Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

Handbook of Clay Science
Edited by F. Bergaya, B.K.G. Theng and G. Lagaly
Developments in Clay Science, Vol. 1
r 2006 Published by Elsevier Ltd.
1021
Chapter 13.1
LAYERED DOUBLE HYDROXIDES
C. FORANO
a
, T. HIBINO
b
, F. LEROUX
a
AND
C. TAVIOT-GUE
´
HO
a
a
Laboratoire de Mate
´
riaux Inorganiques, CNRS UMR 6002, Universite
´
Blaise Pascal,
F-63177 Aubie
`
re Cedex, France
b
Ecological Materials Group, AIST, 16-1 Onogawa, Tsukuba 305-8569, Japan
13.1.1. DEFINITIONS
Among the group of minerals referred to as ‘Non-Silicate Oxides and Hydroxides’
(Newman, 1987), the ‘layered double hydroxides’ (LDH) have many physical and
chemical properties that are surprisingly similar to those of clay minerals. Their layered
structure, wide chemical compositions (due to variable isomorphous substitution of
metallic cations), variable layer charge density, ion-exchange properties, reactive in-
terlayer space, swelling in water, and rheological and colloidal properties make LDH
clay-like. But because of their anion-exchange properties, LDH were referred to as
‘anionic clays’.
As hydrotalcite is one of the most representative mineral of the group, LDH were
also called ‘hydrotalcite-like compounds’ (HTlc). The structure of hydrotalcite is
related to that of brucite, Mg(OH)
2
in which some of the Mg
2+
cations in the layer
structure were replaced by Al
3+
. Carbonate anions are intercalated between the
layers to maintain electroneutrality. The chemical formula of hydrotalcite may
therefore be given as Mg
0.75
Al
0.25
(OH)
2
(CO
3
)
0.5
0.5H
2
O, and abbreviated to
[Mg–Al–CO
3
] or [Mg–Al].
The general formulae for other members of the family, based on a combination of
divalent and trivalent metal cations, can be written as [M
II
1x
M
III
x
(OH)
2
][X
q
x/q
nH
2
O] or [M
II
–M
III
–X] or [M
II
–M
III
], where [M
II
1x
M
III
x
(OH)
2
] ([M
II
–M
III
]) repre-
sents the layer, and [X
q
x/q
nH
2
O] the interlayer composition (Figs. 13.1.1 and
13.1.2). Extension to multicomponent systems may be expressed as
[M
II
–M
0
II
–M
III
–M
00
III
–X–Y]. Tetravalent cations such as Zr
4+
and Sn
4+
can also
be incorporated (Velu et al., 1999a). Cation radius (size) is an important parameter
in LDH formation. The LDH structure is not stable when the ionic radius of M(II) is
o0.06 nm. With large cations such as Ca
2+
, the hydrotalcite-type structure trans-
forms into that of hydrocalumite.
DOI: 10.1016/S1572-4352(05)01039-1
13.1.2. NATURAL OCCURRENCE
Hydrotalcite, Mg
6
Al
2
(OH)
16
(CO
3
) 4H
2
O, is a white hydrous mineral with a
rhombohedral crystalline system, a low hardness (2.00), and a low density (2.06).
