Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

(Ioannou and Dimirkou, 1997). Anions can be adsorbed in larger amounts by ligand
exchange than by counterion binding because ligand exchange also operates when
the surface is uncharged or even negatively charged.
In the case of multivalent anions, the dominance of a certain species in solution at
a distinct range of pH values does not imply that this species is the major surface
species. The presence of the differently charged anions in the aqueous solution is
determined by the dissociation constants (pK
a
values), whereas the formation of the
surface species also depends on the surface complexing constants. The constant
capacitance model, one of the models used to describe surface complexation
(Goldberg, 1992), indicates that the proportion of neutral species [SH
2
PO
4
](S¼ sur-
face) decreases to zero at pH 7, while the proportion of [SHPO
4
–
] species increases to
a maximum at pH 5.5, and [SPO
4
2–
] is the sole species at pH>8 (Ioannou and
Dimirkou, 1997). Arsenate adsorption showed a maximum around pH 6(Manning
and Goldberg, 1996).
Multivalent cations may form insoluble salts with anions, making it uncertain
whether the adsorption of the anions proceeds as a true anion exchange or by
precipitation (Rao and Murti, 1987).
Weiss et al. (1956) found that the anion exchange capacity depends on the di-
ameter of the montmorillonite particles if their shape is assumed to be almost reg-
ular. This would be expected if the anion exchange occurs on the edges. From the
adsorption of fluoride ions, the authors derived average diameters of montmorillo-
nite particles of 300–450 mm. By contrast, most of the fluoride ions are adsorbed at
the basal (planar) surface of kaolinite particles, and the anion exchange capacity
depends on the thickness of these particles.
Although the an ion exchange capacity of clay minerals is small compared to their
CEC, the adsorption of anions is of great importance to soil fertility and plant
nutrition. The adsorption of phosphate by soil and soil minerals recei ved particular
attention (Parfitt, 1978; Bowden et al., 1980; Fox, 1980; Theng et al., 1982). Wend-
elbo and Rosenqvist (1987) observed that sulphate can exert a dispersive effect on
clay soils, intermediate between chloride and phosphate. Phosphate, oligopho s-
phates, polyphosphates, and many other polyanions are capable of stabilising clay
mineral dispersions against coagulation and flocculation (see Chapters 5 and 10.1).
Methods for determining the adsorption of phosphate and chloride were de-
scribed by Bain and Smith (1987).
12.10.2. DEFINITION OF CEC AND FACTORS INFLUENCING ITS
MEASUREMENT
The CEC of clays and clay minerals may be defined as the quantity of cations
available for exchange at a given pH (Table 12.10.3), and is traditionally expressed in
milliequivalents (meq)/100 g of calcined clay (Bergaya and Vayer, 1997). Thus, the
Chapter 12.10: Cation and Anion Exchange988
pH value during the exchange reaction has to be specified. Various units were used in
the literature to express CEC. Although CEC values are often given in terms of dried
clay, the drying conditions (105, 110, 140 1C, or higher temperature) are not always
specified (van Olphe n, 1951a, 1977; van Olphen and Fripiat, 1979). The unit of CEC
recommended by the IUPAC is cmol(+)/kg, which is numerically equivalent to meq/
100 g. Only a few papers mention the pH during the exchange reaction.
The exchange capacity is usually determined at neutrality (pH ¼ 7). Table 12.10.3
lists the CEC of some clay minerals, showing the broad range of values. This is
because cation exchange is influenced by many factors, such as the nature of the
exchangeable cations, particle size (grinding can change the CEC), temperature, and
phase conditions (dilute or concentrated aqueous solutions, organic solvents, solid-
state reactions). As a result, CEC is difficult to determine accurately. In any case,
CEC determination requires the complete replacement of all exchangeable cations by
‘index’ cations that are not present in the clay mineral sample.
12.10.3. ORIGIN OF CEC
The CEC of clay minerals is generally related to the layer charge. The measured
CEC is eq uivalent to the layer charge only when all the charge-compensating cations
are exchangeable. The CEC corresponds to the summation of two types of charges
arising from
(i) Layer (isomorphous) substitution if the compensating cations are not fixed on the
clay mineral and are exchangeable by other cations. This structural charge is also
called constant or permanent and is generated by ion substitutions or site va-
cancies in the octahedral and/or tetrahedral sheets (Gast, 1977) (see Chapter 2).
