Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.


434 Marina Ruths and Jacob N. Israelachvili
between dispersed colloidal particles by adsorption of a thin layer of material with
dielectric properties close to those of the surrounding medium (matching of refrac-
tive index), or by adsorption of a polymer that gives a steric repulsive force that
keeps the particles separated at a distance where the magnitudeof the van der Waals
attraction is negligible. Thermal motion will then keep the particles dispersed.
9.4.2 Electrostatic and Ion Correlation Forces
Most surfaces in contact with a highly polar liquid (such as water) acquire a sur-
face charge, either by dissociation of ions from the surface into the solution or by
preferential adsorption of certain ions from the solution. The surface charge is bal-
anced by a layer of oppositely charged ions (counterions) in the solution at some
small distance from the surface (see [3]). In dilute solution, this distance is the De-
bye length, κ
−1
, which is purely a property of the electrolyte solution. The Debye
length falls with increasing ionic strength (i.e., with the molar concentration M
i
and
valency z
i
) of the ions in solution:
κ
−1
=
⎛
⎜
⎜
⎜
⎜
⎜
⎜
⎜
⎜
⎜
⎝
εε
0
k
B
T
e
2
N
A
.
i
z
2
i
M
i
⎞
⎟
⎟
⎟
⎟
⎟
⎟
⎟
⎟
⎟
⎠
1/2
, (9.11)
where e is the electronic charge. For example, for 1:1 electrolytes at 25
◦
C, κ
−1
=
0.304nm/
√
M
1:1
,whereM
i
is given in M (moldm
−3
). κ
−1
is thus about 10 nm in
a 1 mM NaCl solution and 0.3 nm in a 1 M solution. In totally pure water at pH 7,
where M
i
= 10
−7
M, κ
−1
is 960nm, or about 1 µm. The Debye length also relates the
surface charge density σ of a surface to the electrostatic surface potential ψ
0
via the
Grahame equation, which for 1:1 electrolytes can be expressed as:
σ =
8εε
0
k
B
T sinh
(
eψ
0
/2k
B
T
)
×
M
1:1
. (9.12)
Sincethe Debyelength isa measure of thethicknessof the diffuseatmosphereof
counterions near a charged surface, it also determines the range of the electrostatic
“double-layer” interaction between two charged surfaces. The electrostatic double-
layer interaction is an entropic effect that arises upon decreasing the thickness of the
liquid film containing the dissolved ions. Because of the attractive force between
the dissolved ions and opposite charges on the surfaces, the ions stay between the
surfaces, but an osmotic repulsion arises as their concentration increases. The long-