Snarum, Norway, is the original site of occurrence but the mineral was found in New
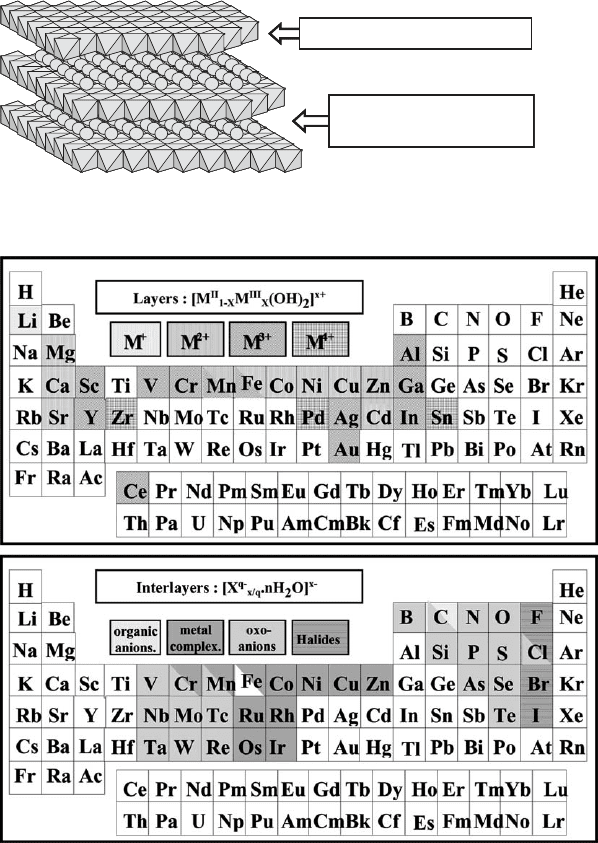
Layers : [M
II
1-X
M
III
X
(OH)
2
]
x+
Interlayer Domains :
[X
X/q
nH
2
O]
x-
Fig. 13.1.1. Structure of layered double hydroxides.
Fig. 13.1.2. Elements entering in the composition of natural and synthetic anionic clays.
Chapter 13.1: Layered Double Hydroxides1022

South Wales and Tasmania, Australia. HTlc are rare in nature, and are often as-
sociated with serpentine and calcite. The principal minerals belonging to the hy-
drotalcite and manaseite groups are presented in Table 13.1.1.
Although natural stocks of hydrotalcite are limited, the formation in soils of
mixed metal-Al hydroxide phases on the surface of phyllosilicates and gibbsite
crystallites was shown by XAFS (Sc heidegger et al., 1997, 1998). Most metals in the
first transition series can be incorporated into the hydroxyl sheet of the hydrotalcit e-
like structure. Thus, the formation of mixed metal-Al secondary precipitates may be
a general reaction mechanism for transition metal adsorption to clay minerals.
LDH with the general chemical formula [Fe
II
(1x)
Fe
III
x
(OH)
2
]
x+
[X
q
x/q nH
2
O]
(X
q
¼ SO
2
4
,CO
2
3
or Cl
) constitute the green rust family. Their occurrence in
hydromorphic soils was shown by Mo
¨
ssbauer and Raman spectroscopy (Trolard et
al., 1997). These minerals play a central role in controlling the solubility and transport
of iron in soil solutions and aquifers (Ge
´
nin et al., 1988, 1998) and more generally in
the biogeochemistry of Fe. They may also control some redox processes in aquifers
and participate in the transformation of various pollutants (Ge
´
nin et al., 2001).
Another subgroup related to hydrotalcite is the hydrocalumite group whose rep-
resentative is Ca
2
Al(OH)
6
Cl 3H
2
O and more generally for other members of the
group [Ca
2
M
III
(OH)
6
]
x+
[X
q
x/q nH
2
O] with M
3+
¼ Al
3+
,Fe
3+
,Cr
3+
,Ga
3+
, and
X
q
¼ SO
2
4
,CO
2
3
,orCl
. Hydrocalumite is a rare, hydrated Ca
2+
aluminate,
Table 13.1.1. Minerals of the manasseite and hydrotalcite group
Mineral Formula Space group
Layer composition Interlayer composition
M
II
M
III
(OH) X nH
2
O
Hydrotalcite Mg
6
Al
2
(OH)
16
(CO
3
) 4(H
2
O) R-3m (-3 2/m)
Stichtite Mg
6
Cr
2
(OH)
16
(CO
3
) 4(H
2
O) R-3m (-3 2/m)
Desautelsite Mg
6
Mn
2
(OH)
16
(CO
3
) 4(H
2
O) R3m or R-3m Trig
Pyroaurite Mg
6
Fe
2
(OH)
16
(CO
3
) 4(H
2
