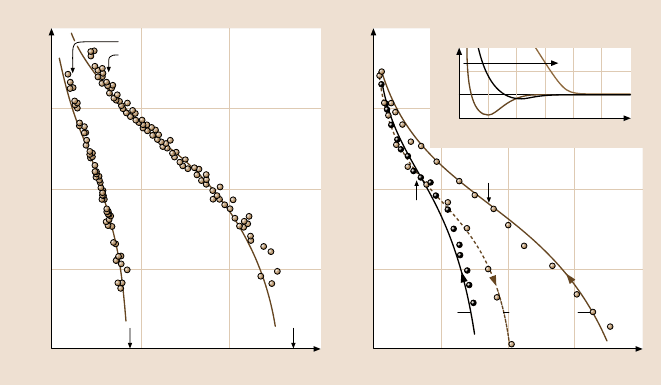
Fig. 9.8a,b. Experimentally determined forces in systems of two interacting polymer layers:
(a) Polystyrene brush layers grafted via an adsorbing chain-end group onto mica surfaces in
toluene (a good solvent for polystyrene). Left curve:MW= 26,000 g/mol, R
F
= 12 nm. Right
curve:MW= 140,000 g/mol, R
F
= 32 nm. Both force curves were reversible on approach
and separation. The solid curves are theoretical fits using the Alexander–de Gennes theory
with the following measured parameters: spacing between attachments sites: s = 8.5nm,
brush thickness: L = 22.5 nm and 65 nm, respectively. (adapted from [203]). (b) Polyethy-
lene oxide layers physisorbed onto mica from 150 µg/ml solution in aqueous 0.1 M KNO
3
(a good solvent for polyethylene oxide). Main figure: Equilibrium forces at full coverage
after ∼ 16 h adsorption time. Left curve:MW= 160,000 g/mol, R
g
= 32 nm. Right curve:
MW = 1,100,000 g/mol, R
g
= 86 nm. Note the hysteresis (irreversibility) on approach and
separation for this physisorbed layer, in contrast to the absence of hysteresis with grafted
chains in case (a). The solid curves are based on a modified form of the Alexander–de Gennes
theory. Inset in (b): evolution of the forces with the time allowed for the higher MW poly-
mer to adsorb from solution. Note the gradual reduction in the attractive bridging component.
(adapted from [204–206]; after [3]; copyright 1991, with permission from Elsevier Science)
9 Surface Forces and Nanorheology of Molecularly Thin Films 447
regimes [213]. In the mushroom regime, where the coverage is sufficiently low that
there is no overlap between neighboring chains, the thickness of the adsorbed layer
is proportional to n
1/2
(i.e., to R
g
) at theta conditions and to n
3/5
in a good solvent.
Several models [213–218] have been developed for the extension and interac-
tions between two brushes (strongly stretched grafted chains). They are based on
a balance between osmotic pressure within the brush layers (uncompressed and
compressed) and the elastic energy of the chains and differ mainly in the assump-
tions of the segment density profile, which can be a step function or parabolic. At
high coverage (in the brush regime), where the chains will avoid overlapping each
other, the thickness of the layer is proportional to n.
Experimentalworkon bothmonodisperse[27,28,203,219]andpolydisperse[30,
220] systems at different solvent conditions has confirmed the expected range
and magnitude of the repulsive interactions resulting from compression of densely
packed grafted layers.
9.4.6 Thermal Fluctuation Forces
If a surface is not rigid but very soft or even fluid-like, this can act to smear out
any oscillatory solvation force. This is because the thermal fluctuations of such in-
terfaces make them dynamically “rough” at any instant, even though they may be
perfectly smooth on a time average. The types of surfaces that fall into this category
are fluid-like amphiphilic surfaces of micelles, bilayers, emulsions, soap films, etc.,
but also solid colloidal particle surfaces that are coated with surfactant monolayers,
as occurs in lubricating oils, paints, toners, etc.
These thermal fluctuation forces (also called entropic or steric forces) are usu-
ally short range and repulsive and are very effective at stabilizing the attractive van
der Waals forces at some small but finite separation. This can reduce the adhesion
energy or force by up to three orders of magnitude. It is mainly for this reason that
fluid-like micelles and bilayers, biological membranes, emulsion droplets, or gas
bubbles adhere to each other only very weakly.