range electrostatic interaction energy at large separations (weak overlap) between
two similarly charged molecules or surfaces is typically repulsive and is roughly an
exponentially decaying function of D:
E(D) ≈+C
ES
e
−κD
, (9.13)
where C
ES
is a constant that depends on the geometry of the interacting surfaces, on
their surface charge density, and the solution conditions (Table 9.3). We see that the
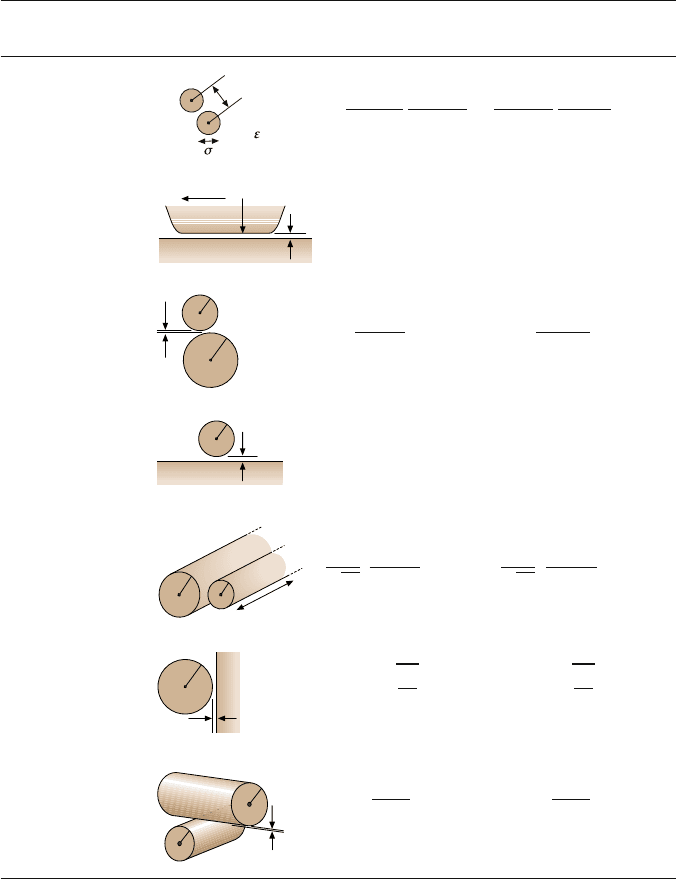
9 Surface Forces and Nanorheology of Molecularly Thin Films 435
Table 9.3. Electrical double-layer interaction energy E(D) and force (F = −dE/ dD) between
macroscopic bodies
Geometry of bodies Electric double-layer interaction
with surfaces D apart (D R)EnergyE Force F
Two ions or
small molecules
r σ
r
Solven
t
z
1
e
z
2
e
+z
1
z
2
e
2
4πεε
0
r
e
−κ(r−σ)
(
1+ κσ
)
+z
1
z
2
e
2
4πεε
0
r
2
(
1+κr
)
(
1+κσ
)
e
−κ(r−σ)
Two flat surfaces
(per unit area)
r D
r
D
Area = Sr
2
(
κ/2π
)
Ze
−κD
κ
2
/2π
Ze
−κD
Two spheres or
macromolecules
of radii R
1
and R
2
R
1
,R
2
D
D
R
1
R
2
R
1
R
2
R
1
+R
2
Ze
−κD
κ
R
1
R
2
R
1
+ R
2
Ze
−κD
Sphere or macro-
molecule of radius
R near a flat surface
R D
R
D
RZe
−κD
κRZe
−κD
Two parallel
cylinders or rods
of radii R
1
and R
2
(per unit length)
R
1
,R
2
D
Length
R
1
R
2
κ
1/2
√
2π
R
1
R
2
R
1
+R
2
1/2
Ze
−κD
κ
3/2
√
2π
R
1
R
2
R
1
+R
2
1/2
Ze
−κD
Cylinder of
radius R
near a flat surface
(per unit length)
R D
D
R
κ
1/2
R
2π
Ze
−κD
κ
3/2
R
2π
Ze
−κD
Two cylinders or
filaments of radii R
1
and R
2
crossed at 90
◦
R
1
,R
2
D
R
1
R
2
D
R
1
R
2
Ze
−κD
κ
R
1
R
2
Ze
−κD
The interaction energy and force for bodies of different geometries is based on the Poisson–
Boltzmann equation (a continuum, mean-field theory). Equation (9.14) gives the interac-
tion constant Z (in terms of the surface potential ψ
0
) for the interaction between simi-
larly charged (ionized) surfaces in aqueous solutions of monovalent electrolyte. It can also
be expressed in terms of the surface charge density σ by applying the Grahame equation
(9.12) (after [60], with permission)
436 Marina Ruths and Jacob N. Israelachvili
Debyelength is the decaylength of theinteraction energybetweentwo surfaces(and
of the mean potential away from one surface).C
ES
can be determined by solving the
so-called Poisson–Boltzmann equation or by using other theories [119–123]. The
equations in Table 9.3 are expressed in terms of a constant, Z,definedas
Z = 64πεε
0
(k
B
T/e)
2
tanh
2
zeψ
0
/(4k
B
T)
, (9.14)
which depends only on the properties of the surfaces.