In most cases, the compensating cations are exchangeable. Specific interactions
with the silicate layers can impede quantitative exchange of the compensating
cations. An example is potassium ions in illites and micas, which are ‘fixed’ in the
ditrigonal cavities (Verburg and Baveye, 1994). Some cations undergo chemical
reactions altering the expected classical exchange behaviour (see Chapter 5).
Table 12.10.3. CEC of clay minerals in cmol(+)/kg( ¼ meq/100 g) (Weiss, 1958b; Grim,
1968)
Kaolinite 3–15
Halloysite 2H
2
O 5–10
Halloysite 4H
2
O 40–50
Montmorillonite 70–120
Vermiculite 130–210
Illite 10–40
Micas (biotite, muscovite) up to 5
Chlorite 10–40
Sepiolite, palygorskite 20–30
12.10.3. Origin of Cec 989
(ii) Coordination of the cations at the edges of the silicate layers is, depending on the
pH, completed by H
3
O
+
,H
2
O, or OH
(see Chapter 5). This pH-dependent
charge varies with the nature of the solution (ionic strength, pH) due to the
reactions:
MOH þ H
2
O$MO
þ H
3
O
þ
MOH þ H
3
O
þ
$MOH
þ
2
þ H
2
O
with M ¼ Si
4+
,Al
3+
,orMg
2+
. For other possible reactions, see Tournassat
et al. (2004). The contribution of the variable charge to the total charge depends
on particle morphology and the ratio of the edge/basal surface area. In case of
smectites, the pH-dependent charge varies between 10 and 20% of the total
charge (Anderson and Sposito, 1991). In kaolinites, the layers are aggregated to
larger particles, and the edges represent a larger fraction of the total surface area
(see Chapter 12.9). As kaolinites have little layer substitution, their structural
charge is relatively low, and the CEC is mainly attributed to the edge charges
(Gast, 1977).
12.10.4. CEC MEASUREMENTS
The CEC of clay minerals can be determined by a variety of established methods
(Van Olphen, 1951a, Weiss, 1958a, 1958b; Grim, 1968; Bain and Smith, 1987).
The choice of technique depends on the magnitude of the expected CEC, the nature
of the charge-compensating cations, and the available quantity of the sample. Some
methods are tedious and entail theoretical uncertainties and practical difficulties. It is
difficult to recommend a universal method for different clays and clay minerals.
The most widely used methods involve the displacement of the interlayer cations
with the index cations in a known volume of solution and the analytical determi-
nation of the cations by standard techniques such as atomic absorption, spectro-
photometry, or titration.
A. Classical Methods
These methods are based on the exchange between the cations at the clay mineral
surface and the index cations in solution. The measurements are influenced by a
number of experimental variables, including pH, temperature, solution ionic
strength, and nature of the index cation as well as by factors relating to the clay
mineral such as nature of the compensating cations, particle morphology and size,
and surface area/volume ratio of the particles (Grim, 1968). The two last factors are
important when the pH-dependent charge is high compared with the permanent
charge.
Chapter 12.10: Cation and Anion Exchange990
B. Influence of Exchangeable and Index Cations
The exchange reaction of clay minerals is stoichiometric in relation to the charges.
As discussed above, the different cations have neither the same replaceability nor the
same replacing power. For measuring CEC, it is important to use cations that are
preferred or held more tightly on the surface than the cations being replaced. In some
cases, the cations are fix ed on the clay mineral and are not displaced by other cations
even when an excess of highly preferred cations is added. Typical examples are
potassium ions in micas (see Section 12.10.1) and many organic cations.
Clay minerals show a strong preference for organic cations (Theng et al., 1967).
Several authors (see Section 12.10.5) used organic cations such as methylene blue
and crystal violet as index cations. Such cations are strongly adsorbed by clay min-
erals, their binding coefficients (Nir et al., 1986) are very high compared with alkali
or alkali-earth cations (Table 12.10.1).
When exchanging cations such as NH
4
+
or Ba
2+
are used, the procedure needs
several incubation and separation cycles. In this case the CEC measurements are also
influenced by further factors like the number of washings and the nature of the
washing solvent.