Because of their short range it was, and still is, commonly believed that these
forces arise from water orderingor “structuring”effects at surfaces,and that they re-
flect some unique or characteristic property of water. However, it is now known that
these repulsive forces also exist in other liquids [221, 222]. Moreover, they appear
to become stronger with increasing temperature, which is unlikely if the force orig-
inated from molecular ordering effects at surfaces. Recent experiments, theory, and
computer simulations [223–226] have shown that these repulsive forces have an en-
tropic origin arising from the osmotic repulsion between exposed thermally mobile
surface groups once these overlap in a liquid. These phenomena include undulating
and peristaltic forces between membranes or bilayers, and, on the molecular scale,
protrusion and head-groupoverlap forces where the interactions are also influenced
by hydration forces.
448 Marina Ruths and Jacob N. Israelachvili
9.5 Adhesion and Capillary Forces
9.5.1 Capillary Forces
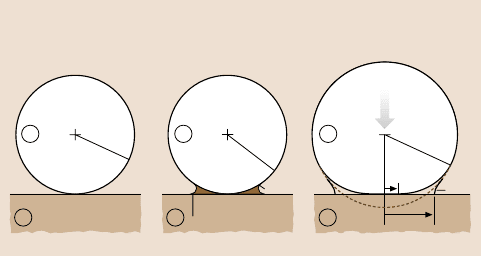
When considering the adhesion of two solid surfaces or particles in air or in a liquid,
it is easy to overlook or underestimate the important role of capillary forces, i.e.,
forces arising from the Laplace pressure of curved menisci formed by condensation
of a liquid between and around two adhering surfaces (Fig. 9.9).
The adhesion force between a non-deformablespherical particle of radius R and
a flat surface in an inert atmosphere (Fig. 9.9a) is
F
ad
= 4πRγ
SV
. (9.20)
But in an atmosphere containing a condensable vapor, the expression above is re-
placed by
F
ad
= 4πR(γ
LV
cosθ + γ
SL
) , (9.21)
where the first term is due to the Laplace pressure of the meniscus and the second
is due to the direct adhesion of the two contacting solids within the liquid. Note that
the aboveequation does not containthe radius of curvature,r, of the liquid meniscus
(see Fig. 9.9b). This is because for smaller r the Laplace pressure γ
LV
/r increases,
but the area over which it acts decreasesby the same amount, so the two effects can-
cel out. Experiments with inert liquids, such as hydrocarbons, condensing between
two mica surfaces indicate that (9.21) is valid for values of r as small as 1–2nm,
Configuration
at equilibrium
and pull-off
a)
R
b)
r
θ
Equilibrium
c)
r
r
JKR
F
R
R
1
2
1
2
1
2
H
e
r
t
z
Fig. 9.9a–c. Adhesion and capillaryforces: (a) a non-deforming sphere on a rigid, flat surface
in an inert atmosphere and (b) in a vapor that can “capillary condense” around the contact
zone. At equilibrium, the concave radius, r, of the liquid meniscus is given by the Kelvin
equation. For a concave meniscus to form, the contact angle θ hastobelessthan90
◦
.Inthe
case of hydrophobic surfaces surrounded by water, a vapor cavity can form between the sur-
faces. As long as the surfaces are perfectly smooth, the contribution of the meniscus to the ad-
hesion force is independent of r (after [1] with permission). (c) Elastically deformable sphere
on a rigid flat surface in the absence (Hertz) and presence (JKR) of adhesion ((a)and(c)
after [3]; copyright 1991, with permission from Elsevier Science)

9 Surface Forces and Nanorheology of Molecularly Thin Films 449
correspondingto vapor pressures as low as 40% of saturation [148,227,228].Capil-
lary condensationalso occursin binary liquidsystems, e.g.,when waterdissolvedin
hydrocarbon liquids condenses around two contacting hydrophilic surfaces or when
a vapor cavity forms in water around two hydrophobic surfaces. In the case of wa-
ter condensing from vapor or from oil, it also appears that the bulk value of γ
LV
is
applicable for meniscus radii as small as 2nm.
The capillary condensation of liquids, especially water, from vapor can have
additional effects on the physical state of the contact zone. For example, if the sur-
faces contain ions, these will diffuse and build up within the liquid bridge, thereby
changing the chemical composition of the contact zone, as well as influencing the
adhesion.In the case of surfaces coveredwith surfactant or polymer molecules (am-
phiphilic surfaces),the molecules can turn over on exposureto humid air, so that the
surface nonpolar groups become replaced by polar groups, which renders the sur-
faces hydrophilic. When two such surfaces come into contact, water will condense
around the contact zone and the adhesion force will also be affected – generally
increasing well above the value expected for inert hydrophobic surfaces. It is ap-
parent that adhesion in vapor or a solvent is often largely determined by capillary
forces arisingfrom the condensationof liquidthat maybe present only in very small
quantities, e.g., 10–20% of saturation in the vapor, or 20ppm in the solvent.
