Bhushan B. Nanotribology and Nanomechanics: An Introduction
Подождите немного. Документ загружается.

464 Marina Ruths and Jacob N. Israelachvili
vary between 1 and 0, it determines whether a particular friction force will be large
or close to zero. Molecular simulations offer the best way to understand and predict
the magnitude of ε, but the complex multibody nature of the problem makes simple
conclusions difficult to draw [299–302]. Some of the basic physics of the energy
transfer and dissipation of the molecular collisions can be drawn from simplified
models such as a 1D three-body system [268].
Finally, the above equation may also be expressed in terms of the friction
force F:
F = S
c
A = C
1
A+ C
2
L . (9.37)
Equations similar to (9.36) and (9.37) were previously derived by Derjaguin [303,
304] and by Briscoe and Evans [305], where the constants C
1
and C
2
were inter-
preted somewhat differently than in this model.
In the absence of any attractive interfacial force, we haveC
1
≈0, and the second
term in (9.36)and (9.37) should dominate(Fig. 9.15).Such situations typically arise
when surfaces repel each other across the lubricating liquid film. In such cases, the
total frictional force should be low and should increase linearly with the external
load according to
F = C
2
L . (9.38)
Friction force
Load–5 30
60
50
40
30
20
10
0
0 5 10 15 20 25 460 500 540 10,000 12,000 14,000
120
100
80
3,200
2,400
1,600
800
L = 0
JKR
Hertz
Amontons
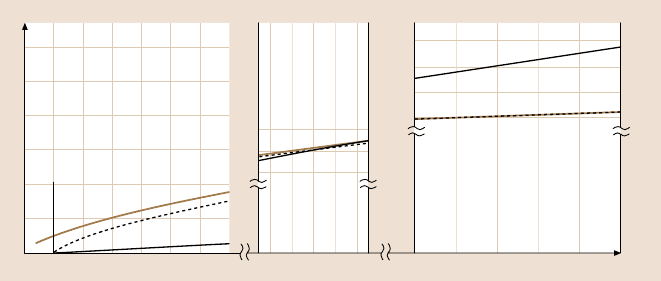
Fig. 9.15. Friction as a function of load for smooth surfaces. At low loads, the friction is dom-
inated by the C
1
A term of (9.37). The adhesion contribution (JKR curve) is most prominent
near zero load where the Hertzian and Amontons’ contributions to the friction are minimal.
As the load increases, the adhesion contribution becomes smaller as the JKR and Hertz curves
converge. In this range of loads, the linear C
2
L contribution surpasses the area contribution to
the friction. At much higher loads the explicit load dependence of the friction dominates the
interactions, and the observed behavior approaches Amontons’ law. It is interesting to note
that for smooth surfaces the pressure over the contact area does not increase as rapidly as the
load. This is because as the load is increased, the surfaces deform to increase the surface area
and thus moderate the contact pressure (after [307], with permission of Kluwer Academic
Publishers)
9 Surface Forces and Nanorheology of Molecularly Thin Films 465
An example of such lubricated sliding occurs when two mica surfaces slide in water
or in salt solution (see Fig. 9.20a), where the short-range “hydration” forces be-
tween the surfaces are repulsive. Thus, for sliding in 0.5M KCl it was found that
C
2
= 0.015 [283]. Another case where repulsive surfaces eliminate the adhesive
contribution to friction is for polymer chains attached to surfaces at one end and
swollen by a good solvent [219]. For this class of systems, C
2
< 0.001 for a finite
range of polymer layer compressions (normal loads, L). The low friction between
the surfaces in this regime is attributed to the entropic repulsion between the oppos-
ing brush layers with a minimum of entanglement between the two layers. However,
with higher normal loads, the brush layers become compressed and begin to entan-
gle, which results in higher friction (see [306]).
It is important to note that (9.38) has exactly the same form as Amontons’ Law
F = μL , (9.39)
where μ is the coefficient of friction.
