Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

Villie
´
ras, F., Chamerois, M., Bardot, F., Michot, L.J., 2002b. Evaluation of wetting properties
of powders from gas adsorption experiments. In: Mittal, K.L. (Ed.), Contact Angle, Wett-
ability and Adhesion, vol. 2. VSP, Utrecht, pp. 435–447.
Villie
´
ras, F., Michot, L.J., Bardot, F., Cases, J.M., Franc- ois, M., Rudzinski, W., 1997a. An
improved derivative isotherm summation method to study surface heterogeneity of clay
minerals. Langmuir 13, 1104–1117.
Villie
´
ras, F., Michot, L.J., Bardot, F., Chamerois, M., Eypert-Blaison, C., Franc-ois, M.,
Ge
´
rard, G., Cases, J.M., 2002a. Surface heterogeneity of minerals. Comptes Rendus
Geosciences 334, 597–609.
Villie
´
ras, F., Michot, L.J., Cases, J.M., Berend, I., Bardot, F., Franc- ois, M., Ge
´
rard, G.,
Yvon, J., 1997b. Static and dynamic studies of the energetic surface heterogeneity of clay
minerals. In: Rudzinski, W., Steele, W.A., Zgrablich, G. (Eds.), Equilibria and Dynamics
of Gas Adsorption on Heterogeneous Solid Surfaces. Elsevier, Amsterdam, pp. 573–623.
Chapter 12.9: Surface Area and Porosity978
Handbook of Clay Science
Edited by F. Bergaya, B.K.G. Theng and G. Lagaly
Developments in Clay Science, Vol. 1
r 2006 Elsevier Ltd. All rights reserved.
979
Chapter 12.10
CATION AND ANION EXCHANGE
F. BERGAYA
a
, G. LAGALY
b
AND M. VAYER
a
a
CRMD, CNRS-Universite
´
d’Orle
´
ans, F-45071 Orle
´
ans Cedex 2, France
b
Institut fu
¨
r Anorganische Chemie, Universita
¨
t Kiel, D-24118 Kiel, Germany
Thomson (1850), Way (1852), Johnson (1859),andvan Bemmelen (1888) pio-
neered the study of cation exchange in soils. Since that time much progress was made
in our understanding of this phenomenon. The upsurge of interest in such issues as
fertilizer efficiency or contaminant mobility in the environment contributed to the
large increase in publications on cation exchange in soils, particularly with respect to
the clay fraction of soils. Ion exchange is of fundamental and practical importance to
soil studies and all fields in which clay materials feat ure. Indeed, the ability of
colloidal particles (including clay minerals) to retai n and exchange positively charged
ions is ‘perhaps the most important chemical property of natural porous media’
(Verburg and Baveye, 1994 ). This property has a controlling influence on the mo-
bility of positively charged chemical sp ecies in soil, such as potassium (from fer-
tilizers) and heavy metal ions (see Chapter 11.1) as well as on the geochemical cycling
of cations in general.
This chapter gives a brief survey of cation and anion exchange, with particular
reference to the definition and determination of the cation exchange capacity (CEC)
of clay minerals. The main questions are concerned with (i) the best method to
measure CEC and (ii) the real meaning of the measured CEC under specified con-
ditions.
12.10.1. ION EXCHANGE REACTIONS
A. Cation Exchange Equilibria
A
+
+ B
+
B
+
+ A
+
DOI: 10.1016/S1572-4352(05)01036-6
Cation exchange equilibria are often illustrated in diagrams by plotting the con-
centration ð
c
A
þ
Þ or equivalent fraction ð
w
A
þ
Þ of A
+
at the surface vs. the concen-
tration ðc
A
þ
Þ or equivalent fraction ðw
A
þ
Þ of this ion in the equilibrium solution. For
monovalent cations the equivalent fractions are given by
w
A
þ
¼
m
A
þ
=ð
m
A
þ
þ
m
B
þ
Þ
w
B
þ
¼
m
B
þ
=ð
m
A
þ
þ
m
B
þ
Þ
ð1Þ
where m denotes the concentration (in mol per volume or area unit) of the specified
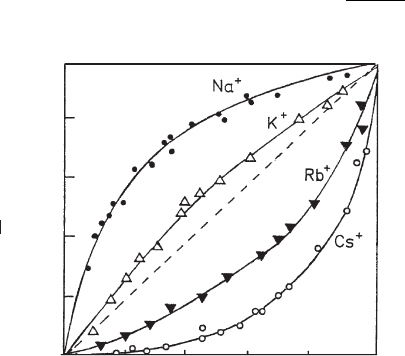
cations at the surface and in solution. Fig. 12.10.1 shows the exchange of ammonium
ions on montmorillonite by sodium, potassium, rubidium, and caesium ions (Martin
and Laudelout, 1963). The diagonal of the ‘square plot’ (broken line) indicates no
preference for either cation. In practice, the exchange shows a certain preference of
one cation over another as indicated by the deviation of the curve from the diagonal.