9.5.2 Adhesion Mechanics
Two bodies in contact deform as a result of surface forces and/or applied normal
forces. For the simplest case of two interacting elastic spheres (a model that is eas-
ily extended to an elastic sphere interacting with an undeformable surface, or vice
versa) and in the absence of attractive surface forces, the vertical central displace-
ment (compression) was derived by Hertz [229] (Fig. 9.9c). In this model, the dis-
placement and the contact area are equal to zero when no external force (load) is
applied, i.e., at the points of contact and of separation. The contact area A increases
with normal force or load as L
2/3
.
In systems where attractive surface forces are present between the surfaces, the
deformations are more complicated. Modern theories of the adhesion mechanics
of two contacting solid surfaces are based on the Johnson–Kendall–Roberts (JKR)
theory [15, 230], or on the Derjaguin–Muller–Toporov (DMT) theory [231–233].
The JKR theory is applicable to easily deformable, large bodies with high surface
energy, whereas the DMT theory better describes very small and hard bodies with
low surface energy [234]. The intermediate regime has also been described [235].
In the JKR theory, two spheres of radii R
1
and R
2
, bulk elastic modulus K,and
surfaceenergy γ will flatten due to attractivesurface forces when in contactat no ex-
ternalload.Thecontactarea willincreaseunderan externalload L ornormalforce F,
such that at mechanical equilibrium the radius of the contact area, r,isgivenby
r
3
=
R
K
F + 6πRγ +
!
12πRγF + (6πRγ)
2
, (9.22)
450 Marina Ruths and Jacob N. Israelachvili
where R = R
1
R
2
/(R
1
+ R
2
). In the absence of surface energy, γ, equation (9.22) is
reduced to the expression for the radius of the contact area in the Hertz model.
Another important result of the JKR theory gives the adhesion force or “pull-off”
2
0 200
D (μm)
100
1
0
r (μm)
Undeformed
surface
Surface separation D (nm)
20 120
250
200
150
100
50
0
40 60 80 100
Radial distance r (μm)
Surface separation D (nm)
060
250
200
150
100
50
0
10 20 30 40
Radial distance r (μm)
50
–0.5
0.5
1
0
z
x –0.5
0.5
1
0
z
x
a) b)
L = 0.01N
L = 0.02N
L = 0.05N
Asymptotic
curve at
L = 0.05N
L = 0.21N
R = 1.55cm
0.25°
c) d)
L = –0.005N
L = 0.01N
L = 0.12N
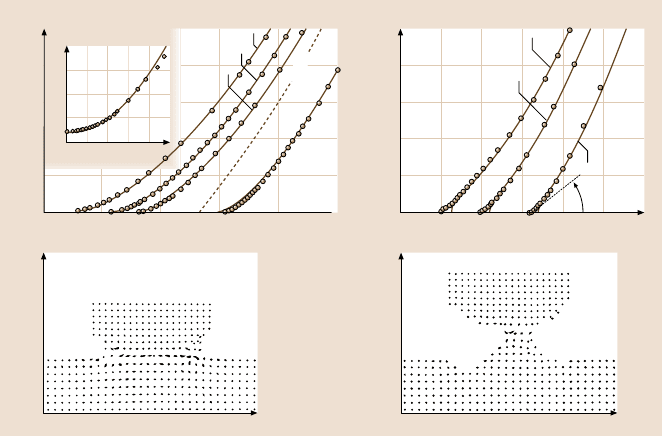
Fig. 9.10a–d. Experimental and computer simulation data on contact mechanics for ideal
Hertz and JKR contacts. (a) Measured profiles of surfaces in nonadhesive contact (circles)
compared with Hertz profiles (continuous curves). The system was mica surfaces in a con-
centrated KCl solution in which they do not adhere. When not in contact, the surface shape is
accurately described by a sphere of radius R = 1.55 cm (inset). The applied loads were 0.01,
0.02, 0.05, and 0.21 N. The last profile was measured in a different region of the surfaces
where the local radius of curvature was 1.45 cm. The Hertz profiles correspond to central
displacements of δ = 66.5, 124, 173, and 441 nm. The dashed line shows the shape of the un-
deformed sphere corresponding to the curve at a load of 0.05 N; it fits the experimental points
at larger distances (not shown). (b) Surface profiles measured with adhesive contact (mica
surfaces adhering in dry nitrogen gas) at applied loads of −0.005, 0.01, and 0.12 N. The con-
tinuous lines are JKR profiles obtained by adjusting the central displacement in each case to
get the best fit to points at larger distances. The values are δ = −4.2, 75.6, and 256 nm. Note
that the scales of this figure exaggerate the apparent angle at the junction of the surfaces. This
angle, which is insensitive to load, is only about 0.25
◦
.(c)and(d) Molecular dynamics simu-
lation illustrating the formation of a connective neck between an Ni tip (topmost eight layers)
and an Au substrate. The figures show the atomic configuration in a slice through the system
at indentation (c) and during separation (d). Note the crystalline structure of the neck. Dis-
tances are given in units of x and z,wherex = 1andz = 1 correspond to 6.12 nm. ((a)and(b)
after [236]; copyright 1987, with permission from Elsevier Science; (c)and(d) after [112],
with kind permission from Kluwer Academic Publishers)
9 Surface Forces and Nanorheology of Molecularly Thin Films 451
force:
F
ad
= −3πRγ
S
, (9.23)
where the surface energy, γ
S
, is defined through W = 2γ
S
,whereW is the reversible
work of adhesion. Note that, according to the JKR theory, a finite elastic modu-
lus, K, while having an effect on the load–area curve, has no effect on the adhesion
force, an interesting and unexpected result that has nevertheless been verified exper-
imentally [15,236–238].