C. Influence of pH
The pH of the solution has two impacts:
(i) The pH at which the exchange reaction takes place as well as the nature of the
unbuffered or buffered solution determine the type of measured charge: perma-
nent or pH dependant charge. Thus, the pH during the exchange has to be
carefully measured and controlled. Grim (1968) proposed to determine the CEC
at pH ¼ 7 assuming that only the exchangeable cations on permanent sites will
participate in the exchange reaction. Amacher et al. (1990) proposed to deter-
mine the CEC in soils at different conditions in order to determine not only the
exchangeable cations but also the exchangeable cations at acidic sites.
(ii) The pH de termines also the form of the ions present in solution. Depending on
pH, many multivalent cations form oligomeric and polymeric hydroxo meta l
ions (see Chapter 5) and also at pH ¼ 7 the cations form different complexes.
The estimation of CEC from analytically determined amounts of cations pre-
supposes that the charge of these cations is known. If different forms of cations
are bound, the numerical value of the CEC is not correct (Bache, 1976 ).
12.10.5. ANALYTICAL TECHNIQUES
The basic steps of CEC determination starting from an X-clay are illustrated in
Fig. 12.10.5. We distinguish two kinds of determinations: the first is based on
quantitative analysis (E) after the replacement of exchangeable cations X by the
index cation A or B; the second consists of titrations (T) performed during the
12.10.5. Analytical Techniques 991
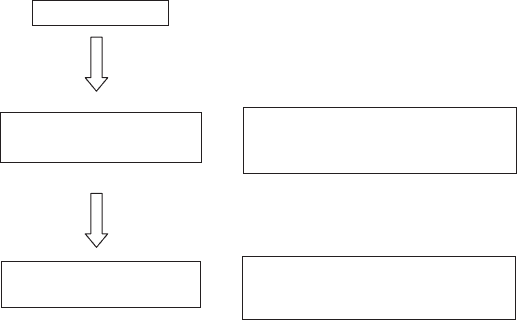
addition of the index cation A or B and supposes that the replacement of the initial
exchangeable cations X by the index cations A or B occurs immediately.
The first step is the replacement of all cations X on the clay mineral by the index
cations A when the solution containing cations A is added to the clay miner al.
During this step, the CEC is evaluated (T1) by following some physical properties of
the dispersion or the supernatant solution (after sedimentation) such as conductivity
(Dakshinamurti and Bhavanarayana, 1982), surface tensi on when a cationic surfact-
ant is the index cation (Burrafato and Miano, 1993), or the appearance of flocculat-
ion (Pleysier et al., 1986).
When all cations X are replaced (in some cases, total exchange needs several cycles
and thus is very time consuming), the CEC is derived by analysing either the excess
of cations A in solution (E1), the released cations X in solution (E2), or the index
cations A retained by the clay mineral (E3). E1 and E2 are complementary methods.
If E2 is used, the total volume of washing solution must be collected and considered.
Generally, it is the same for E1; in this case we consider the quantity and not the
concentration. Method E3 needs the complete removal of the non-adsorbed index
cations A by washing. The sampl es are generally washed with organic solvents. Small
amounts of salts can be retained if the organic solvent is not carefully chosen, in
which case the CEC is not correctly measured (Francis and Grigal, 1971). All E3
methods cited in the literature require sophisticated instruments such as radioactivity
counters or microwave equipment, or are completely destructive.
Other methods are based on the replacement of the cations A by the index cations
B in a second step. During this step, the CEC can be evaluated (T2) in the same way
as by T1. Either the excess of cations B remaining in solution (E4) is measured, or the
amount of the index cations A released into the solution is determined (E5). CEC
determination by measuring the amount of index cations B fixed on the clay mineral
(E6) was never reported. For both steps, classical analytical techniques such as
Addition of a solution with A-cations
T1: Titration of A-cations
X-clay
+
Solution with A-cations and X-cations
E1: analysis of A-cations in excess
E2: analysis of released X-cations
Solution with A-cations and B-cations
E4: analysis of B-cations in excess
E5: analysis of released A-cations
A-clay
E3: analysis of the A-cations
B-clay
E6: analysis of the B-cations
Addition of a solution with B-cations
T2: Titration of B-cations
+
Fig. 12.10.5. Basic steps of CEC determinations.
