Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

but is laterally uncorrelated despite the highly ordered stacking of the layers (3R).
This random anionic layer stacking was attributed to the high degree of degeneracy
(26-fold) of carbonate ion sites. Bellotto et al. (1996a) examined the layer stacking
arrangement of MgAl, MgGa, and NiAl by XRD analysis with the DIFFAX pro-
gram. The occurrence of faults is quantified by a ‘fault pro bability’, describing the
probability for a specific layer sequence to be arranged in rhombohedral or hex-
agonal stacking. The stacking arrangement is random for the solids investigated,
except for MgAl2CO
2
3
, which shows a preference for the rhombohe dral polytype.
This feature is assigned to a predominantly Coulombic layer–interlayer bondin g.
The stacking sequence is not affected by hydrother mal treatments at 470 K, which
only increased crystallinity. The higher entropy of random sequences can account for
their therm al stability.
D. Guest– Host Interactions
Interactions between guests (anions) and host (the brucite-like layer) are controlled
by electrostatic interactions and hydrogen bonding between the hydroxyl surface
groups and the anions. The interaction depends on the ordering of the metal cations
in the layer, and the chemical nature of the anion. For ordered LDH structures
such as [Li–Al], the anions are aligned perpendicular to the Li
+
–Li
+
axis (Fogg and
O
0
Hare, 1999), showing the key role of electrostatic interactions in this structure.
Generally, oxoanions strongly interact with the hydroxyl surface groups and a
dense packing of the anions is often achieved. Thi s can be explained in terms of
symmetry compatibility between the oxoanions polyedra and the octahedral layers
of the host structure. The various hexagonal or rhomboedral stacking of the LDH
layers define octahedral, prismatic, and tetrahedral interlayer crystallographic sites,
which can ideally accommodate monomeric oxoanions. Optimal hydrogen bonds are
formed when the Td or Oh units are arranged face to face or corner to corner. As an
example, the structure of the [Zn–Cr–SO
4
] interlayer domains is described as an
ordered arrangement of alternate inverse interlayer SO
4
tetrahedra. The tetrahedra
retain their C
3
-axis perpendicular to the layer, with one oxygen pointing to a metal
cation of the octahedral layer and the other three oxygens facing three OH grou ps of
the opposite layer. The short hydrogen bond lengths of 0.293 and 0.271 nm indicate
strong interactions betw een the layers and sulphate anions, resulting in a 0.892 nm
basal spacing (Khaldi et al., 1997). The structural series of XO
4
-containing LDH
([Zn–Cr–SO
4
], [Zn–Al–SO
4
], [Cu–Cr–SO
4
], [Zn–Al–CrO
4
], [Cu–Cr–CrO
4
],
[Zn–Cr–Cr
2
O
7
], and [Cu–Cr–Cr
2
O
7
]) shows basal spacings close to the d value of
[Zn–Cr–SO
4
]. In all these phases, the oxoanions are orientated similar to the SO
2
4
ions in the reference material. Similar d values obtain even with the more flexible
Cr
2
O
7
because the anions can lie flat and parallel to the layers (Bigey et al., 1997).
When interlayer anions strongly interact with the layers, modification of molec -
ular dynamic is expected. Intercalation of CO
2
3
; SO
2
4
; and ClO
4
into [Mg–Al]
LDH lowers the symmetry of the anions due to hydrogen bonding with intercalated
13.1.5. Structure 1041
water molecules and OH groups as shown by Fourier transform infrared (FTIR) and
Raman spectroscopy (Kloprogge et al., 2002). On the other hand, the NO
3
anions
seem to be randomly distributed in the interlayer space because their vibration bands
remain unaffected during intercalation.