O) R-3m (-3 2/m)
Takovite Ni
6
Al
2
(OH)
16
(CO
3
,OH) 4(H
2
O) R-3m (-3 2/m)
Reevesite Ni
6
Fe
2
(OH)
16
(CO
3
) 4(H
2
O) R-3m (-3 2/m)
Comblainite Ni
x
Co
1x
(OH)
2
(CO
3
)
(1x)/2
n(H
2
O) R-3m (-3 2/m)
Sergeevite Ca
2
Mg
11
(OH)
4
(CO
3
)
9
(HCO
3
)
4
6(H
2
O) R32 3 2
Honessite Ni
6
Fe
2
(OH)
16
(SO
4
) 4H
2
O R-3m (-3 2/m)
Caresite (Fe,Mg)
4
Al
2
(OH)
12
(CO
3
) 3H
2
O P3112 or P321(2 3 2)
Charmarite Mn
2+
4
Al
2
(OH)
12
(CO
3
) 3H
2
O P6322 (6 2 2)
Iowaite Mg
6
Fe
2
(OH)
16
Cl
2
4H
2
O R-3m (-3 2/m)
Meixnerite Mg
6
Al
2
(OH)
16
(OH)
2
4H
2
O R-3m (-3 2/m)
Wermlandite (Ca,Mg)
7
(Al,Fe)
2
(OH)
18
(SO
4
)
2
12H
2
O P-3c1 (-3 2/m)
Woodallite Mg
6
(Cr,Fe)
2
(OH)
16
Cl
2
4H
2
O R-3m (-3 2/m)
Zincowoodwardite Zn
1x
Al
x
(OH)
2
(SO
4
)
x/2
n(H
2
O) P3- or R-3m (-3z)
13.1.2. Natural Occurrence 1023

occurring as bladed crystals in a wide cavity of a phonolitic rock at Montalto di
Castro, Italy (Passaglia and Sacerdoti, 1988) or in limestone inclusions in basalt from
Bellerberg, near Ettringen (Germany), and from Boissejour (France) (Fischer et al.,
1980). The structure of hydrocalumite is based on an ordered arrangement of Ca
2+
and M
3+
ions, in the corrugated brucite-like layers leading to a monoclinic crystal
lattice (Rousselot et al., 2002). These compounds can form during hydration of
cement compounds (Kuzel and Baier, 1996). They are being intensively investigated
for the immobilization of toxic cations or the optimization of concrete prop erties.
13.1.3. SYNTHETIC LDH
A. Chemical Composition of the Layers
A wide range of M
2+
/M
3+
combinations of the layers was found (Fig. 13.1.2; Tables
13.1.2 and 13.1.3). Only one example is known with a monovalent cation,
LiAl
2
(OH)
6
X nH
2
O. Velu et al. (1997a, 1999a, 1999b, 2000a) and Tichit et al.
(2002a) showed that tetravalent metal cations can be incorporated to some extent.
LDH are not limited to binary combinations of metal cations; ternary, quaternary,
and multicomponent LDH can also be synthesized. Multicomponent LDH are in-
teresting precursors for the preparation of finely divided mixed oxides with an ho-
mogeneous distribution of metal (Jiratova et al., 2002). Partial substitution of metal
can be used to tune the catalytic properties of the material. When Mg is partially
replaced by Cu or Fe in the hydrotalcite-like layer, using the coprecipitation method,
the LDH display a selective effect on the synthesis of methylamines (Carja et al., 2002).