The above approximate expressions are accurate only for surface separations
larger than about one Debye length. At smaller separations one must use numer-
ical solutions of the Poisson–Boltzmann equation to obtain the exact interaction
potential, for which there are no simple expressions. In the limit of small D, it can
be shown that the interaction energy depends on whether the surfaces remain at
constant potential ψ
0
(as assumed in the above equations) or at constant charge σ
(when the repulsion exceeds that predicted by the above equations), or somewhere
between these limits. In the “constant charge limit” the total number of counterions
in the compressed film does not change as D is decreased, whereas at constant po-
tential, the concentration of counterions is constant. The limiting pressure (or force
per unit area) at constant charge is the osmotic pressure of the confined ions:
F = k
B
T ×ion number density = 2σk
B
T/(zeD), for D κ
−1
.
(9.15)
That is, as D → 0 the double-layer pressure at constant surface charge becomes in-
finitely repulsive and independent of the salt concentration (at constant potential the
force instead becomes a constant at small D). However, at small separations, the
van der Waals attraction (which goes as D
−2
between two spheres or as D
−3
be-
tween two planar surfaces, see Table 9.2) wins out over the double-layer repulsion,
unless some other short-range interaction becomes dominant (see Sect. 9.4.4). This
is the theoretical prediction that forms the basis of the so-called Derjaguin–Landau–
Verwey–Overbeek (DLVO) theory [119,124],illustrated in Fig. 9.6.
Because of the different distance dependence of the van der Waals and electro-
static interactions, the total force law, as described by the DLVO theory, can show
severalminima and maxima. Typically, the depth of the outer (secondary)minimum
is a few k
B
T, which is enough to cause reversible flocculation of particles from an
aqueous dispersion. If the force barrier between the secondary and primary mini-
mum is lowered, for example, by increasing the electrolyte concentration, particles
can be irreversibly coagulated in the primary minimum. In practice, other forces
(described in the following sections) often appear at very small separations, so that
the full force law between two surfaces or colloidal particles in solution can be more
complex than might be expected from the DLVO theory.
There are situations when the double-layer interaction can be attractive at short
range even between surfaces of similar charge, especially in systems with charge
regulation due to dissociation of chargeable groups on the surfaces [123,125]; ion
condensation[126], whichmay lowerthe effectivesurface chargedensity in systems
containingdi-and trivalentcounterions;or ioncorrelation,which is an additionalvan
der Waals-like attraction due to mobile and therefore highly polarizablecounterions
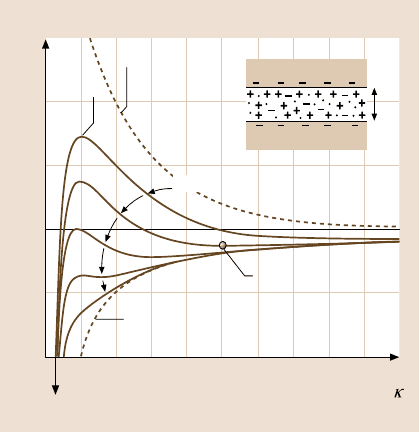
9 Surface Forces and Nanorheology of Molecularly Thin Films 437
Interaction energy E
Normalized distance D
010
1.5
1.0
0.5
0.0
–0.5
–1.0
123456789
Primary minimum adhesion at D|0
D
Net DLVO interaction
Double-layer
repulsion
Force
barrier
High σ
Low σ
vdW attraction
Secondary minimum
Fig. 9.6. Schematic plots of the DLVO interaction potential energy E between two flat,
charged surfaces [or, according to the Derjaguin approximation, (9.3), the force F be-
tween two curved surfaces] as a function of the surface separation normalized by the De-
bye length, κ
−1
. The van der Waals attraction (inverse power-law dependence on D) together
with the repulsive electrostatic “double-layer” force (roughly exponential) at different sur-
face charge σ [or potential, see (9.12)] determine the net interaction potential in aqueous
electrolyte solution (after [60] with permission)
located at the surface [127]. The ion correlation (or charge fluctuation) force be-
comes significant at separations below 4 nm and increases with the surface charge
density σ and the valency z of the counterions. Computer simulations have shown
that,at high chargedensity and formonovalentcounterions,the ion correlationforce
can reduce the effective double-layer repulsion by 10–15%. With divalent counte-
rions, the ion correlation force was found to exceed the double-layer repulsion and
the total force then became attractive at a separation below 2 nm even in dilute elec-
trolyte solution [128]. Experimentally, such short-range attractive forces have been
found between charged bilayers [129,130] and also in other systems [131].