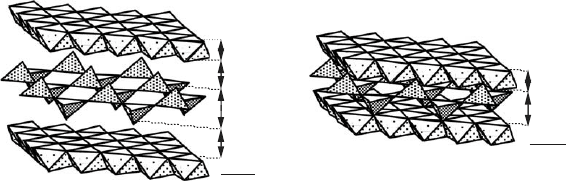
Cell parameter refinement for intercalat ed silicate anions [Zn–Cr–SiO
4
] gives the
typical hexagonal 3R lattice as for [Zn–Cr–Cl] (Schu
¨
tz and Biloen, 1987; Depe
`
ge
et al., 1996). Additional reflections of the [Zn–Al–SiO
4
] phase are attributed to a
superstructural arrangement of the silicate species in the LDH interlayer space. The
XRD pattern can be indexed in the 1H stacking hexagonal mode with a unit
cell parameter a ¼ a
o
ffiffiffi
3
p
. The
29
Si NMR chemical shifts are similar to those in
phyllosilicates, suggesting that the SiO
4
4
groups in the LDH phases are arranged
similarly, and that the silicon environment is either of type Si(OSi)
3y
(OM)
y
(OH)
or Si(OSi)
3y
(OM)
y
(OM
0
) (with typically M ¼ Al and M
0
in octahedral site)
(Fig. 13.1. 4 ).
Very short basal spacings are obtained with voluminous anions such as V
10
O
6
28
because of a flat orientation of the decaoctahedra building block of V
10
O
6
28
. The
limited size of the oxometallate anions and their low charge density do not allow the
formation of interlayer microporosity. Indeed, the basal spacing of LDH containing
oxopolyanions ne ver surpasses 1.4 nm, whereas intercalation of Al
13
hydroxocations
into clay minerals yields a distinctly greater interlayer expansion (Pinnavaia, 1987).
Organic anions always interact with their anionic groups (2CO
2
; 2SO
3
;
2PO
2
3
; 2OSO
3
; 2OPO
2
3
) being strongly hydrogen bonded to the surface hy-
droxyl groups, while their hydrophobic hydrocarbon chains are pushed far away
from the hydrophilic layer surface, and adopt the lowest energy conformation (Meyn
et al., 1990). a, o-Dianionic molecules can bridge two adjacent layers, giving a basal
spacing proportional to the length of the hydrocarbon chain. With monovalent long-
chain anions, either a bilayer or an interdigitated arrangement is formed. As a result,
the basal spacing is usually greater than 1.5 nm (Sanz and Serratosa, 1984; Meyn et
al., 1990; Bonnet et al., 1996; Pre
´
vot et al., 1998; Costantino et al., 1999).
4.6 Å
2.0 Å
2.7 Å
2.7 Å
d calc = 12.0 Å
d exp = 11.85 Å
2.0 Å
4.6 Å
d calc = 6.6 Å
d exp = 7.65 Å
Fig. 13.1.4. Polycondensation of silicate layers in double layered hydroxides.
Chapter 13.1: Layered Double Hydroxides1042
In particular, the interlayer packing depends on the relation between the charge
and size of the organic anions and the equivalent area of the layer. When the cross-
sectional area of the anions is similar to the equivalent area, optimal packing is
obtained. This is the case for dodecylsulphate-containing [Zn
2
Al] LDH. The anions
adopt the same packing as in their sodium salt, with electrostatic and hydrogen
bonding between anionic head groups and the surface OH groups and van der Waals
bonding between lateral hydrocarbon chains. Guest–guest interactions are then de-
termined by the layer cha rge density, which is a determining factor in the adsorption
of neutral molecules (alkyl amines and alcohols) in alkylsulphate-containing LDH
(Boehm et al., 1977 ; Kopka et al., 1988; Meyn et al., 1990, 1993), catalysis reactions,
and in situ polymerization (Leroux and Besse, 2001).
E. Analytical Methods
Since synthetic LDH are commonly not highly crystalline, various and co mplemen-
tary techniques are needed to get structural information.
XRD
Diffraction peaks for syn thetic LDH are usually refined in R-3m space group in
rhomboedral symmetry, and the parameters calculated according to the equation
1=d
2
¼½4ðh
2
þ hk þ k
2
Þ=3a
2
þl
2
=c
2
. The basal spacing is expected to decrease with
increasing layer charge density (and vice versa) due to enhanced electrostatic
attraction between hydroxide layer s and interlayer anions. For an ideal in-plane
octahedral assembly, the size of the octahedra sharing edges is related to the metal-
oxygen distance (d) by the relation a ¼
ffiffiffi
2
p
dðM2OÞ and the latter to the ionic radius
(r) by the relation d(M–O) ¼ (1x) r(M
2+
)+xr(M
3+
). The variation of the lattice
parameter a with the M(III) content is given by da=dx ¼
ffiffiffi
2
p
½rðM
2þ
ÞrðM
3þ
Þ.
This relation is often used to assess the cation substitution in LDH.