Chapter 12.10: Cation and Anion Exchange992
volumetry, atomic absorption spectrometry, inductively coupled plasma emission
spectrometry (ICPES), etc. are used.
The selection of a particular method is determined (i) by the choice of the index
cations and (ii) more simply, by the techniques available in the laboratory. CEC
determinations using different index cations are described below.
A. Exchange with Protons
Hissink (1924) measured the acidity of soils, and derived their CEC from titration.
Later, Glae ser (1946) and Grim (1968) proposed methods based on the acid–base
titration of H
+
-exchanged clay minerals. These methods are no longer in use because
the preparation of H
+
-clay miner als needs special care. Further, it is impossible to
reach constant pH because the presence of protons leads to autotransformation of
the minerals (see Chapter 5).
B. Exchange with Organic Cations
Since organic cations are preferentially exchanged over inorganic cations, they (es-
pecially methylene blue) were widely used for determining CEC as well as specific
surface areas (Brindley and Thompson, 1970; Hang and Brindley, 1970; API, 1990;
Rytwo et al., 1991; Kahr and Madsen, 1995) (see Chapter 7.3). Although total
exchange of the inorganic cations needs only one incubation cycle, the determination
of CEC by adsorption of methylene blue (MB) seems questionable because the
adsorbed MB cations can assume more than one orientation at the clay miner al
surface (Hang and Brindley, 1970). The surface area of the flat-lying monovalent MB
cation is about 1.32 nm
2
. For highly charged clay minerals, the interlayer area per
charge can be smaller than the area occupied by M B, and hence the amount ad-
sorbed may no longer be equal to the CEC.
Brindley and Thompson (1970) and Hang and Brindley (1970) derived the CEC of
clay minerals from saturation adsorption of MB but this is only valid for the Li
+
-and
Na
+
-exchanged forms. Rytwo et al. (1991) and Kahr and Madsen (1995) showed that
clay minerals adsorb MB in excess of the CEC. Accordingly, Rytwo et al. (1991)
proposed to determine the CEC from the amount of cations released into solution, while
Kahr and Madsen (1995) used the point where the MB added to the clay dispersion is
no longer adsorbed. This method requires the clay minerals to be in the Na
+
form.
C. Exchange with Ammonium Ions
Several methods use NH
4
+
as the index cation. The method involving leaching
with ammonium acetate and subsequent replacement of the NH
4
+
ions from ex-
change sites is widely used, and often serves as a standard or reference procedure
(Peech, 1945; Mackenzie, 1951; Fraser and Russell, 1969; Busenberg and Clemen cy,
1973; Greenhill and Peverill, 1977; Gillmann et al., 1983). However, the method is
12.10.5. Analytical Techniques 993
time-consuming because the exchange with NH
4
+
involves five to six steps. In ad-
dition, the CEC is generally determined by analysing the washed clay using tech-
niques that are sometimes laborious. However, the method has the following
advantages: (i) the exchange can be accomplished at a well-defined pH using buffered
solutions (pH 7 for NH
4
OAc and pH 8.5 for NH
4
Cl/NH
3
) and (ii) several techniques
can be used to determine the nitrogen content (Kjeldahl titration, acidimetry, am-
monium sensitive electrodes, colorimetry).
D. Exchange with Alkali or Alkaline-Earth Cations
The use of alkali or alkaline earth cations such as Cs
+
(Hillier and Clayton, 1992),
Ba
2+
(Bache, 1970), Ca
2+
,Na
+
,K
+
, and Sr
2+
as index cations A or B is also very
time-consuming as several cycles are needed to reach complete exchange. Different
analytical techniques are used such as conductivity (Mortland and Mellor, 1954),
electrochemical measurements with specific electrodes (Malcolm and Kennedy, 1969;
Chiu et al., 1990), nephelometry for small quantities (Adams and Evans, 1979), or
isotopic techniques (Bache, 1970; Francis and Grigal, 1971; Brouwer et al., 1983;
Maes et al., 1985) all of which are not commonly available in laboratories.