9.4.3 Solvation and Structural Forces
When a liquid is confined within a restricted space, for example, a very thin film
between two surfaces, it ceases to behave as a structureless continuum. At small
surface separations (below about ten molecular diameters), the van der Waals force
between two surfaces or even two solute molecules in a liquid (solvent) is no longer
a smoothly varying attraction. Instead, there arises an additional “solvation” force
that generally oscillates between attraction andrepulsion with distance, with a perio-
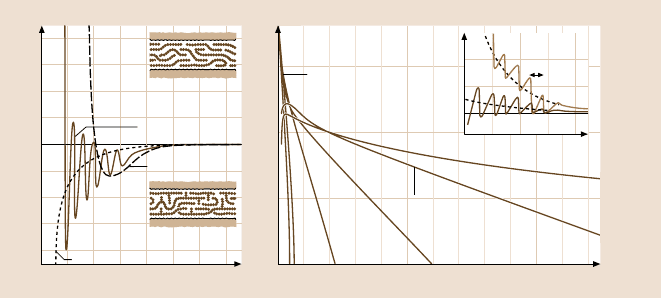
438 Marina Ruths and Jacob N. Israelachvili
dicity equal to some mean dimension σ of the liquid molecules [132]. Figure 9.7a
shows the force law between two smooth mica surfaces across the hydrocarbon li-
quid tetradecane, whose inert, chain-like molecules have a width of σ ≈ 0.4nm.
The short-range oscillatory force law is related to the “density distribution func-
tion” and “potential of mean force” characteristic of intermolecular interactions in
liquids. These forces arise from the confining effects that the two surfaces have
on liquid molecules, forcing them to order into quasi-discrete layers. Such lay-
ers are energetically or entropically favored and correspond to the minima in the
free energy, whereas fractional layers are disfavored (energy maxima). This effect
is quite general and arises in all simple liquids when they are confined between
Force/Radius F/R (mN/m)
Distance D (nm)
0
4
3
2
1
0
–1
–2
–3
–4
1234567
Repulsive force/Radius F/R (mN/m)
Distance D (nm)
0
10
1
0.1
0.01
50 100
1
10
–1
10
–2
Energy
E (mJ/m
2
)
10
7
0
012
D (nm)
Repulsive pressure
P(N/m
2
)
Linear alkanes
Branched alkanes
vdW
Hydration
force
DLVO
force
10
–1
110
–2
M10
–3
M10
–4
M
10
–5
M
10
–3
M
1M KCl
0.25 nm
a) b)
Fig. 9.7. (a) Solid curve: Forces measured between two mica surfaces across saturated lin-
ear chain alkanes such as n-tetradecane and n-hexadecane [133,134]. The 0.4-nm periodicity
of the oscillations indicates that the molecules are preferentially oriented parallel to the sur-
faces, as shown schematically in the upper insert. The theoretical continuum van der Waals
attraction is shown as a dotted curve. Dashed curve: Smooth, non-oscillatory force law ex-
hibited by irregularly shaped alkanes (such as 2-methyloctadecane) that cannot order into
well-defined layers (lower insert) [134,135]. Similar non-oscillatory forces are also observed
between “rough” surfaces, even when these interact across a saturated linear chain liquid.