Thus, in contact with so dium ions the equivalent fract ion of ammonium ions at the
surface is distinctly higher than that in solution, i.e., NH
4
+
ions are preferentially
adsorbed over Na
+
ions. By contrast, Cs
+
ions are preferred to NH
4
+
ions.
Cation exchange reactions may be described by the law of mass action using the
activities of the ions, rather than their concentrations
K ¼
a
B
þ
a
A
þ
a
A
þ
a
B
þ
ð2Þ
0
1
0.2
0.6
0.4
0.8
0.8 10.60.40.20
NH
4
+
χ
NH
4
+
χ
Fig. 12.10.1. Exchange of ammonium ions by alkali ions on montmorillonite (Camp Berteau)
in 0.05 M solutions at 25 1C.
w
NH
þ
4
and w
NH
þ
4
are the molar fractions (Eq. 1) of ammonium ions
at the surface and in the equilibrium solution (Martin and Laudelout, 1963). From Jasmund
and Lagaly (1993).
Chapter 12.10: Cation and Anion Exchange980
where a
A
þ
and a
B
þ
are the activities of ions A
+
or B
+
in solution,
a
A
þ
and
a
B
þ
the
activities of these ions at the surface of the exchanger, and g is the activity coefficient.
That is, a
A
þ
¼ c
A
þ
g
A
þ
and
a
A
þ
¼
c
A
þ
g
A
þ
: K repres ents the thermodynamic equilib-
rium constant. If the cation exchange is performed in sufficiently dilute solution, the
activity coefficients of the ions in solution g
i
approach unity but this does not apply
to the activity coefficients of the ions at the surfa ce ð
g
i
Þ. This is because the con-
centration of all ions at the surfa ce ði:e:;
c ¼
c
A
þ
þ
c
B
þ
Þ does not decrease when the
solution is diluted.
Using concentrations and activity coefficients, Eq. (2) becomes
K ¼
c
B
þ
g
B
þ
c
A
þ
g
A
þ
c
A
þ
g
A
þ
c
B
þ
g
B
þ
¼ K
S
g
B
þ
g
A
þ
ð3Þ
where K
S
is the selectivity coefficien t. This coefficient is often used instead of the true
equilibrium constant, K, because all parameters in K
S
can be directly measured. As
mentioned above, g
A
þ
and g
B
þ
but not
g
A
þ
and
g
B
þ
approach unity in dilute so-
lutions. It is noteworthy that K
S
is not constant but changes as the exchange reaction
progresses, i.e., with the ratio
c
B
þ
=
c
A
þ
: When B
+
approaches the surface that is
mainly occupied by A
+
, steric effects and the free energy of exchange can be dif-
ferent from the situation when B
+
approaches a surface with B
+
cations occupying
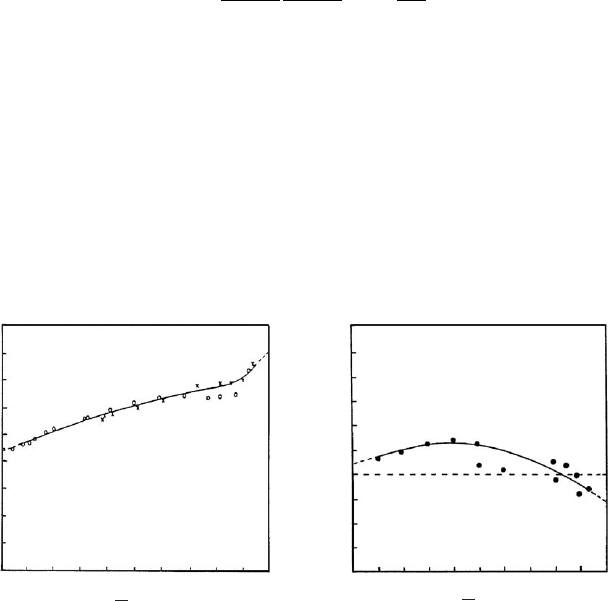
most exchange positions. Fig. 12.10.2 illustrates the variation of K
S
as the amount of
sodium ions on the surface increases for a vermiculite and a montmorillonite (Gast
and Klobe, 1971; Gast, 1972).
