Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

(Palmour and Johnson, 1967). The latter two approaches were subsequently devel-
oped by So
¨
rensen (1978) who devised a special heating procedure called ‘Stepwise
Isothermal Analysis’ (SIA). Here isothermal sections are combined with constant
heating rate sections in such a way as to keep the rate of mass loss or of sintering
between two pre-determined values. This is to combine the interest of an appro-
ximately controlled rate of dehydration or sintering and that of an easy kinetic
processing of the isothermal steps by relatively standard equations.
Fig. 12.11.6 shows the principle by which the thermal behaviour of clay minerals
may be studied incorporating the three improvements indicated above (analytical,
preparative and kinetic) (Rouquerol, 1970), using the technique of ‘Controlled Rate
Evolved Gas Detection’ (CR–EGD).
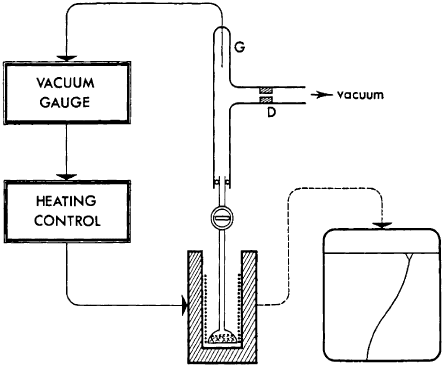
Here, the clay mineral sample is placed on the bottom of a fused silica bulb and is
permanently evacuated through a diaphragm, D. Any water vapour released by
sample causes an increase in the residual pressure (or ‘‘vacuum’’) above the sample,
and therefore, a small pressure drop through the diaphragm. The heating control is
set so as to maintain a constant rate of water vapour release from the clay mineral;
this is obtained by keeping the pressure drop through the diaphragm constant, and
therefore, by simply keeping constant the residual pressure above the sample (since
vacuum is constant downstream). The residual pressure is measured by a vacuum
gauge, G (Pirani, thermocouple or Penning gauge, or capacitance manometer) whose
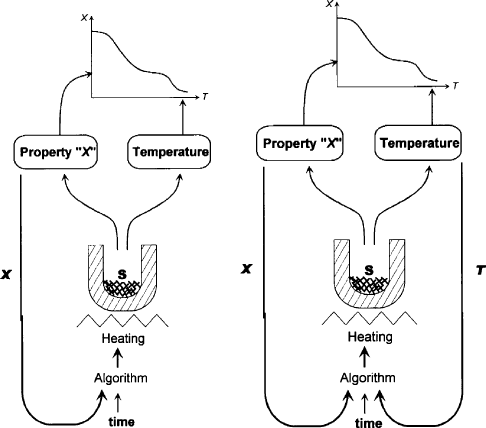
Fig. 12.11.5. Principle of Controlled Rate Thermal Analysis (CRTA, left), a part of the more
general method of Sample-Controlled Thermal Analysis (SCTA, right) where, nevertheless,
only the ‘‘X loop’’ is compulsory, not the ‘‘T loop’’ (Rouquerol, 2003).
12.11.3. Sample-Controlled Thermal Analysis 1009
signal directly feeds the heating control of the furnace (instead of the usual tem-
perature signal provided by a thermocouple or a resistance thermometer). As it will
be seen, this set up is used by several authors for the study of clay minerals.
Another set-up is that of the Constant Rate Thermogravimetry (CR-TG) pro-
posed by Paulik and Paulik (1971) under the name of ‘‘Quasi-Isothermal, Quasi-
Isobaric Derivatography’’ and specially used by Fo
¨
ldvari (1991) for the studies of
clay minerals. The set-up consists of a thermobalance operating at atmospheric
pressure, and controlled in the CRTA mode by feeding the heating control of the
furnace with the derivative dm/dt of the mass signal.