The coherence length along the stacking direction may be estimated from the (0 0 l)
FWHM using the Scherrer formula D
hkl
¼ Kl/b
1/2
cos y, where l is the X-ray wave-
length, y the diffraction angle; b
1/2
the full-width at half-maximum intensity; and K is
a constant usually taken to be equal to 0.9 (CuK
a
radiation). The values are highly
dependent on the LDH composition. For synthetic hydrotalcite (Mg
2
Al) a value of
90 nm is calculated, corresponding roughly to 100 layers (Oriakhi et al., 1997), 37 nm
for Zn
3
Al (Pre
´
vot et al., 1998), and 18 nm for Ni
2
Al (Oriakhi et al., 1997).
EDX is useful for studying the kinetics of formation or exchange reaction. When
time resolved, it allows staging phenomena in some LDH to be observed (Khan and
O’Hare, 2 002).
Neutron Diffraction
Structures of LiAl
2
(OH)
6
X(X¼ Cl, Br, NO
3
) dehydrated phases may be determined
by neutron powder diffraction technique (Besserguenev et al., 1997). These phases
are isomorphous and crystallize in the P6
3
/mcm space group. This technique is also
13.1.5. Structure 1043
applied to improve understanding of the thermal evolution of the Pd/Mg/Al system
(segregation and oxide formation) (Basile et al., 2000).
Inelastic neutron scattering was used to study the interlayer water (Kagunya et al.,
1998). Quasi-elastic neutron scattering showed previously that the water molecules
are not fixed in one location, but exhibi t translational diffusion as well as reorien-
tation motions. The basal spacing of the LDH influences water mobility (Kagunya,
1996).
XAS
Because of the poorly crystalline nature of LDH, XAS was widely used to investigate
LDH formation (Roussel et al., 2001; Yamaguchi et al., 2002). XAS indicates local
ordering for pyroaurite (Vucelic et al., 1997) and other synthetic LDH such as
Co
2
Fe
y
Al
1y
(OH)
6
Cl nH
2
O(Intissar et al., 2002).
EXAFS shows structural changes arising from substitution s as in
(Co
1y
Cu
y
)
2
Al(OH)
6
Cl nH
2
O, where for y ¼ 1 the local structure is similar to that
of bottalackite (Leroux et al., 2002). When the M(II)/M(III) ratio is greatly changed
as in Co
x
Al(OH)
2(1+x)
Cl nH
2
O, the decrease of layer charge density is compensated
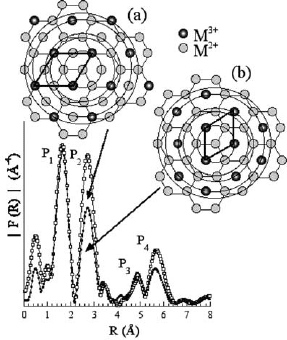
by oxidation Co(II) to Co(III) (Leroux et al., 2001a)(Fig. 13.1.5).
EXAFS is also used to determine the interlayer structure and possible grafting
reactions. For example, the W LIII-edge and Nb K-edge absorption shows that
hexametallate anions, Nb
x
W
6x
O
19
(x+2)
, orient with their C
3
-axis perpendicular to
the host layers (Evans et al., 1996). For CrO
2
4
and Cr
2
O
2
7
, Cr–K edge absorption
Fig. 13.1.5. Moduli of the Fourier transform for Co
n
Al(OH)
2(n+1)
Cl nH
2
O: (a) n ¼ 3 and
(b) n ¼ 2. The distances are not corrected from atomic phases. The ideal cation intralamellar
arrangement is displayed. Co K-edge EXAFS spectra were recorded at the beam line D44
(LURE, Orsay).
Chapter 13.1: Layered Double Hydroxides1044
shows that the decrease in basal spacing is related to grafting of these oxoanions to
the surface OH groups (Malherbe et al., 1999).
Sulphur K-edge absorption was used to determine the degree of interaction of
styrene sulphonate with the ZnAl LDH surface. To illustrate the high selectivity of
the XAS technique, an ill-defined ZnS-like phase was detected after heating to 600 1C
under nitrogen (Moujahid et al., 2003).
Raman and FTIR
The application of both Raman and FTIR spectroscopy to the characterization of
LDH has been reviewed by Kloprogge and Frost (2001) .