Figure 9.14c,d shows the kinetic friction force measured with both SFA and
FFM (friction force microscopy, using AFM) on a system where both surfaces were
covered with a chemically bound benzyltrichlorosilanemonolayer [286]. When im-
mersed in ethanol, the adhesion in this system is low, and very different contact
areas and loads give a linear dependence of F on L with the same friction coeffi-
cients, and F → 0asL → 0. In the FFM measurements (Fig. 9.14d), the plateau in
the data at higher loads suggest a transition in the monolayers, similar to previous
observations on other monolayer systems. The pressure in the contact region in the
SFA is much lower than in the FFM, and no transitions in the friction forces or in the
thickness of the confined monolayers were observed in the SFA experiments (and
no damage to the monolayers or the underlying substrates was observed during the
experiments, indicating that the friction was “wearless”). Despite the difference of
more than six orders of magnitude in the contact radii, pressure, loads, and friction
forces, the measured friction coefficients are practically the same.
At the molecular level a thermodynamic analog of the Coulomb or cobblestone
models (see Sect. 9.7.1) based on the “contact value theorem” [3,283,307] can ex-
plain why F ∝ L also holds at the microscopic or molecular level. In this analysis
we consider the surface molecular groups as being momentarily compressed and
decompressed as the surfaces move along. Under irreversible conditions, which
always occur when a cycle is completed in a finite amount of time, the energy
“lost” in the compression–decompression cycle is dissipated as heat. For two non-
adhering surfaces, the stabilizing pressure P
i
acting locally between any two ele-
mental contact points i of the surfaces may be expressed by the contact value theo-
rem [3]:
P
i
= ρ
i
k
B
T = k
B
T/V
i
, (9.40)
where ρ
i
= V
−1
i
is the local number density (per unit volume) or activity of the in-
teracting entities, be they molecules, atoms, ions or the electron clouds of atoms.
This equation is essentially the osmotic or entropic pressure of a gas of confined
466 Marina Ruths and Jacob N. Israelachvili
molecules. As onesurface movesacross the other,local regionsbecome compressed
and decompressed by a volume ΔV
i
. The work done per cycle can be written as
εP
i
ΔV
i
,whereε (ε ≤ 1) is the fraction of energy per cycle “lost” as heat, as defined
earlier. The energy balance shows that, for each compression–decompressioncycle,
the dissipated energy is related to the friction force by
F
i
x
i
= εP
i
ΔV
i
, (9.41)
where x
i
is the lateral distance moved per cycle, which can be the distance between
asperities or the distance between surface lattice sites. The pressure at each con-
tact junction can be expressed in terms of the local normal load L
i
and local area
of contact A
i
as P
i
= L
i
/A
i
. The volume change over a cycle can thus be expressed
as ΔV
i
= A
i
z
i
,wherez
i
is the vertical distance of confinement. Inserting these into
(9.41), we get
F
i
= εL
i
(z
i
/x
i
) , (9.42)
which is independent of the local contact area A
i
. The total friction force is thus
F =
F
i
=
εL
i
(z
i
/x
i
) = εz
i
/x
i
L
i
= μL , (9.43)
where it is assumed that on average the local values of L
i
and P
i
are independent
of the local slope z
i
/x
i
. Therefore, the friction coefficient μ is a function only of the
average surface topography and the sliding velocity, but is independent of the local
(real) or macroscopic (apparent) contact areas.
While this analysis explains non-adhering surfaces, there is still an additional
explicit contact area contribution for the case of adhering surfaces, as in (9.37).
The distinction between the two cases arises because the initial assumption of the
contact value theorem, (9.40), is incomplete for adhering systems. A more appro-
priate starting equation would reflect the full intermolecular interaction potential,
including the attractive interactions, in addition to the purely repulsive contribu-
tions of (9.40), much as the van der Waals equation of state modifies the ideal gas
law.
9.7.3 Examples of Experimentally Observed Friction of Dry Surfaces
Numerous model systems have been studied with a surface forces apparatus (SFA)
modified for friction experiments (see Sect. 9.2.3).The apparatus allows for control
of load (normal force) and sliding speed, and simultaneous measurement of surface
separation, surface shape, true (molecular)area of contactbetween smooth surfaces,
and friction forces. A variety of both unlubricated and solid- and liquid-lubricated
surfaces have been studied both as smooth single-asperity contacts and after they
have been roughened by shear-induced damage.