12.11.4. APPLICATION OF THERMAL ANALYSIS TO CLAYS
Our aim is not to provide a comprehensive summary of the literature on the
thermal analysis of clay minerals; rather, we will describe the most promising tech-
niques and approaches for future work in this field. In particular, we will show what
can be obtained with careful control of the experimental conditions, i.e., with Sam-
ple-Controlled Thermal Analysis. With the help of microprocessors and microcom-
puters, SCTA now reached an interesting state of maturity.
A. Analytical Aspects
The first objective of a good thermal analysis exp eriment is of course to separate as
much as possible the successive steps of any thermal reaction. In the case of clay
Fig. 12.11.6. Principle of a simple set-up of Controlled Rate Evolved Gas Detection
(CR-EGD) operating with continuous evacuation (Rouquerol, 1970).
Chapter 12.11: Thermal Analysis1010
minerals, this means separating as much as possible the steps of water evolution, i.e.,
separating the various types of water initially present. The improvement brought by
the SCTA approach is illustrated in Fig. 12.11.7 for a natural sepiolite from Vallecas
(Spain).
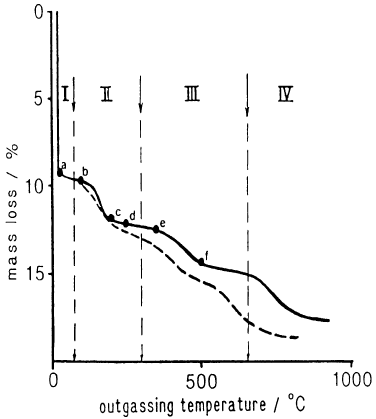
The dashed curve corresponds to a conventional TG experiment (Rautureau and
Mifsud, 1977) whereas, the solid curve was obtained by Controlled Transformation
Rate Evolved Gas Detection. The four steps are mo re clearly separated on the
CR-EGD than the TG curve (Grillet et al., 1988). Step I corresponds to the de-
sorption of zeolitic water. This is followed by the release of water molecules bound to
Mg atoms exposed at layer edges in two successive steps (II and III), before final
dehydroxylation of the mineral occurs (step IV).
The successive steps of the dehydroxylation of montmorillonite are also easily seen
on the CR-EGD trace in Fig. 12.11.8.(Laureiro et al., 1996). The first step up to ca.
350 1C is due to water desorption from the surface of the mineral (BET surface
area ¼ 78 m
2
/g. The second step up to ca. 650 1C is due to dehydroxylation limited by
a two-dimensional diffusion mechanism, while the last step corresponds to sintering.
The separation power of SCTA is also illustrated in Fig. 12.11.9 (Villieras et al.,
1992) showing the CR-EGD curve obtained for a mixture of talc and chlorite. The
dehydroxylation of chlorite apparently occurs in two separate steps (1 and 2), while
talc dehydroxylates in a singl e step (between 800 and 1000
o
C). The SCTA curve can
be used to determine the proportion of chlorite and talc in the sample.
Fig. 12.11.7. CR-EGD curve of a sepiolite from vallecas (solid curve) and corresponding
conventional TG curve (dashed curve) (Grillet et al., 1988).
12.11.4. Application of Thermal Analysis to Clays 1011
SCTA can also be used to characterise the initial state of clay miner als and, more
particularly, to distinguish between similar miner al species.
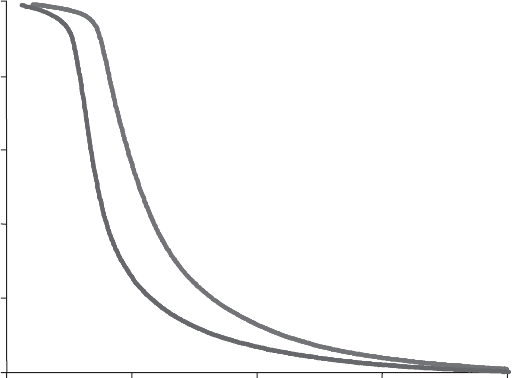
This is illustrated in Fig. 12.11.10 for two kaolinites of different origin (Dion
et al., 1998). Both samples (one from Charentes, France, the other from Keokuk,
UK) have the same grain size and are submitted to the same conditions of therm-
olysis (same rate of dehydration of 4.5 mg h
1
g
1
, and the same residual water va-
pour pressure of 0.45 mbar). The large displacement of one curve from the other may
be ascribed to the larger proportion of defects in the Char entes sample as compared
0
5
10
15
20
25
0 200 400 600 800
Temperature / °C
Time / h
Water desorption
Diffusion
mechanism D2
Sintering
Fig. 12.11.8. CR-EGD trace for the thermolysis of montmorillonite (Laureiro et al., 1996).