FTIR and Raman spectroscopy were used to investigate the local structures of
LDH such as M
6
Al
2
(OH)
13
CO
3
nH
2
O(M¼ Mg, Ni, Co) (Kloprogge and Frost,
1999) and Mg
x
Zn
6x
Al
2
(OH)
16
(CO
3
) 4H
2
O(Johnson et al., 2002). Raman micro-
spectroscopy of hydrocalumites reveal the dynami c disorder resulting from the free
rotation of the nitrate groups around the [0 0 1] axis, and from the half-bonded and
half-free H
2
O molecules (Renaudin and Franc-ois, 1999). The orientation of Co
2+
and Ni
2+
phthalocyanine in the interlayer space was also studied by the Raman
spectroscopy. The results indicate inter actions of the p electrons with the surface,
causing the organic molecules to adopt a flat orientation with respect to the surface
(De Fria et al., 1998).
Raman spectroscopy was also used to investiga te the kinetics and mechanisms of
catalytic acitivity, including the decomposition of H
2
O
2
by a molybdate-exchanged
LDH (Sels et al., 2001), and the epoxidation of geraniol by a vanadium-pillared
LDH (Villa et al., 2001).
FTIR spectro scopy is largely used to follow the alteration of the vibration bands
after intercalation and/or thermal treatment (Rives, 2002; Guo et al., 2002; Villegas
et al., 2002). This technique is also useful for assessing catalytic activity (e.g., cat-
alytic oxidation of sulphides (Choudary et al., 2002) and wet air oxidation of phenol
(Alejandre et al., 2001)), as well as surface acid–base properties. In the latter case, the
shift of a particular vibration band of a probe molecule, such as CDCl
3
or CO
2
,is
used to scale the interactions with basic surface sites, while the absorption bands for
pyridine and NH
3
provide an estimate of Brønsted and Lewis surface acidity (Lopez-
Salinas et al., 1997a, Chisem et al., 1998: Kooli et al., 1997c; Prinetto et al., 2000a).
Solid-State NMR
Different nuclei located either in the inorganic layers or the interlayer space may be
investigated by solid-state NMR spectroscopy.
27
Al NMR is commonly used to
determine the degree of conversion of Al
3+
from octahedral to tetrahedral envi-
ronment during the thermal decomposition or reconstruction of LDH phases
(Rocha et al., 1999).
27
Al and
71
Ga MAS NMR were used to investigate the thermal
decomposition of Mg/Al and Mg/Ga LDH (Aramendia et al., 1999).
Characterization of the interlayer species and their interactions with the host
structure are the subject of numerous NMR studies. The ordering of interlayer water
13.1.5. Structure 1045
and carbonate was studied using
1
H and
13
C NMR techniques (van der Pol et al.,
1994).
Incorporation of C
60
into LDH was established by measuring the spin-lattice
relaxation time ( T
1
)by
13
C NMR. The C
60
guest molecule does not freely rotate as in
the pure solid form (Tseng et al., 1996).
Other notable NMR studies are concerned with intercalated silicates by
29
Si
(Depe
`
ge et al., 1996), phenylphosphonic acid by
31
P(Carlino et al., 1996), styrene
sulphonate and poly(styrene) sulphonate by
13
C CP MAS (Mo ujahid et al., 2002a),
and borate by
11
B(del Arco et al., 2000). Static
51
V NMR spectra indicate hyperfine
magnetic interaction exerted by unpaired electrons of Ni
2+
on the inserted vanadium
nuclei in NiCo LDH (Me
´
ne
´
trier et al., 1997).
NMR experiments on uncommon nuclei were also reported. For example, Hou
and Kirkpatrick (2000) investigated the structures and dynamics of seleno-oxoanions
in LDH phases by solid-state
77
Se NMR. Although selenate is a tetrahedral anion,
the observed
77
Se chemical shift anisotropy is uniaxial when the anion is incorpo-
rated between the LDH layers. Static
35
Cl NMR spectroscopy was performed on
hydrotalcite and hydrocalumite samples (Hou et al., 2002). The collected data in-
dicate that the chlorine environment varies significantly with the hydration state.
27
Al,
35
Cl, and
6,7
Li nuclei in LiAl
2
–Cl LDH phases were studied by Hou and
Kirkpatrick (2001).