Figure 9.16 shows the contact area, A, and friction force, F, both plotted against
the applied load, L, in an experiment in which two molecularly smooth surfaces of
mica in adhesive contact were slid past each other in an atmosphere of dry nitrogen
9 Surface Forces and Nanorheology of Molecularly Thin Films 467
Friction force
F (N)
Normal load L (N)
0 0.3
0.5
0.4
0.3
0.2
0.1
2 u 10
4
10
4
0
0.1 0.2
Dynamic contact
area A
(Pm
2
)
Damage
observed
Negative
load
Damaged
(F=
μL)
Friction force
Contact area
JKR
(F = S
c
A)
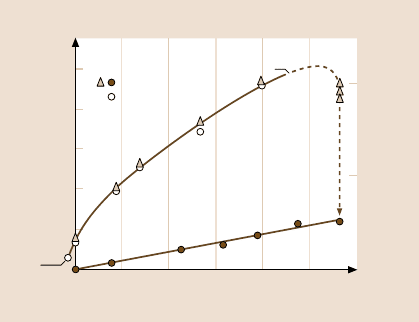
Fig. 9.16. Friction force F and contact area A versus load L for two mica surfaces sliding
in adhesive contact in dry air. The contact area is well described by the JKR theory, (9.22),
even during sliding, and the friction force is found to be directly proportional to this area,
(9.28). The vertical dashed line and arrow show the transition from interfacial to normal
friction with the onset of wear (lower curve). The sliding velocity is 0.2 µms
−1
(after [45],
with permission, copyright 1989 American Society of Mechanical Engineers)
gas. This is an example of the low-load adhesion-controlled limit, which is excel-
lentlydescribed by (9.28).In a number ofdifferent experiments,S
c
was measuredto
be 2.5×10
7
Nm
−2
and to be independent of the sliding velocity [45,308]. Note that
there is a friction force even at negative loads, where the surfaces are still sliding in
adhesive contact.
Figure 9.17 shows the correlation between adhesion hysteresis and friction for
two surfaces consisting of silica films deposited on mica substrates [41]. The fric-
tion between undamaged hydrophobic silica surfaces showed stick–slip both at dry
conditions and at 100% relative humidity.Similar to the mica surfaces in Figs. 9.16,
9.18, and 9.20a, the friction of damaged silica surfaces obeyed Amontons’ law with
a friction coefficient of 0.25–0.3 both at dry conditions and at 55% relative humid-
ity.
The high friction force of unlubricated sliding can often be reduced by treat-
ing the solid surface with a boundary layer of some other solid material that ex-
hibits lower friction, such as a surfactant monolayer, or by ensuring that during
sliding a thin liquid film remains between the surfaces (as will be discussed in
Sect. 9.8). The effectiveness of a solid boundary lubricant layer on reducing the
forces of friction is illustrated in Fig. 9.18. Comparing this with the friction of the
unlubricated/untreated surfaces (Fig. 9.16) shows that the critical shear stress has
been reduced by a factor of about ten: from 2.5×10
7
to 3.5×10
6
Nm
−2
.Atmuch
higher applied loads or pressures, the friction force is proportionalto the load, rather
than the area of contact [298], as expected from (9.27).