0
5
10
15
20
25
30
0 200 400 600 800 1000
Temperature °C
Time / h
Talc
Chlorite
(1)
Chlorite
(2)
Fig. 12.11.9. CR-EGD traces for a mixture of talc with chlorite (Villieras et al., 1992).
Chapter 12.11: Thermal Analysis1012
with the Keokuk material. These structural defects are responsible for lowering the
dehydroxylation temperature.
The high resolution obtainable from a Sample-Controlled experiment allows im-
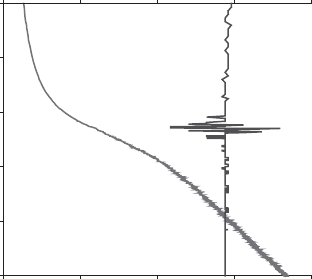
purities or very weak phenomena to be detected. Fig. 12.11.11 shows how the re-
crystallisation of metakaolinite can be detected, in a CR-EGD experiment. This
event is indicated by the simple perturbation of the pressure signal. This perturbation
is directly due to structural reorganisation allowing a very small amoun t (less than
0.1% of the initial water co ntent) of weakly bound water to escape at that inst ant.
B. Preparative Aspects
Sample-Controlled Ther mal Analysis allows samples of much greater homogeneity
to be prepared than is possible with standard temperature-progr ammed heating.
This is because the rate of dehydration can be controlled at a sufficiently low level for
low temperature and pressure gradients to be obtained. These conditions are re-
quired for homogeneity of the final product. This approach is indeed shown to be
most efficient to control the thermal transformation corresponding to the heating of
kaolinite (Dion, 1994; Massiot et al., 1995).
It is worth stressing that this SCTA approach lends itself to industrial application
simply by making use of the SCTA results recorded in the laboratory with a test
sample. One simply needs indeed to reproduce, in the industrial furnace, the same
T ¼ f(t) profile to ensure a controlled rate of dehydration up to the selected
temperature.
0
0.2
0.4
0.6
0.8
1
300 400 500 600 700
Temperature / °C
Extent of Reaciton
Charentes
Keokuk
Fig. 12.11.10. CR-EGD traces for two kaolinites of different origin (Dion et al., 1998).
12.11.4. Application of Thermal Analysis to Clays 1013
C. Kinetic Aspects
The major advantages of using SCTA to study the kinetics of thermolysis for a solid
like a clay mineral are as follows:
(i) the temperature gradients can be reduced at will; the single temperature sensor
in good contact with the sample therefore measur es a ‘meaningful temperature’.