This is because the irregularly shaped surfaces (rather than the liquid) now prevent the liq-
uid molecules from ordering in the gap. (b) Forces measured between charged mica surfaces
in KCl solutions of varying concentrations [20]. In dilute solutions (10
−5
and 10
−4
M), the
measured forces are excellently described by the DLVO theory, based on exact solutions to
the nonlinear Poisson–Boltzmann equation for the electrostatic forces and the Lifshitz theory
for the van der Waals forces (using a Hamaker constant of A
H
= 2.2×10
−20
J). At higher
concentrations, as more hydrated K
+
cations adsorb onto the negatively charged surfaces, an
additional hydration force appears superimposed on the DLVO interaction at distances below
3–4 nm. This force has both an oscillatory and a monotonic component. Insert: Short-range
hydration forces between mica surfaces shown as pressure versus distance. The lower and
upper curves show surfaces 40% and 95% saturated with K
+
ions. At larger separations, the
forces are in good agreement with the DLVO theory (after [3]; copyright 1991, with permis-
sion from Elsevier Science)
9 Surface Forces and Nanorheology of Molecularly Thin Films 439
two smooth, rigid surfaces, both flat and curved. Oscillatory forces do not require
any attractive liquid–liquid or liquid–wall interaction, only two hard walls confin-
ing molecules whose shape is not too irregular and that are free to exchange with
molecules in a bulk liquid reservoir. In the absence of any attractive pressure be-
tween the molecules, the bulk liquid density could be maintained by an external
hydrostatic pressure – in real liquids attractive van der Waals forces play the role of
such an external pressure.
Oscillatory forces are now well understood theoretically, at least for simple liq-
uids, and a number of theoretical studies and computer simulations of various con-
fined liquids (including water) that interact via some form of Lennard–Jones poten-
tial have invariably led to an oscillatory solvation force at surface separations below
a few molecular diameters [136–144]. In a first approximation,the oscillatory force
law may be described by an exponentially decaying cosine function of the form
E ≈ E
0
cos(2πD/σ)e
−D/σ
, (9.16)
where both theory and experiments show that the oscillatory period and the charac-
teristic decay length of the envelope are close to σ.
Once the solvation zones of the two surfaces overlap, the mean liquid density
in the gap is no longer the same as in the bulk liquid. Since the van der Waals in-
teraction depends on the optical properties of the liquid, which in turn depends on
the density, the van der Waals and the oscillatory solvation forces are not strictly
additive. It is more correct to think of the solvation force as the van der Waals
force at small separationswith the molecularpropertiesand density variationsof the
medium taken into account. It is also important to appreciate that solvation forces
do not arise simply because liquid molecules tend to structure into semi-ordered
layers at surfaces. They arise because of the disruption or change of this ordering
during the approach of a second surface. The two effects are related; the greater the
tendency toward structuring at an isolated surface the greater the solvation force be-
tween two such surfaces, but there is a real distinction between the two phenomena
that should be borne in mind.
Oscillatory forces lead to different adhesion values depending on the energy
minimum from which two surfaces are being separated. For an interaction en-
ergy described by (9.16), “quantized” adhesion energies will be E
0
at D = 0(pri-
mary minimum), E
0
/ eatD = σ, E
0
/ e
2
at D = 2σ,etc.E
0
can be thought of
as a depletion force (see Sect. 9.4.5) that is approximately given by the osmotic
limit E
0
≈−k
B
T/σ
2
, which can exceed the contribution to the adhesion energy
in contact from the van der Waals forces (at D
0
≈ 0.15−0.20nm, as discussed in
Sect. 9.3.1, keeping in mind that the Lifshitz theory fails to describe the force law
at intermediate distances). Such multivalued adhesion forces have been observed in
a number of systems, including the interactions of fibers.
Measurements of oscillatory forces between different surfaces across both aque-
ous andnonaqueous liquidshave revealedtheir richness ofproperties[145–149],for
example, their great sensitivity to the shape and rigidity of the solvent molecules,
to the presence of other components, and to the structure of the confining sur-
faces (see Sects. 9.5.3 and 9.9). In particular, the oscillations can be smeared out
440 Marina Ruths and Jacob N. Israelachvili
if the molecules are irregularly shaped (e.g., branched) and therefore unable to pack
into ordered layers, or when the interacting surfaces are rough or fluid-like (see
Sect. 9.4.6).