Further developments in this area would be expecte d with the application of two-
dimensional heteronuclear-correlated MAS NMR spectroscopy, e.g.,
1
H-
27
Al and
27
Al-
31
P correlations (Fernandez et al., 2001; Alba et al., 2001).
57
Fe Mo
¨
ssbauer Spectroscopy
This technique provided information on the mechanisms of oxidation of
Ni(II)–Fe(II) hydroxides in chloride-containing aqueous media leading to a Ni–Fe
pyroaurite-type material (Refait and Ge
´
nin, 1997).
57
Fe Mo
¨
ssbauer spectroscopy
was also us ed to study the in situ behaviour of a hydrated iron-substituted nickel
hydroxide in a Ni/Cd electrochemical cell (Guerlou–Demourgues et al., 1996).
Computer Modeling
By comparison with clay minerals, only a few computer-modelling studies on LDH
materials were reported so far (Newman et al., 2001).
Molecular dynamics computer simulations of Mg/Al hydrotalcite containing Cl
were performed to improve understanding of the structure, hydration, and swelling
behaviour of LDH (W ang et al., 2001; Hou et al., 2002). Similar approache s were
used by Kalinichev and Kirkpatrick (2002) on Friedel salt (Ca
2
Al(OH)
6
Cl 2H
2
O,
hydrocalumite) and ettringite to model some components of crystalline cement
phases. Bonapasta et al. (2001, 2002) modelled the c ross-linking of polyacrylate and
poly(vinyl alcohol) with Ca or Al atoms via the carboxylate or OH groups. This
reaction may be a key factor determining the filling of large voids in macro defect-
free cements.
Chapter 13.1: Layered Double Hydroxides1046
The surface adsorption and intercalation of fluorescein anions into ZnAl hy-
drotalcite were computed based on the structure of the host and the dimensions of
the guest compound (Costantino et al., 2000).
Other models were used to estimate the relative layer rigidity of LDH materials in
comparison to other 2D structures. The dependence on composition of the basal
spacing, determined by XRD, was simulated using an extended version of the dis-
crete finite-layer rigidity model including both intralayer and interlayer rigidity
effects (Solin et al., 1995).
13.1.6. MORPHOLOGY
A. Size and Shape
The shape of LDH particles is obviously de pendent on the method of preparation. In
the standard coprecipitation method both nucleation and growth steps oc cur si-
multaneously during synthesis. Particles are obtained with a high degree of crys-
tallinity leading to well-resolved XRD patterns but with a wide range of sizes. The
control of particle size is more effective by hydrothermal treatment or homogeneous
precipitation using urea hydrolysis. Well-defined plate-like hydrotalcite particles
with regular hexagonal shape and sizes between 2 and 20 mm were prepared (Ogawa
and Kaiho, 2002; Oh et al., 2002). The urea method always gives rise to larger
particles.
Yun and Pinnavaia (1995) compared the effect of coprecipitation conditions on
particle morphology and porosity. Fine-grained particles with rough surfaces, rel-
atively high surface areas, and mesopores of size in the range 5–30 nm in size are
obtained by copr ecipitation at varia ble pH. On the other hand, at constant pH,
larger, well-formed hexagon al particles with a narrower mesopore size distribution
(about 2 nm radius) are obtained. Edge-face particle aggregation and co-facial layer
stacking create interparticle mesopores. Structural defects in [Mg–Ga] were ob-
served by TEM ( Lopez-Salinas et al., 1998). Layers located nea r the edges of the
particles are more loose ly held together than those in the bulk of the particles.
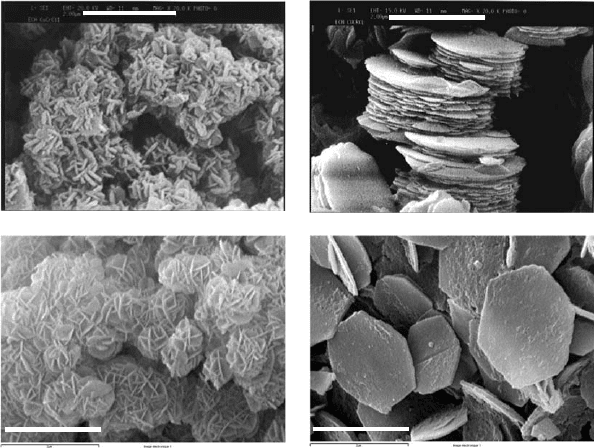
Adachi-Pagano et al. (2003) studie d the effect of preparat ion procedure on particle
size and aggregation of Cu
2
Cr(OH)
6
Cl 2H
2
OandMg
2
Al(OH)
6
(CO
3
)
0.5
2H
2
O
(Fig. 13.1.6). Coprecipitation with long ageing time often favours a sand-rose
morphology.