This is far from being the case in conventional (constant heating rate) thermal
analysis where tempe rature differences from 5 to 50 K are observed between
different points of the sample in obtaining a DTA signal (Liptay, 1973);
(ii) at any time during dehydration the energy of activation may be determined by
the ‘rate-jump method’ (Rouquerol and Rouquerol, 1972). In this method, the
rate of dehydration is made to swing between two values (say, in the ratio 1/3 or
1/4) and the resulting modulated T ¼ f(t) curve is recorded (Fig. 12.11.12). Each
modulation of the experiment provides a set of 2 rates of dehydration, r
1
and r
2
(imposed) and 2 sample temperatures, T
1
and T
2
(measured). During the ‘rate-
jump’ from one temperature to the next, the state of the sample is virtually
unchanged, since the experiment is usually carried out in such a way as to
increase the extent of dehydration by less than 0.1%. This allows Arrhenius’ law
to be applied whi le the dependence of the rate of reaction on its extent is
ignored, i.e., without assuming any f(a) law. Under these conditions, the method
can be called either ‘isoconversional’ or ‘assumptionless’;
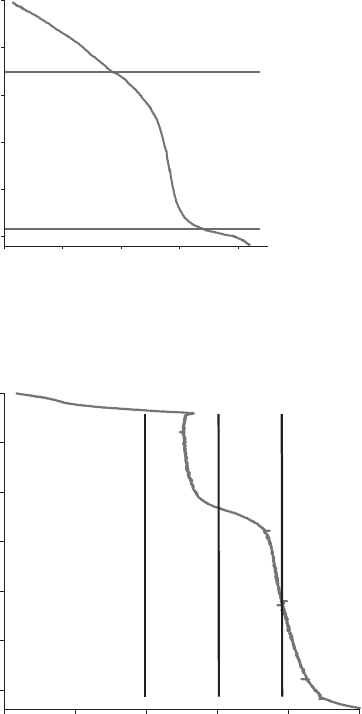
(iii) provided the rate of dehydration is kept constant throughout the experiment,
each dehydration mechanism gives rise to a specific shape of the CRTA curve, as
shown in Fig. 12.11.13 (Criado et al., 1990). These curves allow the major
0
5
10
15
20
25
-50 50 150 250
350
Temperature / °C
Time / h
2.7 2.75 2.8 2.85 2.9
Pressure / a.u.
Fig. 12.11.11. CR-EGD traces (temperature trace and pressure trace with perturbations)
showing transformation from kaolinite to metakaolinite (Dion, 1994).
Chapter 12.11: Thermal Analysis1014
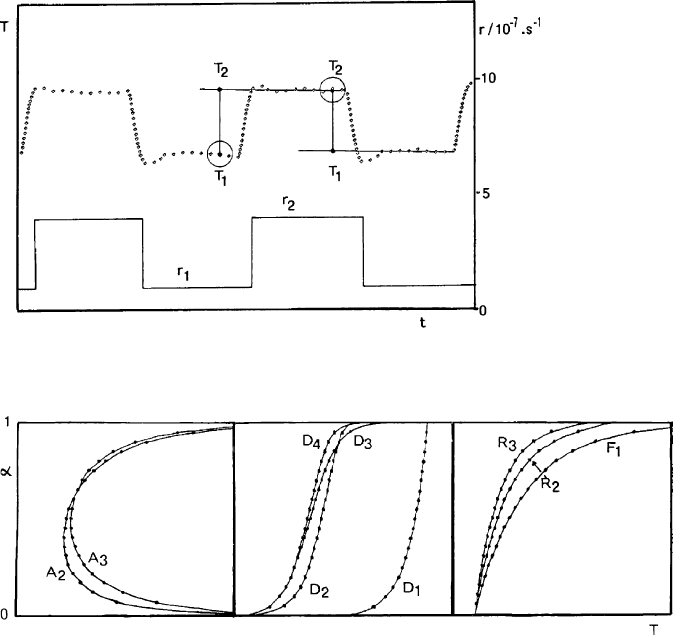
mechanisms to be differentiated; that is, where the rate of the dehydration is
controlled either by germination and growth of nuclei (saddle shaped, with
minimum), or by diffusion (S-sh aped) or by interfacial advancement (no min-
imum, no inflexion);
(iv) as reaction rates can be closely controlled CRTA uniquely provides a means of
passing progressively from a ‘thermodynamic regime’ (at very low rate of de-
hydration, under quasi- equilibrium conditions) to a ‘kinetic regime’ (at a higher
rate which is controlled by heat and/or mass transport phenomena);
(v) as CRTA can separate the successive steps in thermolysis and control the pro-
cess from beginning to end, rate laws within the entire reaction domain may be
established as was done for kaolinite (Nahdi et al., 2002a). This contrasts with
isothermal kinetics where either the first or the final part of the reaction, or both,
is skipped.