E. Exchange with Transition Metal Ions or Organo-Metal Complexes
The last group of methods used hydrates or complexes of transition metal cations as
index cations, such as [Co(H
2
O)
6
]
2+
(Rhodes and Brown, 1994) or [Co(NH
3
)
6
]
3+
(Mantin and Glaeser, 1960), silver thiourea cations (Chhabra et al., 1976; Pleysier
and Juo, 1980; Pleysier et al., 1986; Searle, 1986) and [Ni(en)
n
]
2+
(en ¼ ethylene
diamine) (Cornell and Aksoyoglu, 1991).
Mantin (1969) proposed a novel method to determine the CEC of clay minerals. It
consists of saturating the samples with metal cations (Cu
2+
,Ni
2+
,Zn
2+
,Mn
2+
,
Al
3+
,Fe
3+
), and then adding varied quantities of ethylene diamine to the clay
mineral dispersion. As a result, the complex [M(en)
n
]
p+
is formed in the interlayer
space. The CEC is derived from the quantity of ethyl ene diamine required for
transforming the metal cation completely into the complex. Mantin further sug-
gested the use of [M(en)
n
]
p+
complexes such as [Ni(en)
n
]
2+
for CEC determinations.
The method based on [Cu(en)
2
]
2+
was developed by Bergaya and Vayer (1997),
while Meier and Kahr (1999) proposed the use of [Cu(trien)
3
]
2+
complex to deter-
mine CEC. Ammann et al. (2005) found good agreement between the two methods if
the pH-dependence of the photometric determinations is taken into account.
12.10.6. OTHER TECHNIQUES
The CEC of smectites and vermiculites can be calculated from the layer charge
determined by the alkylammonium method ( Lagaly, 1994). The CEC values so
Chapter 12.10: Cation and Anion Exchange994
derived correspond to the permanent negative layer charge that in the case of
smectites, are 10–20% smaller than the (total) exchange capacity (Lagaly, 1981,
1994). The CEC can also be estimated from the colloidal properties and the sediment
values of clay dispersions in the presence of surfactants (Janek an d Lagaly, 2003). On
the rare occasion when the clay miner al sample is non-interstratified and very pure,
or when its mineralogi cal composition is exactly known, the CEC may be deduced
from chemical analysis.
12.10.7. CONCLUSIONS
The determination of CEC of clay minerals, using [Cu(en)
2
]
2+
and [Cu(trien)]
2+
complexes, is presently the most versatile method.
(1) The procedure is very simple and well suited for routine analysis. No sophis-
ticated chemicals and materials are required. Only glass vessels, stirring devices, a
centrifuge, an d possibly a photometer are needed.
(2) The exchange reaction is fast.
(3) The Cu
2+
complexes can displace all kinds of cations from exchange sites on the
clay minerals, including heavy metal cations. Full quantitative exchange can be
obtained with Na
+
- and Ca
2+
-montmorillonites (Bergaya and Vayer, 1997;
Ammann et al., 2005). This accords with the high equilibrium constant of the
Cu
2+
/Na
+
exchange reaction, indicative of ideal ion-exchange behaviour
(Fletcher and Sposito, 1989).
(4) The results are reproducible over a wide range of CEC values, and good accuracy
may be obtained even with small amounts of samples or clays with low CEC.
(5) The measurement is not restricted to a particular pH (Ammann et al., 2005).
Although the use of metal complexes as index cations may not be universally
applicable, the method allows the CEC of many types of clays and clay minerals to
be determined simply and reliably.
REFERENCES
Abollino, O., Aceto, M., Malandrino, M., Sarzanini, C., Mentasti, E., 2003. Adsorption of
heavy metals on Na-montmorillonite. Effect of pH and organic substances. Water Re-
search 37, 1619–1627.
Adams, J.M., Evans, S., 1979. Determination of the cation-exchange capacity (layer charge) of
small quantities of clays minerals by nephelometry. Clays and Clay Minerals 27, 137–139.
Altin, O., O
¨
zbelge, H.O
¨
., Dog
˘
u, T., 1998. Use of general purpose adsorption isotherms for heavy
metal–clay mineral interactions. Journal of Colloid and Interface Science 198, 130–140.