Morphology is also affected by chemical composition. Partial substitution of
Fe
2+
or Cu
2+
for Mg
2+
in synthetic hydrotalcite leads to a distinct change in
textural properties (Carja et al., 2001). The Cu
2+
-substituted LDH displays a rel-
atively uniform porous structure with enlarged mesopores and decreased specific
surface area compared with the unsubstituted Mg
2+
compound. The Fe
2+
-substi-
tuted LDH develops microporosity, non-uniform particles, decreas ed pore sizes, and
increased specific surface area.
13.1.6. Morphology 1047
Hydrotalcite-like materials with a unique sheet-like morphology and a diameter/
thickness ratios >100 were prepared by reacting MgO, AlOOH, and a mon-
ocarboxylic acid in aqueous media at 80–90 1C for 6 h at pH>7 (Kelkar an d Schutz,
1997a; Kelkar, et al., 1997b). In contrast to conventional hydrotalcites with hex-
agonal particle morphology, these materials have high mechanical strength making
them useful industrially. Acicular particles of [Mg–Al] hydrotalcite were prepared by
coprecipitation, and they show a high endothermic DSC peak and a wide temper-
ature range of decomposition (Ren et al., 2002).
B. Specific Surface Area and Porosity
The specific surface area of a single LDH layer with known composition and struc-
ture may be derived from the formula
S ¼ a
2
ffiffiffi
3
p
10
18
N=M
where N is the Avogadro’s number ð¼ 6:022 10
23
Þ, a the cell parameter (nm), and
M the molecular weight of the unit formula. For Zn
2
Cr(OH)
6
Cl 2H
2
O and
Mg
3
Al(OH)
8
(CO
3
)
0.5
2H
2
O, the calculated S values are 817 and 1285 m
2
/g, respec-
tively. Such high values are not measured because the internal surfaces are often
c)
2 µm
a)
2 µm
b)
2 µm
d)
2 µm
Fig. 13.1.6. SEM of Cu
2
Cr(OH)
6
Cl 2H
2
O prepared by (a) CuO/CrCl
3
-induced hydrolysis
and (b) coprecipitation (V. Pre
´
vot, unpublished results) and Mg
2
Al(OH)
6
(CO3)
0.5
2H
2
O
prepared by (c) coprecipitation and (d) urea method (Adashi-Pagano, et al., 2003).
Chapter 13.1: Layered Double Hydroxides1048
inaccessible. Typical values of the specific surface area measured by the BET tech-
nique range from 20 to 85 m
2
/g (del Arco et al., 1994). For Mg–Al LDH containing
simple anions such as carbonate, chloride, and nitrate, the surface area is mostly less
than 100 m
2
/g ( Pesic et al., 1992; Di Cosimo et al., 1998; Hussein et al., 2001; Inacio
et al., 2001). Hydrothermal crystallization decreases the surface area (Labajos et al.,
1992). Synthesis by coprecipitation in mixed solutions of water and organic solvents
increases the surface area (Malherbe et al., 1997; Inacio et al., 2001).
Nitrogen adsorption–desorption isotherms (type II with a narrow hysteresis) in-
dicate that LDH-containing simple anions are mesoporous with no microporosity
even when large POM pillars are intercalated (Labajos et al., 1992; Pesic et al., 1992;
Inacio et al., 2001). However, [Mg–Al] LDH intercalated by [Fe(CN)
6
] complexes
have an exceptionally high specific surface area of 499 m
2
/g (Nijs et al., 1999) and a
narrow pore size distribution with the majority of pores o0.71 nm. A correlation is
found between the Mg
2+
/Al
3+
ratio and the resulting microporosity after pillaring.
The optimal ratio is about 3.3, resulting in a pillared [Fe(CN)6]–MgA l–LDH with a
specific Langmuir surface area of 499 m
2
/g and a micr opore volume between 0.158
and 0.177 mL/g.