Equation (9.22) and (9.23) provide the framework for analyzing results of ad-
hesion measurements (Fig. 9.10) of contacting solids, known as contact mechan-
ics [230,239], and for studying the effects of surface conditions and time on adhe-
sion energy hysteresis (see Sect. 9.5.4).
The JKR theory has been extended [240,241] to consider rigid or elastic sub-
strates separated by thin compliant layers of very different elastic moduli, a situation
commonly encountered in SFA and AFM experiments. The deformation of the sys-
tem is thenstrongly dependanton the ratioof r to the thickness of the confinedlayer.
At small r (low L), thedeformationoccurs mostly in the thin confined layer,whereas
at large r (large L), it occurs mainly in the substrates. Because of the changing dis-
tribution of traction across the contact, the adhesion force in a layered system is also
modified from that of isotropic systems (9.23) so that it is no longer independent of
the elastic moduli.
9.5.3 Effects of Surface Structure, Roughness, and Lattice Mismatch
In a contact between two rough surfaces, the real area of contact varies with the
applied load in a different manner than between smooth surfaces [243, 244]. For
non-adhering surfaces exhibiting an exponential distribution of elastically deform-
ing asperities (spherical caps of equal radius), it has been shown that the contact
area for rough surfaces increases approximately linearly with the applied normal
force (load), L, instead of as L
2/3
for smooth surfaces [243]. It has also been shown
that for plastically deforming metal microcontacts the real contact area increases
with load as A ∝ L [245, 246]. In systems with attractive surface forces, there is
a competition between this attraction and repulsive forces arising from compression
of high asperities. As a result, the adhesion in such systems can be very low, espe-
cially if the surfaces are not easily deformed[247–249]. The oppositeis possible for
soft (viscoelastic) surfaces where the real (molecular) contact area might be larger
than for two perfectly smooth surfaces [250]. The size of the real contact area at
a given normal force is also an important issue in studies of nanoscale friction, both
of single-asperity contacts (cf. Sect. 9.7) and of contacts between rough surfaces
(cf. Sect. 9.9.2).
Adhesion forces may also vary depending on the commensurability of the crys-
tallographic lattices of the interacting surfaces. McGuiggan and Israelachvili [251]
measured the adhesion between two mica surfaces as a function of the orientation
(twist angle) of their surface lattices. The forces were measured in air, water, and
452 Marina Ruths and Jacob N. Israelachvili
Adhesion energy E (mJ/m
2
)
Angle
θ (deg)
–3
10
9
8
7
6
–2 –1 0 1 2 3 4 5
170 190
10
9
8
7
6
5
180 θ
E
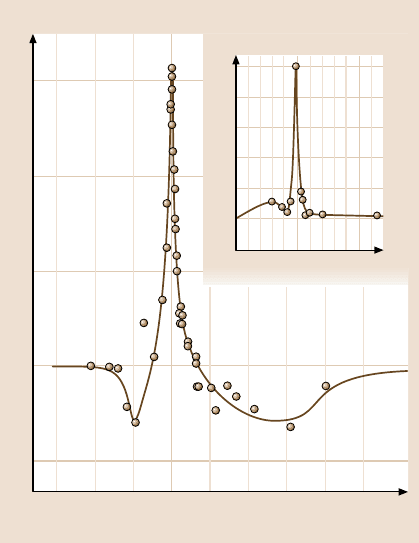
Fig. 9.11. Adhesion energy
for two mica surfaces in con-
tact in water (in the primary
minimum of an oscillatory
force curve) as a function of
the mismatch angle θ about
θ = 0
◦
and 180
◦
between
the mica surface lattices (af-
ter [242] with permission)
an aqueous salt solution where oscillatory structural forces were present. In humid
air, the adhesion was found to be relatively independent of the twist angle θ due to
the adsorption of a 0.4nm thick amorphous layer of organics and water at the inter-
face. In contrast, in water, sharp adhesion peaks (energyminima) occurred at θ = 0
◦
,
±60
◦
, ±120
◦
and 180
◦
, corresponding to the “coincidence” angles of the surface
lattices (Fig. 9.11). As little as ±1
◦
away from these peaks, the energy decreased
by 50%. In aqueous KCl solution, due to potassium ion adsorption the water be-
tween the surfaces becomes ordered, resulting in an oscillatory force profile where
the adhesive minima occur at discrete separations of about 0.25nm, corresponding
to integral numbers of water layers. The whole interaction potential was now found
to depend on the orientation of the surface lattices, and the effect extended at least
four molecular layers.