It is easy to understand how oscillatory forces arise between two flat, plane par-
allel surfaces. Between two curved surfaces, e.g., two spheres, one might imagine
the molecular ordering and oscillatory forces to be smeared out in the same way
that they are smeared out between two randomly rough surfaces (see Sect. 9.5.3);
however, this is not the case. Ordering can occur as long as the curvature or rough-
ness is itself regular or uniform, i.e., not random. This is due to the Derjaguin ap-
proximation (9.3). If the energy between two flat surfaces is given by a decaying
oscillatory function (for example, a cosine function as in (9.16)), then the force (and
energy) between two curved surfaces will also be an oscillatory function of distance
with some phase shift. Likewise, two surfaces with regularly curved regions will
also retain their oscillatory force profile, albeit modified, as long as the corrugations
are truly regular, i.e., periodic. On the other hand, surface roughness, even on the
nanometer scale, can smear out oscillations if the roughness is random and the con-
fined molecules are smaller than the size of the surface asperities [150,151]. If an
organic liquid contains small amounts of water, the expected oscillatory force can
be replaced by a strongly attractive capillary force (see Sect. 9.5.1).
9.4.4 Hydration and Hydrophobic Forces
The forces occurring in water and electrolyte solutionsare morecomplex than those
occurring in nonpolar liquids. According to continuum theories, the attractive van
der Waals force is always expected to win over the repulsive electrostatic “double-
layer” force at small surface separations (Fig. 9.6). However, certain surfaces (usu-
ally oxide or hydroxidesurfaces such as clays or silica) swell spontaneously or repel
each other in aqueous solution, even at high salt concentrations. Yet in all these sys-
tems one would expect the surfaces or particles to remain in strong adhesive con-
tact or coagulate in a primary minimum if the only forces operating were DLVO
forces.
There are many other aqueous systems in which the DLVO theory fails and
where there is an additional short-range force that is not oscillatory but monotonic.
Between hydrophilicsurfaces this force is exponentiallyrepulsiveand is commonly
referred to as the hydration,orstructural, force. The origin and nature of this force
has long been controversial,especially in the colloidal and biological literature. Re-
pulsive hydration forces are believed to arise from strongly hydrogen-bonding sur-
face groups, such as hydrated ions or hydroxyl (−OH) groups, which modify the
hydrogen-bonding network of liquid water adjacent to them. Because this network
is quite extensive in range [152], the resulting interaction force is also of relatively
long range.
Repulsive hydration forces were first extensively studied between clay sur-
faces [153]. More recently, they have been measured in detail between mica and
silica surfaces [20–22, 154], where they have been found to decay exponentially
with decay lengths of about 1nm. Their effective range is 3–5 nm, which is about
9 Surface Forces and Nanorheology of Molecularly Thin Films 441
twice the range of the oscillatory solvation forcein water. Empirically, the hydration
repulsion between two hydrophilic surfaces appears to follow the simple equation
E = E
0
e
−D/λ
0
, (9.17)
where λ
0
≈ 0.6−1.1nm for 1:1 electrolytes and E
0
= 3−30mJm
−2
depending on
the hydration (hydrophilicity) of the surfaces, higher E
0
values generally being as-
sociated with lower λ
0
values.
The interactions between molecularly smooth mica surfaces in dilute electrolyte
solutions obey the DLVO theory (Fig. 9.7b). However, at higher salt concentrations,
specific to each electrolyte, hydrated cations bind to the negativelycharged surfaces
and give rise to a repulsive hydration force [20,21]. This is believed to be due to
the energy needed to dehydrate the bound cations, which presumably retain some
of their water of hydration on binding. This conclusion was arrived at after noting
that thestrengthand rangeof the hydrationforces increasewiththe knownhydration
numbers of the electrolyte cations in the order: Mg
2+
>Ca
2+
>Li
+
∼Na
+
>K
+
>Cs
+
.
Similar trends are observed with other negatively charged colloidal surfaces.