0
10.80.60.40 0.2
3.2
2.4
1.6
0.8
ln K
s
ln K
s
Na
+
χ
10.80.60.40 0.2
Na
+
χ
2.4
1.6
0.8
-1.6
-0.8
0
(a) (b)
Fig. 12.10.2. Variation of the selectivity coefficient K
S
with the molar fraction of sodium ions,
w
Na
þ
, on the clay mineral when the Li
+
cations are exchanged by Na
+
at 25 1C(Gast and
Klobe, 1971; Gast, 1972). From Jasmund and Lagaly (1993). (a) Vermiculite (Transvaal,
South Africa), total electrolyte concentration 0.01 M. (b) Montmorillonite (Chambers;
Arizona, API No. 23), total electrolyte concentration 0.001 M.
12.10.1. Ion Exchange Reactions 981

To obtain the true equilibrium constant, K, the activity coefficients of the cations
on the surface have to be determined. This procedure and the evaluation of the free
enthalpy of exchange (by integrating the function lnK
S
) were describ ed by Gast and
Klobe (1971). The same principles earlier used by Theng et al. (1967) to descri be the
exchange of alkylammonium ions with sodium and calci um ions on montmorillonite.
For the heterovalent exchange
A
+
+ B
2+
B
2+
+ 2A
+
A
+
the thermodynamic equilib rium constant is
K ¼
a
B
2þ a
2
A
þ
a
2
A
þ
a
B
2þ
ð4Þ
Often the equivalent fractions of the ions on the surface were introduced
w
A
þ
¼
m
A
þ
=ð
m
A
þ
þ 2
m
B
2þÞ
w
B
2þ
¼ 2
m
B
2þ
=ð
m
A
þ
þ 2
m
B
2þ
Þ
ð5Þ
where m is expressed in mol per volume or area. The (Gaines–Thomas) selectivity
coefficient is given by
K
s
¼
w
B
2þa
2
A
þ
w
2
A
þ
a
B
2þ
ð6Þ
Soil scientists often use other coefficients such as the Vanselow selectivity coefficient,
K
S,V
(Anderson and Sposito, 1991), or the Gapon coefficient, K
S,G
K
S;V
¼
w
B
2þa
2
A
þ
w
A
þ
a
B
2þ
ð7Þ
K
S;G
¼
w
B
2þa
A
þ
w
A
þ
ffiffiffi
a
p
B
2þ
ð8Þ
The separation factor, a , only considers concentrations
a ¼
c
B
2þc
A
þ
c
A
þ
c
B
2þ
ð9Þ
Chapter 12.10: Cation and Anion Exchange982
The Vanselow and Gapon coefficients are introduced because in some particular
cases (Shainberg et al., 1987) their values show little change with the degree of
exchange.
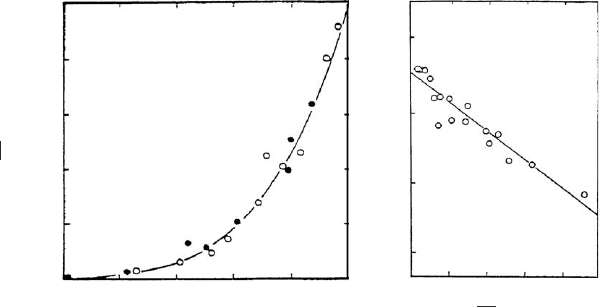
In heterovalent exchange reactions, the more highly charged cations are preferred
over monovalent ions, as indicated by Eq. (4) (Laudelout et al., 1968). This is
illustrated by Fig. 12.10.3 showing the equilibrium (
w
K
þ
vs. w
K
þ
) and selectivity
coefficient for the K
+
/Ca
2+
exchange on montm orillonite (Inoue and Minato,
1979).
Nir and co-workers (Nir et al., 1986, 1994; Margulies et al., 1988; Hirsch et al.,
1989; Rytwo et al., 1996) evaluated the cation-binding coefficients from the exchange
isotherms (Table 12.10.1 ). The procedure consists in solving the electrostatic
Gouy–Chapman equations and calculating the amounts of cations adsorbed as the
sum of the cations residing in the double-layer region and those bound to the sur-
face. The model also accounts explicitly for cation complex ation in solution. By
taking into account the adsorption of CaCl
+
and MgCl
+
, the apparent increase in
CEC with divalent cation concentration is eliminated.