B. M
2+
/M
3+
Ratio Variation
LDH with Variable M
2+
/M
3+
Ratios
In many cases, the M(II)/M(III) ratio may vary according to the coprecipit-
ation conditions and initial concentration of the salts (Table 13.1.3). In the general
Table 13.1.2. Chemical compositions of LDH and optimal pH of coprecipitation
M
II
–M
III
–X pH
form
M
II
/M
III
(R) range M
II
–M
III
–X pH
form
M
II
/M
III
(R) range
[Zn–Al–Cl] 7.0 1.0pRp5.0 [Zn–Cr–Cl] 4.5 RE 2.0
[Zn–Al–Cl] 10.0 1.0p Rp3.0 [Zn–Cr–Cl] 10.0 2.0pRp3.0
[Ni–Cr–Cl] 11.5 1.0pRp3.0 [Mg–Fe–CO
3
] — 2.7pRp5.6
[Ni–Cr–CO
3
] 13.0 1.0pRp2.0 [Ni–Al–ClO
4
] 10.0 1.0pRp3.0
[Cu–Cr–Cl] 5.5 1.6pRp2.3 [Co–Fe–Cl] 9.0 1.8pRp4.0
[Zn–Al–CO
3
] 9.0 1.7pRp2.3 [Co–Fe–CO
3
] 9.0 1.0pRp3.0
[Mg–Al–CO
3
] 8.0 1.0pRp3.0
Chapter 13.1: Layered Double Hydroxides1024

formula of [M
II
1x
M
III
x
(OH)
2
][X
q
x/q
nH
2
O], x gives the molar fraction of M(III)
per total metal
x ¼
P
M
3þ
P
i
M
i
Table 13.1.3. Some references for the preparation of LDH
LDH Anions References
[Mg–Al]
CO
2
3
; NO
3
; Cl
Miyata (1980)
[Mg–Fe]
CO
2
3
Fernandez et al. (1998)
[Mg–Ga]
CO
2
3
; NO
3
; terephtalate
Aramendia et al. (2002), Lopez-
Salinas et al. (1996)
[Mg–Cr] Cl
Boclair et al. (1999)
[Mg–In] NO
3
Aramendia et al. (2002, 2000)
[Mg–CoII–CoIII] NO
3
Zeng et al. (1998)
[Mg–ScIII],
[Mg–MnIII]
Cl
Rousselot et al. (1999)
Co/Mg/Al
CO
2
3
; NO
3
Tichit et al. (1998)
[Mg–Y]
CO
2
3
Ferna
´
ndez et al. (1997)
[Co–Ni–Mg–Al]
CO
2
3
; NO
3
Tichit et al. (2001)
[Mg–Al–Sn],
[Ni–Al–Sn],
[Co–Al–Sn]
CO
2
3
; NO
3
; Cl
Velu et al. (2000a)
[Mg–Al–Zr],
[Ni–Al–Zr],
[Zn–Al–Zr]
CO
2
3
Tichit et al. (2002a)
[Zn–Al]
CO
2
3
Thevenot et al. (1989)
[Zn–Cr]
Cl
,SO
2
4
Martin and Pinnavaia (1986), Khaldi
et al. (1997)
[Ni–Al] Cl
Kwon et al. (1988)
[Cu–Al]
CO
2
3
,NO
3
,
Lwin et al. (2001)
[Mn–Al] Cl
,NO
3
, dicarboxylic acids Aisawa et al. (2002)
[Li–Al]
CO
2
3
Ulibarri et al. (1987)
[Ca–Al], [Ca–Ga],
[Ca–Fe], [Ca–Sc]
Cl
Rousselot et al. (2002)
[Cd–Al]
CO
2
3
; NO
3
;
Vichi and Alves (1997)
[Ba–Al] Cl
Don Wang et al. (1995)
[Ni–V
III
]Cl
Caravaggio et al. (2001)
[Ni–Fe]
CO
2
3
del Arco et al. (1999)
[Co–Fe]
FeðCNÞ
4
6
Bender (2001)
[CoII–CoIII] NO
3
Zapata et al. (2002)
13.1.3. Synthetic LDH 1025
Some natural and synthetic minerals exist with a fixed x value of 1/3. In the majority
of cases x varies in the range 0:10pxp0:33.