Fig. 12.11.12. Modulated T ¼ f(t) curve as recorded when applying the rate-jump procedure
to determine the activation energy.
Fig. 12.11.13. Three main shapes of isokinetic curves, corresponding to different basic mech-
anisms (Criado et al., 1990).
12.11.4. Application of Thermal Analysis to Clays 1015
(vi) finally, the reaction mechanism may be modelled for application to industrial
heating conditions as Nahdi et al. (2002b) done for kaolinite dehydroxylation.
REFERENCES
Caille
`
re, S., He
´
nin, S., 1948. L’analyse thermique et son interpre
´
tation. Proceedings of the
International Congress on Ceramics, Paris, pp. 137–151.
Criado, J.M., Ortega, A., Gotor, F., 1990. Correlation between the shape of controlled rate
thermal analysis curves and the kinetics of solid state reactions. Thermochimica Acta 157,
171–179.
Dion, P., 1994. De
´
shydroxylation de la kaolinite par analyse thermique a
`
vitesse de trans-
formation controˆ le
´
e. Etude de la me
´
takaolinite. Ph.D. thesis. Universite
´
d’Orle
´
ans, France.
Dion, P., Alcover, J.F., Bergaya, F., Ortega, A., Llewellyn, P., Rouquerol, F., 1998. Kinetic
study by controlled transformation rate thermal analysis of the dehydroxylation of ka-
olinite. Clay Minerals 33, 269–276.
Erdey, L., Paulik, F., Paulik, J., 1965. Device for automatic heating control of thermo-
balances, in the case of stepwise isothermal heating. Hungarian Patent N 1 152197, reg-
istered 31 October 1962, published 1 December 1965.
Fo
¨
ldvari, M., 1991. Measurement of different water species in minerals by means of thermal
derivatography. In: Smykatz-Kloss, W., Warne, S.St.J. (Eds.), Thermal Analysis in the
Geosciences. Springer, Berlin, pp. 84–100.
Grillet, Y., Cases, J.M., Franc- ois, M., Rouquerol, J., Poirier, J.E., 1988. Modification of the
porous structure and surface area of sepiolite under vacuum thermal treatment. Clays and
Clay Minerals 36, 233–242.
Laureiro, Y., Jerez, A., Rouquerol, F., Rouquerol, J., 1996. Dehydration kinetics of Wyoming
montmorillonite studied by controlled transformation rate thermal analysis. Thermochi-
mica Acta 278, 165–173.
Le Chatelier, H., 1887. De l’action de la chaleur sur les argiles. Bulletin de la Socie
´
te
´
Franc-aise
de Mine
´
ralogie 10, 204–211.
Liptay, G., 1973. Atlas of thermoanalytical curves. Akademia Kiado, Budapest.
Mackenzie, R.C., 1970. Differential Thermal Analysis, Volume 1. Academic Press, London
and New York.
Massiot, D., Dion, P., Alcover, J.F., Bergaya, F., 1995.
27
Al and
29
Si MAS NMR study of
kaolinite thermal decomposition by controlled rate thermal analysis. Journal American
Chemical Society 78, 2940–2944.
Nahdi, K., Perrin, S., Pijolat, M., Rouquerol, F., Ariguib, N., Ayadi, M., 2002a. Nu-
cleation and anisotropic growth model for isothermal kaolinite dehydroxylation
under controlled water vapour pressure. Physical Chemistry and Chemical Physics 4,
1972–1977.
Nahdi, K., Llewellyn, P., Rouquerol, F., Rouquerol, J., Ariguib, N.K., Ayadi, M.T., 2002b.
Controlled rate thermal analysis of kaolinite dehydroxylation: effect of water vapour
pressure on the mechanism. Thermochimica Acta 390, 123–132.