It has also been appreciated that the structure of the confining surfaces is just as
important as the nature of the liquid for determining the solvation forces [111,150,
151,252–256]. Between two surfaces that are completely flat but “unstructured”,
the liquid molecules will order into layers, but there will be no lateral ordering
within the layers. In other words, there will be positional ordering normal but not
parallel to the surfaces. If the surfaces have a crystalline (periodic) lattice, this may
induce ordering parallel to the surfaces, as well, and the oscillatory force then also
depends on the structure of the surface lattices. Further, if the two lattices have
9 Surface Forces and Nanorheology of Molecularly Thin Films 453
different dimensions(“mismatched” or “incommensurate”lattices ), or if the lattices
are similar but are not in register relative to each other, the oscillatory force law
is further modified [251, 257] and the tribological properties of the film are also
influenced, as discussed in Sect. 9.9 [257,258].
As shown by the experiments, these effects can alter the magnitude of the adhe-
sive minima found at a given separation within the last one or two nanometers of
a thin film by a factor of two. The force barriers (maxima) may also depend on ori-
entation. This could be even more important than the effects on the minima. A high
barrier could prevent two surfaces from coming closer together into a much deeper
adhesive well. Thus the maxima can effectively contribute to determining not only
the final separation of two surfaces, but also their final adhesion. Such considera-
tions should be particularly important for determining the thickness and strength of
intergranular spaces in ceramics, the adhesion forces between colloidal particles in
concentrated electrolyte solution, and the forces between two surfaces in a crack
containing capillary condensed water.
For surfaces that are randomly rough, oscillatory forces become smoothed out
and disappear altogether,to be replaced by a purelymonotonic solvationforce [134,
150,151]. This occurs even if the liquid molecules themselves are perfectly capa-
ble of ordering into layers. The situation of symmetric liquid molecules confined
between rough surfaces is therefore not unlike that of asymmetric molecules be-
tween smooth surfaces (see Sect. 9.4.3 and Fig. 9.7a). To summarize, for there to
be an oscillatory solvation force, the liquid molecules must be able to be correlated
over a reasonably long range. This requires that both the liquid molecules and the
surfaces have a high degree of order or symmetry. If either is missing, so will the
oscillations. Depending on the size of the molecules to be confined, a roughness
of only a few tenths of a nanometer is often sufficient to eliminate any oscillatory
component of the force law [42,150].
9.5.4 Nonequilibrium and Rate-Dependent Interactions: Adhesion Hysteresis
Under ideal conditions the adhesion energy is a well-defined thermodynamic quan-
tity. It is normally denoted by E or W (the work of adhesion) or γ (the surface
tension, where W = 2γ) and gives the reversible work done on bringing two sur-
faces together or the work needed to separate two surfaces from contact. Under
ideal, equilibrium conditions these two quantities are the same, but under most re-
alistic conditions they are not; the work needed to separate two surfaces is always
greater than that originally gained by bringing them together. An understanding of
the molecular mechanisms underlying this phenomenon is essential for understand-
ing many adhesion phenomena, energy dissipation during loading–unloading cy-
cles, contact angle hysteresis, and the molecular mechanisms associated with many
frictional processes.
It is wrong to think that hysteresis arises because of some imperfection in the
system such as rough or chemically heterogeneous surfaces, or because the support-
ing material is viscoelastic. Adhesion hysteresis can arise even between perfectly