Large cations such as Y
3+
may destabilize the LDH structure (Fernandez et al.,
1997), or even impede its formation. Electrostatic M
3+
–M
3+
and M
3+
–M
2+
in-
teractions appear to be a limiting factor for the preparation of LDH with M
3+
substitution rates >0.33. Some exceptions remain: [Li–Al] and some Al-rich [Zn–Al]
HTlc with x ¼ 0:44, obtained by coprecipi tation (Theven ot et al., 1989). Also
[Mg
1x
Ga
x
(OH)
2
](CO
3
)
x/2
mH
2
O with x ¼ 0.072–0.35 (Mg/Ga ¼ 12.91.8) by
coprecipitation (Lopez-Salinas et al., 1996). Attempts to obtain Ga-rich hydrotal-
cites (Mg/Gao1.8) resulted in solids with a constant Mg/Ga ratio of 1.8, which
appears to be the maximum Ga content. During Mg
2+
–Co
2+
precipitation nearly
23% of the Co
2+
ions are oxidized to Co
3+
(Zeng et al., 1998).
LDH with Fixed M
2+
/M
3+
Ratios
The cation layer composition is fixed for some M
II
/M
III
combinations. In the
LiAl
2
(OH)
6
X
1/q
nH
2
O phase, the Li
+
/Al
3+
ratio is fixed at 0.5 caused by ordering
of the cations (Serna et al., 1982; Fogg and O
0
Hare, 1999 ). In hydrocalumite-like
compounds, the large difference in ionic radii (Ca
2+
, 99 pm; Al
3+
, 56 pm; Fe
3+
,
64 pm) leads to a strong distortion of the local Ca
2+
environment from a regular
octahedron to a heptavalent coordination and gives rise to an ordering of the di-
valent and trivalent cations in a corrugated bruc ite-like layer. Cr
3+
-containing LDH
with Mg
2+
,Zn
2+
,Co
2+
,Ni
2+
,orMn
2+
exist preferentially with M
2+
/Cr
3+
¼ 2
(Boclair et al., 1999; Roussel et al., 2000, 2001). A structural pathway involving
direct condensation of hexaaquo zinc(II) complexes with deprotonated Cr
3+
mon-
omeric aquo complexes is proposed to explain the form ation of LDH with a fixed
Zn/Cr ratio of 2. The structural pathway strongly supports the concept of cationic
order in the [Zn–Cr–Cl] LDH sheets. Using a combination of powder XRD and X-ray
absorption (XAS), Vucelic et al. (1997) demonst rated that in synthetic pyroauri te
there is no correlation between Fe(III) cation positions over distances of a few tens
of Angstroms. This signifies a very high level of local ordering, avoiding the existence
of Fe(III)–Fe(III) neighbours. The same situation may apply to other M(II)/M(III )
LDH. On the other hand, Li/Al LDH show long-range cation ordering.
C. Interlayer Anion Composition
A priori, there is no theoretical limit to the intercalation of all types of anions into
the LDH structure. Fig. 13.1.2 shows the large number of elem ents that can be
intercalated in anionic form. Neutral molecules can also be intercalated together
with these anions, giving rise to a very wide range of compositions for the interlayer
domains. The following families of anions can be found:
halides (F
,Cl
,Br
,I
),
non-metal oxoanions (BO
3
3
,CO
3
2
,NO
3
,Si
2
O
5
2
,HPO
4
2
,SO
4
2
,ClO
4
, AsO
4
3
,
SeO
4
2
, BrO
4
, etc.),
Chapter 13.1: Layered Double Hydroxides1026
oxometallate anions (VO
4
3
, CrO
4
2
, MnO
4
,V
10
O
28
6
,Cr
2
O
7
2
,Mo
7
O
24
6
,PW
12
O
40
3
,
etc.),
anionic complexes of transition metals (Fe(CN)
6
2
, etc.),
volatile organic anions (CH
3
COO
,C
6
H
5
COO
,C
12
H
25
COO
,C
2
O
4
2
,
C
6
H
5
SO
3
, etc.),
anionic polymers (PSS, PVS, etc.).