468 Marina Ruths and Jacob N. Israelachvili
Contact radius r
3
(cm
3
u 10
7
)
Load L (mN)
0 120
3.0
2.5
2.0
1.5
1.0
0.5
0
–20 20 40 60 80 100
Sliding velocity v (Pm/s)
0 1.0
90
80
70
60
50
40
30
0.5
20
10
0
Unloading
Loading
100% RH
γ =71± 4 mJ/m
2
γ
R
=15 mJ/m
2
γ
A
=5 mJ/m
2
0% RH
Friction
force
F
s
(mN)
L = 5.5 mN
L = 8.3 mN
L = 0 mN
L = 2.8 mN
0% RH
100% RH
b)
a)
L = 5.5 mN
L = 2.8 mN
L = 0
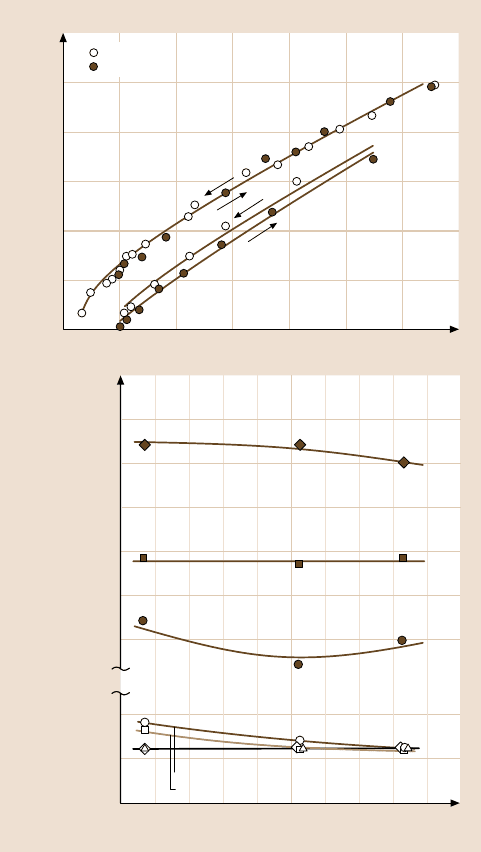
Fig. 9.17. (a) Contact radius r versus externally applied load L for loading and unloading
of two hydrophilic silica surfaces exposed to dry and humid atmospheres. Note that, while
the adhesion is higher in humid air, the hysteresis in the adhesion is higher in dry air. (b)
Effect of velocity on the static friction force F
s
for hydrophobic (heat-treated electron-beam-
evaporated) silica in dry and humid air. The effects of humidity, load, and sliding velocity on
the friction forces, as well as the stick–slip friction of the hydrophobic surfaces, are quali-
tatively consistent with a “friction” phase diagram representation as in Fig. 9.28 (after [41];
copyright 1994, with permission from Elsevier Science)
9 Surface Forces and Nanorheology of Molecularly Thin Films 469
5 u 10
4
4 u 10
4
3 u 10
4
2 u 10
4
10
4
0
Dynamic contact
area A (Pm
2
)
Friction force
F (N)
Normal load L (N)
0 0.6
0.20
0.15
0.10
0.05
0
0.1 0.2 0.3 0.4 0.5
Friction force
Contact area
JKR (F = S
c
A)
Damaged (F = μL)
Water (μ = 0.02)Hertz
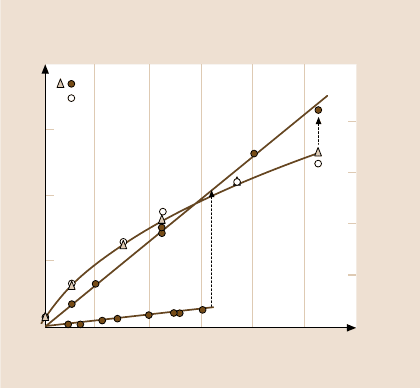
Fig. 9.18. Sliding of mica surfaces, each coated with a 2.5 nm thick monolayer of calcium
stearate surfactant, in the absence of damage (obeying JKR-type boundary friction) and in
the presence of damage (obeying Amontons-type normal friction). Note that both for this
system and for the bare mica in Figs. 9.16 and 9.20a, the friction force obeys Amontons’
law with a friction coefficient of μ ≈ 0.3 after damage occurs. At much higher applied loads,
the undamaged surfaces also follow Amontons-type sliding, but for a different reason: the
dependence on adhesion becomes smaller. Lower line: interfacial sliding with a monolayer
of water between the mica surfaces (load-controlled friction, cf. Fig. 9.20a), shown for com-
parison (after [308]; copyright 1990, with permission from Elsevier Science)
Yamada and Israelachvili [309] studied the adhesion and friction of fluorocar-
bon surfaces(surfactant-coatedboundary lubricantlayers), which were compared to
those of hydrocarbon surfaces. They concluded that well-ordered fluorocarbon sur-
faces have high friction, in spite of their lower adhesion energy (in agreement with
previous findings). The low friction coefficient of Teflon (polytetrafluoroethylene,
PTFE) must, therefore, be due to some effect other than low adhesion. For exam-
ple, the softness of PTFE, which allows material to flow at the interface, which thus
behaves like a fluid lubricant. On a related issue, Luengo et al. [310] found that
C
60
surfaces also exhibited low adhesion but high friction. In both cases the high
friction appears to arise from the bulky surface groups – fluorocarbon compared to
hydrocarbon groups in the former, large fullerene spheres in the latter. Apparently,
the fact that C
60
molecules rotate in their lattice does not make them a good lubri-
cant: the molecules of the opposing surface must still climb over them in order to
slide, and this requires energy that is independent of whether the surface molecules
are fixed or freely rotating. Larger particles such as ∼ 25nm sized nanoparticles
(also known as “inorganic fullerenes”) do appear to produce low friction by behav-
ing like molecular ball bearings, but the potential of this promising new class of
solid lubricant has still to be explored [311].