An alternative thermodynamic model was developed by Kraepiel et al. (1999) who
consider the clay mineral particles as a porous solid bearing permanent charges. In
this model, the Gouy–Chapman diffuse ionic layer is extended to the interlayer
space, while the high concentration of cations and their (differ ent) distribution in the
interlayer space (Kjellander, 1996; Quirk, 2001) are ignored.
0.8
1
0
10.80.60.40.20
0.2
0.4
0.6
K
+
χ
K
+
χ
10.80.60.40.20
K
+
χ
4
2
0
-2
ln
K
s
(a) (b)
Fig. 12.10.3. Exchange of Ca
2+
ions by K
+
ions (
J
) and of K
+
ions by Ca
2+
ions ()at
35 1C. Montmorillonite Aterazawa (Yamagata Pref., Japan) (Inoue and Minato, 1979). From
Jasmund and Lagaly (1993). (a) Equivalent fractions of K
+
ions on montmorillonite,
w
K
þ
; vs.
the equivalent fraction of K
+
ions in the equilibrium solution, w
K
þ
: (b) Variation of the
selectivity coefficient with
w
K
þ
:
12.10.1. Ion Exchange Reactions 983

The exchangeable cations occupy sites of different energy in the interlayer space
(Goulding and Talibudeen, 1980; Brouwer et al., 1983). The formation of ion-ex-
change sites with very high selectivity for caesium ions, induced by (i) repeated
wetting–drying cycles of potassium montmorillonite and (ii) layer charge reduction
using the Hofmann–Klemen effect, was described by Maes et al. (1985).
B. Selectivity
Clay minerals show a preference for larger over smaller inorganic cations. This
tendency, referred to as ‘fixation’ in the soil science lite rature, becomes more pro-
nounced as layer charge increases (Klobe and Gast, 1967; Laudelout et al., 1968;
Gast and Klobe, 1971 ; Sawhney, 1972; Maes and Cremers, 1977; Jasmund and
Lagaly, 1993 ). For smectites, this preference (for larger cations) follows the order:
Cs
þ
4Rb
þ
4K
þ
4Na
þ
4Li
þ
and
Ba
2þ
4Sr
2þ
4Ca
2þ
4Mg
2þ
At higher layer charge (vermiculites) the preference is
Mg
2þ
4Ca
2þ
Sr
2þ
Ba
2þ
As a consequence of the particular interaction between the large alkali ions and the
siloxane surface, these surface sites show the preference for caesium ions over lithium
ions whereas the ionizable surface groups at the edges have a much lower selectivity
for caesium ions (Anderson and Sposito, 1991).
The selectivity of the preferentially adsorbed cations decreases with increasing
degree of coverage by this cation (Gast and Klobe, 1971; Sawhney, 1972; Inoue and
Minato, 1979; McBride, 1979; Bergseth, 1980; Maes et al., 1985; Siantar and Fripiat,
1995; Staunton and Roubaud, 1997).
The increased preference of clay minerals for potassium (and also rubidium and
caesium) ions as layer charge increases (Schwertmann, 1962) is related not only to
the decrease in hydration energy (K
þ
¼ 2314 kJ=mol; Li
þ
¼ 2508 kJ=mol) but also
Table 12.10.1. Binding coefficients for different cations on montmorillonite (in M
–1
)
Li
+
Na
+
K
+
Cs
+
Mg
2+
Ca
2+
Sr
2+
Cd
2+
MB
+
TFT
+
0.6
1
2
200
2
y
4–40
y
5
10
z
10
8y
10
9y
MB
+
¼ methylene blue; TFT
+
¼ thioflavin.
Nir et al. (1986).
y
Rytwo et al. (1996).
z
Hirsch et al. (1989).
y
Margulies et al. (1988).
Chapter 12.10: Cation and Anion Exchange984
to the strongly enhanced van der Waals energy because K
+
can fit closely into the
ditrigonal cavity of the silicate layer. When Li
+
is displaced by K
+
in Wyoming
montmorillonite, the entropy change is negative (DS1 ¼ 2 3 :12 J=K), the (decisive)
value of DH1 is –2.98 kJ/mol and DG1 is –2.05 kJ/mol (Gast and Klobe, 1971; Gast,
1972; Maes and Cremers, 1977; Eberl, 1980; Goulding and Talibudeen, 1980).
The preference of clay minerals for certain cations is caused by several effects.