13.1.7. FUNDAMENTAL PROPERTIES
A. Chemical Stability
Chemical stability is of importance to many practical applications in which LDH
feature. For example, this parameter needs to be assessed when LDH are used as a
sink of radioactive metal ions from nuclear waste repositories (Allda et al., 2002). In
geochemical assessments, the stability of LDH is evaluated in terms of its solubility
in water (Ford et al., 1999; Scheckel et al., 2000). Solubility data for metal hydrox-
ides and LDH were derived from pH titration (Boclair and Braterman, 1998, 1999;
Boclair et al., 1999). The stability of LDH increases in the order Mg
2+
oMn
2+
o
Co
2+
E Ni
2+
oZn
2+
for divalent cations, and Al
3+
oFe
3+
for trivalent cations
(Boclair and Braterman, 1999). This trend accords with the pK
sp
values of the cor-
responding metal hydroxides (K
sp
is the solubility product). Because the pK
sp
of
Mg(OH)
2
is smaller than that of Zn(OH)
2
, Mg-based LDH are more soluble. Thus,
the aqueous solution of a Mg-based LDH is more basic than the corresponding
aqueous solution of a Zn-based LDH. Saturated aqueous solutions of Mg–Al–Cl
LDH and Zn–Al–Cl LDH, deaerated with Ar, have pH values of 8.91 and 6.97,
respectively (Shaw et al., 1990).
In addition to direct measurements of solubi lity, Allda et al. (2002) calculated the
aqueous solubility of an LDH from therm ochemical data. The heat of formation and
the free energy of formation of the carbonate form of an LDH are equal to
the values for a physical mixture of the binary compounds, i.e., the metal hydroxides
and carbonates. Their simple physical mixture model indicates that the aqueous
13.1.7. Fundamental Properties 1049
solubilities of LDH are affected by the interlayer anions. Relative to carbonate,
silicate an d borate decrease solubility, while nitrate and sulphate increase it.
B. Thermal Stability
The thermal stability of LDH was intensely investigated because the thermal de-
composition products are interesting as catalysts. Despite the diversity in compo-
sition most LDH exhibit a similar thermal decomposition behaviour. When heated,
LDH release interlayer water up to ca. 250 1C, followed by dehydroxylation of the
hydroxide layers, and decomposition of the interlayer anions at higher temperatures.
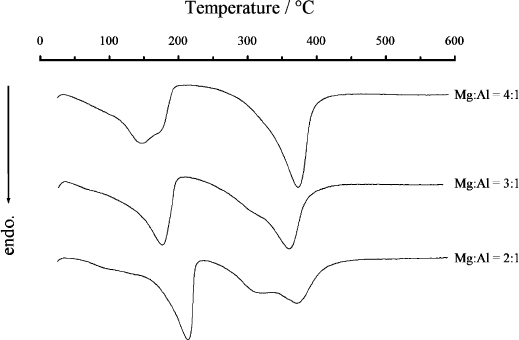
The differential thermal analysis (DTA) curves of Mg–Al–CO
3
LDH show two
major endothermic peaks (Fig. 13.1.7). The first peak (150–2 50 1C) corresponds to
the loss of interlayer water, while the seco nd (250–450 1C) arises from the decom-
position of the hydroxide layers and carbonate anions. In some cases, the second
peak splits into two peaks, the first of which corresponds to the loss of hydroxyl
groups bound to Al, while the second of the split peaks represents the loss of hy-
droxyl groups bound to Mg together with decomposition of carbonate anions
(Miyata, 1980). These weight-loss steps are observed in the corres ponding TG curve.
The XRD patterns indicate that the layer structure doe s not collapse with the loss of
interlayer wat er (Fig. 13.1.8). Valente et al. (2000) measured the decomposition
temperatures of carbonate forms of LDH containing various metal cations under the
same experimental conditions. They found that the thermal stability increases in the
order Co–AloZn–AlE Cu–AloMg–FeENi–AloMg–AlEMg–Cr. The highest de-
composition temperature is ca. 400 1C for Mg–Al LDH and Mg–Cr LDH, and the
lowest decomposition tempe rature is 220 1C for Co–Al LDH.
Fig. 13.1.7. DTA curves of Mg–Al–CO
3
LDHs having various Mg/Al atomic ratios. The Mg/
Al ratio is indicated on each curve.
Chapter 13.1: Layered Double Hydroxides1050