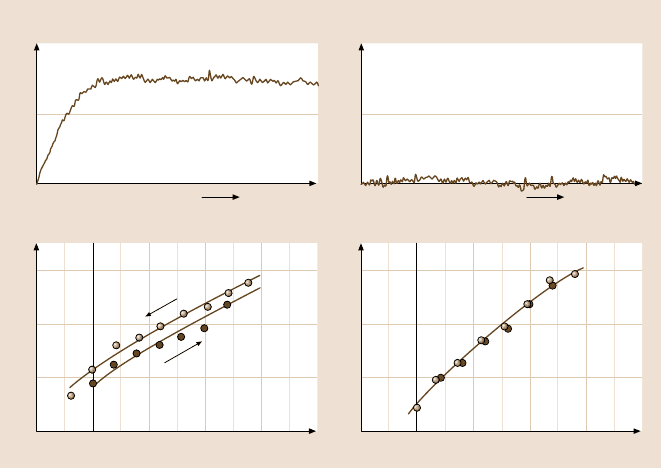
470 Marina Ruths and Jacob N. Israelachvili
Inert air
0 100
1.0
0.5
0
F (mN)
a)
Decane vapor
0 100
1.0
0.5
0
F (mN)
b)
–10 40
3
2
1
0
r
3
(μm)
3
(x 10
–4
)
10 20 300
Load L (mN)
–10 40
3
2
1
0
r
3
(μm)
3
(x 10
–4
)
10 20 300
Load L (mN)
c) d)
Inert air Decane vapor
μ
k
= 0.04
Time, t (s)
μ
k
= 0.003
Time, t (s)
γ
R
= 40 mJ/m
2
γ
A
= 28 mJ/m
2
γ
A
= γ
R
= 21– 24 mJ/m
2
Fig. 9.19. Top: friction traces for two fluid-like calcium alkylbenzene sulfonate monolayer-
coated surfaces at 25
◦
C showing that the friction force is much higher between dry mono-
layers (a) than between monolayers whose fluidity has been enhanced by hydrocarbon pen-
etration from vapor (b). Bottom: Contact radius vs. load (r
3
vs. L) data measured for the
same two surfaces as above and fitted to the JKR equation (9.22), shown by the solid curves.
For dry monolayers (c) the adhesion energy on unloading (γ
R
= 40 mJm
−2
) is greater than
that on loading (γ
R
= 28 mJm
−2
), which is indicative of an adhesion energy hysteresis of
Δγ = γ
R
−γ
A
= 12 mJm
−2
. For monolayers exposed to saturated decane vapor (d) their adhe-
sion hysteresis is zero (γ
A
= γ
R
), and both the loading and unloading data are well fitted by
the thermodynamic value of the surface energy of fluid hydrocarbon chains, γ = 24 mJm
−2
(after [261], with permission; copyright 1993 American Chemical Society)
Figure 9.19 illustrates the relationship between adhesion hysteresis and friction
for surfactant-coated surfaces under different conditions. This effect, however, is
much more general and has been shown to hold for other surfaces as well [41,262,
292,312].