13.1.4. SYNTHESIS
LDH are simple and inexpensive to synthesize on laboratory and industrial scales
(Trifiro and Vaccari, 1996). Many methods allow the preparation of materials with
tailored physical and chemical properties suitable for many applications. For a cat-
alytic system, whose activity results from a cooperative effect between the active
phase and mixed oxides support, a precursor containing all the components homo-
geneously distributed in the same phase may be a suitable choice. LDH are such types
of precursors. The metal cations are homogeneously distributed inside the brucite
sheets; calcination and reduction yield well-dispersed, small and stable metal particles
on mixed oxides support (Das and Srivastava, 2002). As an example, the reduction of
Pd-containing Mg–Al LDH leads to Pd/MgAl oxide bifunctional catalysts. Another
way to introduce Pd in the LDH structure is anion exchange with the [PdCl
2
(OH)
2
]
2
complex (Carpentier et al., 2002). These two methods appear quite different in terms
of Pd loading or structural changes in the reduction step. Table 13.1.2 shows the large
number of elements that can be incorporated into the LDH structure.
The preparation, propert ies, and applications of LDH were well documented
(Cavani et al., 1991; Miyata, 1991; de Roy et al., 1992 ; Mascol o, 1995; Trifiro and
Vaccari, 1996; Rives and Ulibarri, 1999; Hibino et al., 1999; Vaccari, 1999a; Sels et
al., 200 1 ; Rives, 2001; Newman and Jones, 2002). Here we give a general overview of
typical methods of synthesis.
A. Coprecipitation
LDH are readily prepared by the addition of a base to solutions containing a mixture
of M(II) and M(III). In this titration or variable-pH coprecipitation method, M(III)
hydroxides or hydrous oxides are initially formed, and further addition of base
results in coprecipitation or conversion into LDH. Boclair et al. (1999) reported a
well-defined transition step between con stant-pH precipitation of the M(III) hy-
droxides (M ¼ Al, Fe) and the mixed [M(II)–M(III)] precipitates where
M(II) ¼ Mg
2+
,Zn
2+
,Co
2+
,Ni
2+
,Mn
2+
. The conversion of M(OH)
3
(or
MO(OH)) to LDH proceeds by a dissolu tion/precipitation mechanism. In
[M(II)–Cr
3+
] systems (M(II) ¼ Zn
2+
,Co
2+
, and Ni
2+
), the absence of pH tran-
sition is indicative of the preferentia l precipitation of LDH over Cr(OH)
3
(Boclair
et al., 1999).
13.1.4. Synthesis 1027
To obtain LDH with high chemical homogeneity, coprecipitation at constant pH
is recommended. It allows the preparation of a great number of LDH with CO
3
2
,
Cl
,orNO
3
anions as precursors for subsequent reactions (anion-exchange reac-
tions, thermal decomposition, noble metal impregnations, etc.). The pH is kept
constant during the reaction by the simultaneous addition of a base solution (NaOH,
KOH, and NH
4
OH) and a mixed metal salt solution:
ð1 xÞM
II
X
q
2=q
þ xM
III
X
q
3=q
þ 2NaOH þ nH
2
O !
M
II
1x
M
III
x
ðOHÞ
2
X
q
x=q
nH
2
O þ 2NaX
q
1=q
Crepaldi et al. (2000a) demonstrated that materials prepared by this method show
interesting properties for technological applications, including high crystallinit y,
small particle size, high specific surface area, and high average pore diameter.
The pH of coprecipitation has a crucial effect on the chemical, structural, and
textural properties of the phases. For instance, powder XRD of the [Zn–Al–Cl]
samples with variable Zn/Al ratio shows that only the LDH phase crystallizes at
neutral pH. The X-ray pattern shows good resolution when Zn
2+
/Al
3+
E 3. At pH
10.0 and for a Zn
2+
/Al
3+
ratioX3, Zn–Al–Cl coexists with Zn(OH)
2
, while for a
Zn
2+
/Al
3+
ratiop1, the excess Al
3+
ions crystallize as bayerite (Al(OH)
3
) The best
crystalline phase is obtained for Zn
2+
/Al
3
p3, whatever the pH. In the case of pure
[Ni–Cr–Cl] and [Ni–Cr–CO
3
] phases, LDH with a M
II
/M
III
ratio changing from 1.0
to 3.0 and 1.0 to 2.0, respectively, were obtained only after subsequent hydrothermal
treatment.