Palmour, H., Johnson, D.R., 1967. Phenomenological model for rate-controlled sintering. In:
Kuczynski, G.C., Hooton, N.A., Gibbon, C.F. (Eds.), Sintering and Related Phenomena.
Gordon and Breach, New York, p. 779.
Chapter 12.11: Thermal Analysis1016
Paulik, J., Paulik, F., 1971. Quasi-isothermal thermogravimetry. Analytica Chimica Acta 56,
328–331.
Rautureau, M., Mifsud, A., 1977. E
´
tude par microscopie e
´
lectronique des diffe
´
rents e
´
tats
d’hydratation de la se
´
piolite. Clay Minerals 12, 309–318.
Roberts-Austen, W.C., 1899. Proceedings of the Institute of Mechanical Engineering (Lon-
don), 1, 35–102.
Rouquerol, J., 1964. Me
´
thode d’analyse thermique sous faible pression et a
`
vitesse de de
´
com-
position constante. Bulletin de la Socie
´
te
´
Chimique de France 31–32.
Rouquerol, J., 1970. L’analyse thermique a
`
vitesse de de
´
composition constante. Journal of
Thermal Analysis 2, 123–140.
Rouquerol, F., Rouquerol, J., 1972. Activation energy of a thermolysis: conditions for a
significant measurement under very low pressures. In: Thermal Analysis. Proceedings of
the 3rd International Conference on Thermal Analysis, Davos, Switzerland, Vol. 1,
Birkhauser, Basel-Stuttgart, pp. 373–377.
Rouquerol, J., 2003. A general introduction to SCTA and to rate-controlled SCTA. Journal of
Thermoanalysis 72, 1081–1086.
So
¨
rensen, O.T., 1978. Thermogravimetric studies of non-stoichiometric cerium oxides under
isothermal and quasi-isothermal conditions. Journal of Thermoanalysis 13, 429–437.
Villieras, F., Yvon, J., Cases, J.M., Zimmermann, J.L., Baeza, R., 1992. Dosage et localisation
du fer 11 dans le talc et la chlorite par analyse spectrome
´
trique du gaz de thermolyse. CR
Acad. Sci, Paris 315, 121–1206.
Wendlandt, W.W., Collins, L.W., 1976. Thermal Analysis. Benchmark Papers in Analytical
Chemistry, Volume 2. Dowden, Hutchinson & Ross, Stroudsburg, PA, p. 13.
References 1017
Handbook of Clay Science
Edited by Bergaya, Theng and Lagaly
Developments in Clay Science, Vol. I
r 2006 Elsevier Ltd. All rights reserved.
1019
Chapter 13
SOME OTHER MATERIALS RELATED TO CLAYS
F. BERGAYA
a
, B.K.G. THENG
b
AND G. LAGALY
c
a
CRMD, CNRS-Universite
´
d’Orle
´
ans, F-45071 Orle
´
ans Cedex 2, France
b
Landcare Research, Palmerst on North, New Zealand
c
Institut fu
¨
r Anorganische Chemie, Universita
¨
t Kiel, D-24118 Kiel, Germany
In this chapter the layered double hydroxides (LDH) called anionic clays
(Chapter 13.1), three-dimensional zeolites (Chapter 13.2), which share several com-
mon properties with two-dimensional phyllosilicates, and finally the hydrated ce-
ment phases that benefit from the knowledge of clay mineral properties are discussed
(Chapter 13.3). Many other layer silicates and a large diversity of non-silicate layered
materials are not discussed even if the properties are sometimes quite similar
(Schwieger and Lagaly, 2004).
REFERENCE
Schwieger, W., Lagaly, G., 2004. Alkali silicates and crystalline silicic acids. In: Auerbach,
S.M., Carrado, K.A., Dutta, P.K. (Eds.), Handbook of Layered Materials. Marcel Dekker,
New York, pp. 541–629.
DOI: 10.1016/S1572-4352(05)01038-X