Direct comparisonsbetween absoluteadhesion energiesand friction forcesshow
little correlation. In some cases, higher adhesion energies for the same system un-
der different conditions correspond to lower friction forces. For example, for hy-
drophilic silica surfaces (Fig. 9.17) it was found that with increasing relative humid-
ity the adhesion energy increases, but the adhesion energy hysteresis measured in
a loading–unloading cycle decreases, as does the friction force [41]. For hydropho-
bic silica surfaces under dry conditions, the friction at load L = 5.5mN was F =
75mN. For the same sample, the adhesion energy hysteresis was Δγ = 10mJm
−2
,
with a contact area of A ≈ 10
−8
m
2
at the same load. Assuming a value for the char-
9 Surface Forces and Nanorheology of Molecularly Thin Films 471
acteristic distance σ on the order of one lattice spacing, σ ≈ 1nm, and inserting
these values into (9.32), the friction force is predicted to be F ≈ 100mN for the ki-
netic friction force, which is close to the measured value of 75mN. Alternatively, we
may conclude that the dissipation factor is ε = 0.75, i.e., that almost all the energy
is dissipated as heat at each molecular collision.
A liquid lubricant film (Sect. 9.8.3) is usually much more effective at lowering
the friction of two surfaces than a solid boundary lubricant layer. However, to use
a liquid lubricant successfully, it must “wet” the surfaces, that is, it should have
ahighaffinity for the surfaces, so that not all the liquid molecules become squeezed
out when the surfaces come close together, even under a large compressive load.
Another important requirement is that the liquid film remains a liquid under tribo-
logical conditions, i.e., that it does not epitaxially solidify between the surfaces.
Effective lubrication usually requires that the lubricant be injected between the
surfaces, but in some cases the liquid can be made to condense from the vapor. This
is illustrated in Fig. 9.20a for two untreated mica surfaces sliding with a thin layer
of water between them. A monomolecular film of water (of thickness 0.25nm per
surface)has reducedS
c
fromits valuefor drysurfaces(Fig. 9.16)by a factorof more
than 30, which may be compared with the factor of ten attained with the boundary
lubricant layer (of thickness 2.5nm per surface) in Fig. 9.18. Water appears to have
unusual lubricating properties and usually gives wearless friction with no stick–
slip [313].
The effectiveness of a water film only 0.25nm thick to lower the friction force
by more than an order of magnitude is attributed to the “hydrophilicity” of the mica
surface(micais “wetted”by water)and tothe existenceof a stronglyrepulsiveshort-
range hydration force between such surfaces in aqueous solutions, which effectively
removes the adhesion-controlled contribution to the friction force [283]. It is also
interesting that a 0.25nm thick water film between two mica surfaces is sufficient
to bring the coefficient of friction down to 0.01–0.02, a value that corresponds to
the unusually low friction of ice. Clearly, a single monolayer of water can be a very
good lubricant – much better than most other monomolecular liquid films – for
reasons that will be discussed in Sect. 9.9. A linear dependence of F on L has also
been observedfor mica surfacesseparated bycertain hydrocarbonliquids[275,285].
Figure 9.20b shows the kinetic friction forces measured at a high velocity across
thin films of squalane, a branched hydrocarbon liquid (C
30
H
62
), which is a model
for lubricating oils. Very low adhesive forces are measured between mica surfaces
across this liquid [285] and the film thickness decreased monotonically with load.
The friction force at a given load was found to be velocity-dependent, whereas the
contact area was not [285].