The coprecipitation method is sometimes limited by competitive reactions such as
precipitation of metal salts in the case of oxoanions with high metal ion affinity (e.g.,
phosphate and oxometallates). Anion exchange of the Cl
or NO
3
LDH precursor
then appears to be an alternative method.
B. The Urea Method
During the standard coprecipitation, supersaturation of the precipitating agent
(OH
) is reached rapidly and maintained throughout the reaction. This leads to the
continuous nucleation of mixed hydroxides simultaneous with the growing and
Oswald ageing (aggregation) of the particles, resulting in a wide particle size dis-
tribution. By using a base retardant as precipitating agent, the nucleation step can be
separated from particle growth, an d ageing is prevented from the beginning. Co-
precipitation using urea as the base was developed to prepare monodisperse par-
ticles. Urea is a very weak Brønsted base (pK
b
¼ 13:8), is highly soluble in water.
According to Shaw and Bordeaux (1955), hydrolysis of urea proceeds in two
steps: (i) formation of ammonium cyan ate (NH
4
CNO) as the rate-determining step;
and (ii) fast hydrolysis of the cyanate into ammonium carbonate:
COðNH
2
Þ
2
þ 2H
2
O ! 2NH
þ
4
þ CO
2
3
Chapter 13.1: Layered Double Hydroxides1028
The hydrolysis rate of urea can be controlled by temperature. The rate constant
increases about 200 times when the temperature is increased from 60 to 100 1C.
Large platelets of well-crystallized hydrotalcite with hexagonal shape are obtained
by this method (Oh, et al., 2002; Ogawa and Kaiho, et al., 2002; Adachi-Pagano et
al., 2003). The reaction temperature and the concentration of reactants control the
particle size. Monodisperse particles between 1 and 5 mm, and up to 20 mm are
obtained. M
3
2+
Al(OH)
8
(CO
3
)
0.5
nH
2
O(M
2+
¼ Mg
2+
,Ni
2+
,Zn
2+
) LDH were
synthesized by Costantino et al. (1998).
C. Induced Hydrolysis
When oxides such as ZnO, NiO, and CuO are contacted dropwise with acidic so-
lutions of trivalent metal salts such as AlCl
3
or CrCl
3
, the oxides are progressively
dissolved, and LDH are precipitated provided the pH is buffered by the oxide and/or
hydroxide suspension (de Roy et al., 1992):
M
II
O þ xM
III
X
q
3=q
þðn þ 1ÞH
2
O ! M
II
1x
M
III
x
ðOHÞ
2
X
q
x=q
nH
2
O þ xM
II
X
q
2=q
This synthesis was first used to prepare [Zn–Cr–Cl] LDH but was then extended to
other systems, in particular [Zn–Cr–Cl], [Zn–Cr–NO
3
], [Zn–Al–Cl], and
[Zn–Al–NO
3
](Boehm et al., 1977). More recently, a new [Zn–Cr] LDH,
[Zn
7
Cr
4
(OH)
22
](CO
3
)
2
5H
2
O, was prepared by reaction of a perchlorate solution
of the hydrolytic dimer [(H
2
O)
4
Cr(m-OH)
2
Cr(H
2
O)
4
]
4+
with ZnO and subsequent
anion exchange with Na
2
CO
3
(Gutmann and Mueller, 1996).
Induced hydrolysis is not limited to reactions between di- and trivalent catio ns but
can involve divalent–divalent, divale nt–tetravalent and trivalent–trivalen t species
(Taylor, 1984).
D. Reconstruction
Miyata (1980) was the first to describe the reconstruction of the original LDH
structure by hydration of the calcined LDH. This unique property, ascribable to a
structural memory effect, can be used as a general preparation method of LDH. In
the first step the LDH containing a volatile anion is calcined into a mixture of oxides
and then to rehydrated in an aqueous solution containing the anion to be inter-
calated. The method was used for the preparation of several LDH (Kooli et al., 1994,
1995, 1997b) and the intercalation of several oxoanions (CrO
4
2
,HPO
4
2
,HVO
4
2
,
SiO
3
2
, HGaO
3
2
, and SO
4
2
) into [Mg–Al] (Tsugio et al., 1986).