While the hydration force between two mica surfaces is overall repulsive below
a distance of 4nm, it is not always monotonicbelow about1.5nm but exhibits oscil-
lations of mean periodicity of 0.25±0.03nm, roughly equal to the diameter of the
water molecule. This is shown in the insert in Fig. 9.7b, where we may note that the
first three minima at D= 0, 0.28,and 0.56nm occur at negativeenergies,a resultthat
rationalizes observations on certain colloidal systems. For example, clay platelets
such as montmorillonite often repel each other increasingly strongly as they come
closer together,but they are also knownto stack into stable aggregates with water in-
terlayers of typical thickness 0.25 and 0.55nm between them [155,156], suggestive
of a turnabout in the force law from a monotonic repulsion to discretized attraction.
In chemistry we would refer to such structures as stable hydrates of fixed stoichiom-
etry, whereas in physics we may think of them as experiencing an oscillatory force.
Both surface force and clay swelling experiments have shown that hydration
forces can be modified or “regulated” by exchanging ions of different hydration on
surfaces, an effect that has important practical applications in controlling the stabil-
ity of colloidal dispersions. It has long been known that colloidal particles can be
precipitated (coagulated or flocculated) by increasing the electrolyte concentration,
an effect that was traditionally attributed to the reduced screening of the electro-
static double-layer repulsion between the particles due to the reduced Debye length.
However, there are many examples where colloids are stabilized at high salt con-
centrations, not at low concentrations. This effect is now recognized as being due
to the increased hydration repulsion experiencedby certain surfaces when they bind
highly hydrated ions at higher salt concentrations. Hydration regulation of adhesion
and interparticle forces is an important practical method for controlling various pro-
cesses such as clay swelling [155,156], ceramicprocessing and rheology [157,158],
material fracture [157], and colloidal particle and bubble coalescence [159].
Water appears to be unique in having a solvation (hydration) force that exhibits
both a monotonic and an oscillatory component. Between hydrophilic surfaces the
442 Marina Ruths and Jacob N. Israelachvili
monotonic component is repulsive (Fig. 9.7b), but between hydrophobicsurfaces it
is attractive and the final adhesion is much greater than expected from the Lifshitz
theory.
A hydrophobicsurface is one that is inert to water in the sense that it cannotbind
to water molecules via ionic or hydrogen bonds. Hydrocarbons and fluorocarbons
are hydrophobic, as is air, and the strongly attractive hydrophobic force has many
important manifestations and consequences such as the low solubility or miscibility
of water and oil molecules, micellization, protein folding, strong adhesion and rapid
coagulationof hydrophobicsurfaces,non-wettingof water on hydrophobicsurfaces,
and hydrophobic particle attachment to rising air bubbles (the basic principle of
froth flotation).
In recent years, there has been a steady accumulation of experimental data on
the forcelawsbetween varioushydrophobicsurfacesin aqueoussolution [160–178].
These studies have found that the force law between two macroscopic hydrophobic
surfaces is of surprisingly long range, decaying exponentially with a characteristic
decay lengthof 1–2 nmin the separationrange of0–10nm,and then moregradually
further out. The hydrophobicforce can befar stronger than the van der Waals attrac-
tion, especially between hydrocarbon surfaces in water, for which the Hamaker con-
stant is quite small. The magnitude of the hydrophobic attraction has been found to
decrease with the decreasing hydrophobicity (increasing hydrophilicity) of lecithin
lipid bilayer surfaces [31] and silanated surfaces [168], whereas examples of the
opposite trend have been shown for some Langmuir–Blodgett-deposited monolay-
ers [179]. An apparent correlation has been found between high stability of the
hydrophobic surface (as measured by its contact angle hysteresis) and the absence
of a long-rangepart of the attractive force [180].
For two surfaces in water the purely hydrophobic interaction energy (ignoring
DLVO and oscillatory forces) in the range 0–10nm is given by
E = −2γ e
−D/λ
0
, (9.18)
where typically λ
0
= 1−2nm,andγ = 10−50mJm
−2
. The higher valuecorresponds
to the interfacial energy of a pure hydrocarbon–waterinterface.