Amacher, M.C., Henderson, R.E., Breithaupt, M.D., Seale, C.L., Labauve, J.M., 1990. Un-
buffered and buffered salt methods for exchangeable cations and effective cation exchange
capacity by potentiometric titration using divalent cation electrodes. Soil Science Society of
America Journal 54, 1036–1042.
12.10.7. Conclusions 995
Ammann, L., Bergaya, F., Lagaly, G., 2005. Determination of the cation exchange capacity of
clays with copper complexes revisited. Clay Minerals 40, 441–453.
Anderson, S.J., Sposito, G., 1991. Cesium-adsorption method for measuring accessible struc-
tural surface charge. Soil Science Society of America Journal 55, 1569–1576.
API, 1990. Recommended practice standard procedure for laboratory testing drilling fluids.
API Recommended Practice 13, 1.
Auboiroux, M., Melou, F., Bergaya, F., Touray, J.C., 1998. Hard and soft acid–base model
applied to bivalent cation selectivity on a 2:1 clay mineral. Clays and Clay Minerals 46,
546–555.
Bache, B.W., 1970. Barium isotope method for measuring cation-exchange capacity of soils
and clays. Journal of the Science of Food and Agriculture 21, 169–171.
Bache, B.W., 1976. The measurement of cation exchange capacity of soils. Journal of the
Science of Food and Agriculture 27, 273–280.
Bain, D.C., Smith, B.F.L., 1987. Chemical analysis. In: Wilson, M.J. (Ed.), A Handbook of
Determinative Methods in Clay Mineralogy. Blackie, Glasgow, pp. 248–274.
Beneke, K., Lagaly, G., 1982. The brittle mica-like KNiAsO
4
and its organic derivatives. Clay
Minerals 17, 175–183.
Bereket, G., Arog
˘
uz, A.Z., O
¨
zel, M.Z., 1997. Removal of Pb(II), Cd(II), Cu(II), and Zn(II)
from aqueous solutions by adsorption on bentonite. Journal of Colloid and Interface
Science 187, 338–343.
Bergaya, F., Vayer, M., 1997. CEC of clays: measurement by adsorption of a copper ethylene-
diamine complex. Applied Clay Science 12, 275–280.
Bergseth, H., 1980. Selektivita
¨
t von Illit, Vermiculit und Smectit gegenu
¨
ber Cu
2+
,Pb
2+
,
Zn
2+
,Cd
2+
und Mn
2+
. Acta Agricultura Scandinavica 30, 460–468.
Bergseth, H., 1985. Selektierungsvermo
¨
gen eines Eisenoxidhydroxids und einiger Ton-
minerale gegenu
¨
ber Phosphat und Sulfationen. Acta Agricultura Scandinavica 35,
375–388.
Bowden, J.W., Posner, A.M., Quirk, J.P., 1980. Adsorption and charging phenomena in
variable charge soils. In: Theng, B.K.G. (Ed.), Soils with Variable Charge. New Zealand
Society of Soil Science, Lower Hutt, pp. 147–166.
Brindley, G.W., Thompson, T.D., 1970. Methylene blue adsorption by montmorillonite: de-
termination of surface areas and exchange capacity with different initial cation saturations.
Israel Journal of Chemistry 8, 409–415.
Brouwer, E., Baeyens, B., Maes, A., Cremers, A., 1983. Cesium and rubidium ion equilibria in
illite clay. Journal of Physical Chemistry 87, 1213–1219.
Burrafato, G., Miano, F., 1993. Determination of the cation exchange of clays by surface
tension measurements. Clay Minerals 28, 475–481.
Busenberg, E., Clemency, C.V., 1973. Determination of the cation exchange capacity of clays
and soils using an ammonia electrode. Clays and Clay Minerals 21, 213–218.
Chhabra, R., Pleysier, J., Cremers, A., 1976. The measurement of CEC and exchangeable
cations in soils: a new method. In: Bailey, S.W. (Ed.), Proceedings of the International Clay
Conference 1975. Applied Publishing, Wilmette, IL, pp. 439–449.
Chiu, Y.C., Huang, N.L., Uang, C.M., Huang, J.F., 1990. Determination of cation exchange
capacity of clays minerals. Colloids and Surfaces 46, 327–337.