The calcination conditions (temperatur e, rate, and duratio n) are important pa-
rameters determining structure recovery. The hydrotalcite structure is reconstructed
at controlled water vapour pressure by 24 h rehydration, provided that the thermal
treatment of the precursors does not exceed 600 1C(Rocha et al., 1999). Samples
calcined at 750 1C recover their original structure if they are equilibrated for 3 days.
13.1.4. Synthesis 1029
Tetrahedral Al generated by the decomposition of the parent mineral is converted
again into octahedral Al in the brucite layer. Kinetic data (Millange et al., 2000),
using the Avrami–Erofe’ev nucleation-growth model, are consistent with dissolution
of the mixed oxide and crystallization of the LDH from solution. Using SEM and
XRD techniques, Stanimirova et al. (2001) confirmed the dissolution/reconstruction
process. This mechanism is not related to a memory effect as assumed previously.
Nevertheless, some limits of reversibility were observed. Repeated calcination/
hydration cycles with hydrotalcite decrease the content of interlayer carbonate an-
ions an d increasing extraction of Al
3+
from the brucite layers. Ther e is also pro-
gressive segregation of the MgAl
2
O
4
spinel phase the formation of which is unusual
at these soft conditions of calcination (Hibino and Tsunashima, 1998). Nor can the
reconstruction method be used for all M
II
–M
III
combinations. Reconstruction of
Fe
3+
-containing hydrotalcites is limited by the formation of MgFe
2
O
4
spinel, which
appears even at low content of Fe
3+
. In the case of [Mg–Al–Y], reconstruction leads
to segregation of Y
3+
-containing oxides and [Mg–Al] LDH (Fernandez et al., 1997).
In the case of [Zn–Al] LDH, restoration of the hydrotalcite-like structure is inde-
pendent of the Zn/Al ratios for samples calcined between 300 and 400 1C; however, a
second phase, Al hydroxide or Zn oxide, is detected. At temperatures above 600 1C,
the formation of the spinel ZnAl
2
O
4
prevents any reconstruction. The rehydrated
phases have Zn/Al ratios close to 2, irrespective of the composi tion of the starting
material (Kooli et al., 1997a).
This method is suitable for the preparation of hybrid LDH with large organic
anions such as dyes. For instance, phenolphthalein was intercalated into Zn–Al
LDH (Lat terini et al., 2002).
E. Sol-Gel Technique
The sol-gel process was first explored by Lopez et al. (1996) in the preparation of
Mg–Al type samples. The sol-gel hydrotalcites show thermal stability up to 550 1C
(Lopez et al., 1997). However, LDH samples prepared by coprecipi tation are more
crystalline than those prepared by the sol-gel method. The marked increase in spe-
cific surface area is ascribed to the increase in mesopore volume. The textural prop-
erties of the calcined samples are not appreciably influenced by the method of
synthesis (Aramendia et al., 2002).
As an example, Mg/M(III) (M ¼ Al, Ga, In) LDH were prepared from magne-
sium ethoxide and the acetylaceonates of the trivalent metals. However, the method
usually described is not exactly a sol-gel approach, since the alkoxide is first dis-
solved in an alcohol/acid mixed solution (EtOH/HCl, 35% in aqueous solution). A
solution containing acetone and the acetylacetonate of M(III) is then added, and the
pH is adjusted to 10 with aqueous ammonia (Prinetto et al., 2000b).
Similar conditions were used for the preparation of Mg/Al (hydrotalcite) and Ni/
Al (takovite). The nature of the acid used during the first step, either HNO
3
or HCl,
is of great importance (Prinetto et al., 2000a). With the sol-gel method samples with
Chapter 13.1: Layered Double Hydroxides1030