These include hydration of the cations at the surface and in solution (entr opy!),
electrostatic cation–surface and cation–cation inter actions, interaction between the
water molecules and the surface, and the polarizability or hard and soft acid–base
(HSAB) character of the cations (Xu and Harsh, 1992; Auboiroux et al., 1998).
Entropic effects are also often decisive (Laudelout et al., 1968; Gast and Klobe,
1971; Gast, 1972; Maes and Cremers, 1977; Inoue and Minato, 1979; McBride, 1979;
Jasmund and Lagaly, 1993). An influence of the oxidation state of the structural iron
was also observed (see Chapter 8).
The structure of the silicate layers (localization of charges, orientation of OH
dipoles, rotation of tetrahedra) should have a strong e ffect when the layer separation
is small as in the case of micas (Kodama et al., 1974). Potassium ions in micas can be
exchanged by other cations only under particular conditions, e.g., by complexing the
displaced K
+
in very dilute solutions (Scott et al., 1973), or reacting with barium
ions at 80–120 1C(Reichenbach and Rich, 1969; Reichenb ach, 1973). The latter
process is accompanied by a decrease in layer charge (Beneke and Lagaly, 1982).
The cation exchange reaction in micas often proceeds as a co-operative process
(Reichenbach and Rich, 1969; Reich enbach, 1973).
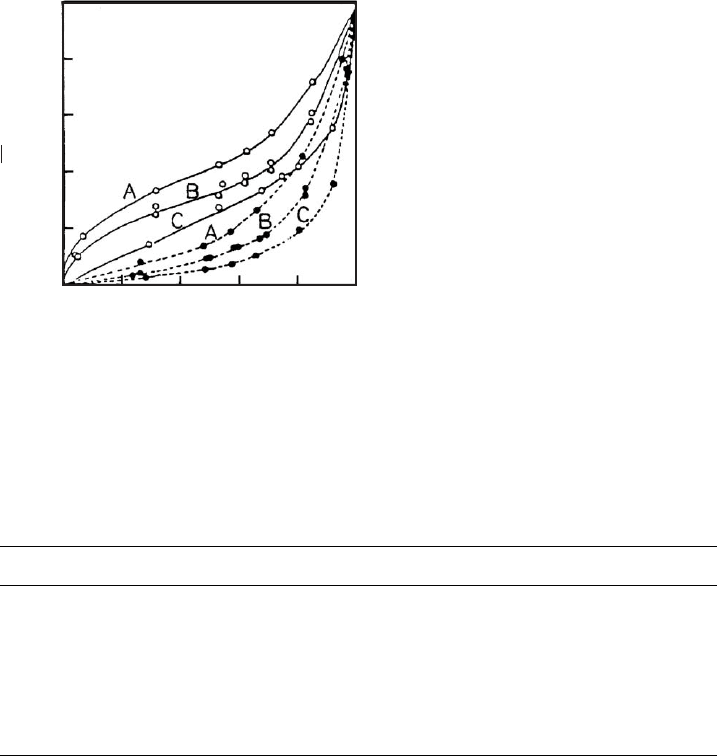
C. Hysteresis
Exchange reactions on clay minerals typically show hysteresis effects. This is illus-
trated in Fig. 12.10.4 for the K
+
/Ca
2+
exchange on montmorillonite induced by
drying. The curves A, B, and C (dotted) represent the exchange of Ca
2+
by K
+
at
three different total concentrations. For K
+
-montmorillonite that was dried at
80 1 C, the exchange of K
+
by Ca
2+
follows the solid curves indicating that the K
+
/
Ca
2+
ratios on the surface are higher than those obtained during the exchange of
Ca
2+
by K
+
(Inoue and Minato, 1979 ).
The thermodynamics of binary exchange of cations on clay minerals and soils was
reviewed by Verburg and Bav eye (1994). The model proposed takes into account the
clay mineral type, state of hydration, and electrolyte concentration, at least qua l-
itatively. The exchangeable cation s are classified into three groups (Table 12.10.2). If
exchange occurs between cations of different groups, hysteresis appears due to one of
several mechanisms: heterogeneous distribution of charges or surface sites, site in-
accessibility caused by coagulation or flocculation (formation of aggregates), clay
mineral dehydration, differences in cation hydration, and osmotic or extensive in-
terlayer swelling (see Chapters 5 and 13.2). A satisfactory explanation of hysteresis
effects is still lacking.