At a separation below 10nm, the hydrophobic force appears to be insensitive
or only weakly sensitive to changes in the type and concentration of electrolyte
ions in the solution. The absence of a “screening” effect by ions attests to the non-
electrostatic origin of this interaction. In contrast, some experiments have shown
that, at separations greater than 10nm, the attraction does depend on the intervening
electrolyte, and that in dilute solutions, or solutions containing divalent ions, it can
continue to exceed the van der Waals attraction out to separations of 80nm [165,
181]. Recent research suggests that the interactions at very long range might not be
a “hydrophobic”force since they are influenced by the presence of dissolved gas in
the solution [176,177], the stability of the hydrophobic surface [178,180], and, on
some types of surfaces, bridging submicroscopic bubbles [172–174].
The long-range nature of the hydrophobicinteraction has a numberof important
consequences. It accounts for the rapid coagulation of hydrophobic particles in wa-
ter and may also account for the rapid folding of proteins. It also explains the ease
9 Surface Forces and Nanorheology of Molecularly Thin Films 443
with which water films rupture on hydrophobic surfaces. In this case, the van der
Waals force across the water film is repulsive and therefore favors wetting, but this
is more than offset by the attractive hydrophobicinteraction acting between the two
hydrophobic phases across water. Hydrophobic forces are increasingly being im-
plicated in the adhesion and fusion of biological membranes and cells. It is known
that both osmotic and electric-field stresses enhance membranefusion, an effect that
may be due to the concomitant increase in the hydrophobic area exposed between
two adjacent surfaces.
Fromthe previousdiscussionwecan inferthat hydrationand hydrophobicforces
are not of a simple nature. These interactions are probably the most important, yet
the least understood of all the forces in aqueous solutions. The unusual properties
of water and the nature of the surfaces (including their homogeneity and stability)
appear to be equally important. Some particle surfaces can have their hydration
forces regulated, for example, by ion exchange. Others appear to be intrinsically
hydrophilic (e.g., silica) and cannot be coagulated by changing the ionic condition,
but can be rendered hydrophobic by chemically modifying their surface groups.
For example, on heating silica to above 600
◦
C, two adjacent surface silanol (−OH)
groups release a water molecule and form a hydrophobic siloxane (−O−) group,
whence the repulsive hydration force changes into an attractive hydrophobic force.
How do these exponentially decaying repulsive or attractive forces arise?
Theoretical work and computer simulations [138,140, 182,183] suggest that the
solvation forces in water should be purely oscillatory, whereas other theoretical
studies [184–191] suggest a monotonically exponential repulsion or attraction, pos-
sibly superimposed on an oscillatory force. The latter is consistent with experimen-
tal findings, as shown in the inset to Fig. 9.7b, where it appears that the oscillatory
force is simply additivewith the monotonic hydration and DLVO forces, suggesting
that these arise from essentially different mechanisms. It has been suggested that
for a sufficiently solvophilic surface, there could be “hydration”-like forces also in
nonaqueous systems [190].
It is probable that the short-range hydration force between all smooth, rigid, or
crystalline surfaces (e.g., mineral surfaces such as mica) has an oscillatory com-
ponent. This may or may not be superimposed on a monotonic force due to image
interactions [186], dipole–dipole interactions [191], and/or structural or hydrogen-
bonding interactions [184,185].
Like the repulsive hydration force, the origin of the hydrophobic force is still
unknown. Luzar et al. [188] carried out a Monte Carlo simulation of the interaction
between two hydrophobic surfaces across water at separations below 1.5nm.They
obtained a decaying oscillatory force superimposed on a monotonically attractive
curve. In more recent computational and experimental work [192–195], it has been
suggested that hydrophobic surfaces generate a depleted region of water around
them, and that a long-range attractive force due to depletion arises between two
such surfaces. Such a difference in density might also cause boundary slip of water
at hydrophic surfaces [51,196,197].