Cornell, R.M., Aksoyoglu, E.S., 1991. Simultaneous determination of the cation exchange
capacity and the exchangeable cations on marl. Clay Minerals 26, 567–570.
Chapter 12.10: Cation and Anion Exchange996
Dakshinamurti, C., Bhavanarayana, M., 1982. An isoconductivity method for determining the
cation exchange capacity of soils and clays. Catena 9, 175–179.
Eberl, D.D., 1980. Alkali cation selectivity and fixation by clay minerals. Clays and Clay
Minerals 28, 161–172.
El-Batouti, M., Sadek, O.M., Assaad, F.F., 2003. Kinetics and thermodynamic studies of
copper exchange on Na-montmorillonite clay mineral. Journal of Colloid and Interface
Science 259, 223–227.
Ferris, A.P., Jepson, W.B., 1975. The exchange capacities of kaolinite and the preparation of
homoionic clays. Journal of Colloid and Interface Science 51, 245–259.
Fletcher, P., Sposito, G., 1989. The chemical modelling of clay–electrolyte interactions for
montmorillonite. Clay Minerals 24, 375–391.
Fox, R.L., 1980. Soils with variable charge: agronomic and fertility aspects. In: Theng, B.K.G. (Ed.),
Soils with Variable Charge. New Zealand Society of Soil Science, Lower Hutt, pp. 195–224.
Francis, C.W., Grigal, D.F., 1971. A rapid and simple procedure using Sr 85 for determining
cation exchange capacities of soils and clays. Soil Science 112, 17–21.
Fraser, A.R., Russell, J.D., 1969. A spectrophotometric method for determination of cation
exchange capacity of clay minerals. Clay Minerals 8, 229–230.
Gast, R.G., 1972. Alkali metal exchange on Chambers montmorillonite. Soil Sience Society of
America Proceedings 36, 14–19.
Gast, R.G., 1977. Surface and colloid chemistry. In: Dixon, J.B., Weed, S.B. (Eds.), Minerals
in Soil Environments. Soil Science Society of America, Madison, WI, pp. 27–73.
Gast, R.G., Klobe, D., 1971. Sodium–lithium exchange equilibria on vermiculite at 25 1C and
50 1C. Clays and Clay Minerals 19, 311–319.
Gillmann, G.P., Bruce, R.C., Davey, B.G., Kimble, J.M., Searle, P.L., Skjemstad, J.O., 1983.
A comparison of methods used for determination of cation exchange capacity. Commu-
nications in Soil Science and Plant Analysis 14, 1005–1014.
Glaeser, R., 1946. De
´
termination de la capacite
´
d’e
´
change de base dans la montmorillonite.
Comptes Rendus de l
0
Acade
´
mie des Sciences, Paris, vol. 222, pp. 1179–1181.
Goldberg, S., 1992. Use of surface complexation models in soil chemical systems. Advances in
Agronomy 47, 233–329.
Goulding, K.W.T., Talibudeen, O., 1980. Heterogeneity of cation-exchange sites for K–Ca
exchange in aluminosilicates. Journal of Colloid and Interface Science 78, 15–24.
Greenhill, N.B., Peverill, K.I., 1977. Determination of cation exchange capacity of soils using
ammonia and chloride electrodes. Communications in Soil Science and Plant Analysis 8,
579–589.
Grim, R.E., 1968. Clay Mineralogy, 2nd edition. McGraw-Hill, New York.
Hang, P.T., Brindley, G.W., 1970. Methylene blue adsorption by clay minerals. Determination
of surface areas and cation exchange capacities (Clay—organic studies XVIII). Clays and
Clay Minerals 18, 203–212.
Hanna, M.T., Zaghloul, A.A., El-Batouti, M., El-Shazly, S.A., 1995. Solvent effect on the
kinetics of the exchange of copper ions on Na-montmorillonite. Journal of Colloid and
Interface Science 176, 418–421.
Hillier, S., Clayton, T., 1992. Cation exchange ‘staining ‘ of clay minerals in thin-section for
electron microscopy. Clay Minerals 27, 379–384.
Hirsch, D., Nir, S., Banin, A., 1989. Prediction of cadmium complexation in solution and
adsorption to montmorillonite. Soil Science Society of America Journal 53, 716–721.
References 997