12.10.1. Ion Exchange Reactions 985
D. Heavy Metal Ion Adsorption
The exchange adsorption of heavy metal cations by clay minerals was widely studied
because of environmental concerns about heavy metal pollution (see Chapter 11.1)
(Nagy and Ko
´
nya, 1988; Siantar and Fripiat, 1995; Altin et al., 1998; Bereket et al.,
1997; Kodama and Komarneni, 1999; Schlegel et al., 1999a, 1999b; Strawn and
Sparks, 1999; Abollino et al., 2003). The amount of multivalent heavy metal cations
adsorbed often exceeds the CEC of the clay mineral. One reason is that multivalent
cations can be bound by equimolar, rather than equivalent, exchange (Weiss, 1958c;
0.8
0.6
0.4
0.2
0
10.80.60.40.20
1
K
+
χ
K
+
χ
Fig. 12.10.4. Hysteresis of the Ca
2+
/K
+
exchange on montmorillonite after drying at 80 1 C.
Dotted curves denote exchange of Ca
2+
by K
+
, while full curves describe exchange of K
+
by
Ca
2+
. Total electrolyte concentrations (eq/L): A, 0.1; B, 0.05; C, 0.01. Montmorillonite as in
Fig. 3 (Inoue and Minato, 1979). From Jasmund and Lagaly (1993).
Table 12.10.2. Classification of cations in three groups. Hysteresis was documented for binary
reactions involving cations from different groups (Verburg and Baveye, 1994)
Group 1 Group 2 Group 3
Na
+
K
+
Ca
2+
Li
+
Rb
+
Ba
2+
Cs
+
Sr
2+
NH
4
+
Mg
2+
Mn
2+
Cu
2+
Ni
2+
Chapter 12.10: Cation and Anion Exchange986

Rytwo et al., 1996; Tournassat et al., 2004.):
A
+
BX
+
+ 2B
2+
+2X
-
+ 2A
+
A
+
BX
+
In addition , the heavy metal ions can be precipitated at the surface in the form of
(hydr)oxides, hydroxy carbonates, or other basic salts (Siantar and Fripiat, 1995)
(for surface precipitation see Chapter 5). In analysing cation exchange data it is
therefore important to separate the exchange reaction from accompanying processes
such as adsorption, surface precipitation, and dissolution (Nagy and Ko
´
nya, 1988;
Schlegel et al., 199 9b ; Tournassat et al., 2004).
The presence of complexing compounds promotes the exchange of interlayer
cations in clay minerals (Ko
¨
ster et al., 1973; Maes et al., 1982; Rytwo et al., 1996;
Ko
´
nya and Nagy, 1998; Ko
´
nya et al., 1998; Abollino et al., 2003). The influence
of solvents on the exchange process was investigated by Hanna et al. (1995) and
El-Batouti et al. (2003).
E. Anion Exchange
Clay minerals also have a certain capacity for anion exchange. For anions, such as
chloride and nitrate, the anion exchange capacity amounts to a few cmol()/kg. By
contrast, up to 20–30 cmol()/kg of phosphate and arsenate can be adsorbed by
kaolinite and montmorillonite (Muljadi et al., 1966a, 1966b, 1966c; Grim, 1968;
Parfitt, 1978; Bergseth, 1985; Violante and Pigna, 2002) and distinctly more by
allophanes (Theng et al., 1982). Kaolinite and montmorillonite can also adsorb
10–20 and 20–30 cmol()/kg of fluoride ions, respectively (Weiss et al., 1956).
Clay minerals can adsorb anions by three different mechanisms:
(1) By electrostatic interaction with particle edges when these are positively charged
(see Chapter 5) (van Olphen, 1951b; Schofield and Samson, 1954; Ferris and
Jepson, 1975).
(2) By exchanging structural OH groups at the edges, and also on basal (planar)
surfaces of kaolinite (Mulja di et al., 1966a, 1966b, 1966c; Parfitt, 1978; Theng
et al., 1982). This process is referred to as ‘ligand exchange’ or ‘specific adsorp-
tion’ although the latter term is outmoded (see Fig. 5.2 in Chapter 5).
(3) By accompanying multivalent cations at exchange positions (see Section D).
In mechanism (1) the extent of anion exchange decreases with increasing pH and
approaches zero at the point of zero net pro ton charge, p.z.n.p.c. (see Chapter 5).
The amount of anions bound by ligand exchange should increase to a maximum
around the p.z.n.p.c. However, the adsorption of anions as counterions in an acidic
medium may decrease with increasing pH as it is generally observed for phosphate
12.10.1. Ion Exchange Reactions 987