Dry polymer layers (Fig. 9.21) typically show a high initial static friction (“stic-
tion”) as sliding commences from rest in adhesive contact. The development of the
friction force after a change in sliding direction, a gradual transition from stick–
slip to smooth sliding, is shown in Fig. 9.21. A correlation between adhesion hys-
teresis and friction similar to that observed for silica surfaces in Fig. 9.17 can be
seen for dry polymer layers below their glass-transition temperature. As shown in
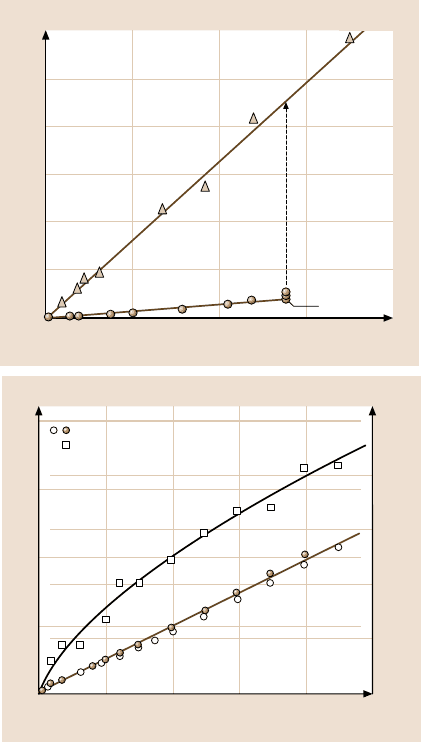
472 Marina Ruths and Jacob N. Israelachvili
Friction force F (N)
Normal load L (N)
0
0.4
0.12
0.10
0.08
0.06
0.04
0.02
0
0.1 0.2 0.3
a)
μ = 0.02
μ = 0.33
Surface
damage
Friction force F (mN)
Load L (mN)
0
2.0
1.5
1.0
0.5
0.0
b)
2500
2000
1500
1000
500
0
Contact area A (Pm
2
)
246810
Hertz
Friction force
Contact area
F = PL
Fig. 9.20. Load-controlled friction. (a) Two mica surfaces sliding past each other while im-
mersed in a 0.01 M KCl salt solution (nonadhesive conditions). The water film is molecularly
thin, 0.25 to 0.5 nm thick, and the interfacial friction force is very low: S
c
≈ 5×10
5
Nm
−2
,
μ ≈ 0.02 (before damage occurs). After the surfaces have become damaged, the friction co-
efficient is about 0.3 (after [308]; copyright 1990, with permission from Elsevier Science)
(b) Steady-state friction force and contact area measured on a confined squalane film between
two undamaged mica surfaces at v = 0.6 µm/s in the smooth sliding regime (no stick–slip).
Open circles show F obtained on loading (increasing L), solid circles show unloading. Both
data sets are straight lines passing through the origin, as shown by the brown line (μ = 0.12).
The black curve is a fit of the Hertz equation (cf. Sect. 9.5.2 and [3]) to the A vs. L data (open
squares)usingK = 10
10
N/m
2
, R= 2 cm. The thickness D varies monotonically from D = 2.5
to D = 1.7 nm as the load increases from L = 0toL = 10 mN (adapted from [285]; copyright
2003 American Physical Society)

9 Surface Forces and Nanorheology of Molecularly Thin Films 473
Friction force
TimeTime
Friction force
a) b)
Fig. 9.21. Typical friction traces showing how the friction force varies with the sliding time
for two symmetric, glassy polymer films under dry conditions. Qualitative features that are
common to both polystyrene and polyvinyl benzyl chloride: (a) Decaying stick–slip motion
is observed until smooth sliding is attained if the motion continues for a sufficiently long
distance. (b) Smooth sliding observed at sufficiently high speeds. Similar observations have
been made by Berthoud et al. [314] in measurements on polymethyl methacrylate (after [262],
with permission; copyright 2002 American Association for the Advancement of Science)
Fig. 9.12b,c, the adhesion hysteresis for polystyrene surfaces can be increased by
irradiation to induce scission of chains, and it has been found that the steady-state
friction force (kinetic friction) shows a similar increase with irradiation time [262].
Figure 9.22 shows an example of a computer simulation of the sliding of two
unlubricated silicon surfaces (modeled as a tip sliding over a planar surface) [112].
The sliding proceeds through a series of stick–slip events, and information on the
friction force and the local order of the initially crystalline surfaces can be obtained.
Similar studies for cold-welding systems [112] have demonstrated the occurrence
of shear or friction damage within the sliding surface (tip) as the lowest layer of it
adheres to thebottom surface.Recent computer simulationshaveaddressed manyof
(111)
(1
–
21)(1
–
21)
(111)
a) b)
Fig. 9.22. Computer simulation of the sliding of two contacting Si surfaces (a tip and
a flat surface). Shown are particle trajectories in a constant-force simulation, F
z,external
=
−2.15×10
−8
N, viewed along the (101) direction just before (a)andafter(b) a stick–slip
event for a large, initally ordered, dynamic tip (after [112] with permission of Kluwer Aca-
demic Publishers)
