Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

Hissink, D.J., 1924. Base exchange in soils. Transactions of the Faraday Society 20, 550–566.
Inoue, A., Minato, H., 1979. Ca–K exchange reaction and interstratification in montmorillo-
nite. Clays and Clay Minerals 27, 393–401.
Ioannou, A., Dimirkou, A., 1997. Phosphate adsorption on hematite, kaolinite, and kaolinite-
hematite systems as described by a constant capacitance model. Journal of Colloid and
Interface Science 192, 119–128.
Janek, M., Lagaly, G., 2003. Interaction of a cationic surfactant with bentonite: a colloid
chemistry study. Colloid and Polymer Science 281, 293–301.
Jasmund, K., Lagaly, G. (Eds.), 1993, Tonminerale und Tone. Struktur, Eigenschaften, An-
wendung und Einsatz in Industrie und Umwelt. Steinkopff Verlag, Darmstadt.
Johnson, S.W., 1859. On some points of agricultural science. American Journal of Science and
Arts, Series 2 28, 71–85.
Kahr, G., Madsen, F.T., 1995. Determination of the cation exchange capacity and the surface
area of bentonite, illite and kaolinite by methylene blue adsorption. Applied Clay Science 9,
327–336.
Kjellander, R., 1996. Ion–ion correlations and effective charges in electrolyte and macroion
systems. Berichte der Bunsengesellschaft fu
¨
r Physikalische Chemie 100, 894–904.
Klobe, W.D., Gast, R.G., 1967. Reactions affecting cation exchange kinetics in vermiculite.
Soil Science Society of America Proceedings 31, 744–749.
Kodama, H., Ross, G.J., Toshimichi, J., Robert, J.W., 1974. Effect of layer charge location on
potassium exchange and hydration of mica. American Mineralogist 59, 491–495.
Kodama, T., Komarneni, S., 1999. Na-4-mica: Cd
2+
,Ni
2+
,Co
2+
,Mn
2+
, and Zn
2+
ion
exchange. Journal of Materials Chemistry 9, 533–539.
Ko
´
nya, J., Nagy, N.M., 1998. The effect of complexing-forming agent (EDTA) on the ex-
change of manganese ions on calcium-montmorillonite I. Reaction scheme and cal-
cium–montmorillonite–Na
2
EDTA system. Colloids and Surfaces A136, 299–310.
Ko
´
nya, J., Nagy, N.M., Kira
´
ly, R., Gelencse
´
r, J., 1998. The effect of complexing-forming
agent (EDTA) on the exchange of manganese ions on calcium-montmorillonite II. Cal-
cium–montmorillonite–Mn(ClO
4
)
2
–Na
2
EDTA system. Colloids and Surfaces 136, 311–319.
Ko
¨
ster, H.M., Kohler, E.E., Krahl, J., Kro
¨
ger, J., Vogt, K., 1973. Vera
¨
nderungen am Mont-
morillonit durch Einwirkung von 0,1 m AeDTE-Lo
¨
sungen, 1 n NaCl-Lo
¨
sung und 0.1 n
Salzsa
¨
ure. Neues Jahrbuch Mineralogische Abhandlungen 119, 83–100.
Kraepiel, A.M.L., Keller, K., Morel, F.M.M., 1999. A model for metal ion adsorption on
montmorillonite. Journal of Colloid and Interface Science 210, 43–54.
Lagaly, G., 1981. Characterization of clays by organic compounds. Clay Minerals 16, 1–21.
Lagaly, G., 1994. Layer charge determination by alkylammonium ions. In: Mermut, A.R.
(Ed.), Layer Charge Characteristics of 2:1 Silicate Clay Minerals. CMS Workshop Lec-
tures, vol. 6. The Clay Minerals Society, Boulder, CO, pp. 1–46.
Laudelout, H., van Bladel, R., Bolt, G.H., Page, A.L., 1968. Thermodynamics of heterovalent
cation exchange reactions in a montmorillonite clay. Transactions of the Faraday Society
64, 1477–1488.
Mackenzie, R.C., 1951. A micromethod for determination of cation exchange capacity of clay.
Journal of Colloid Science 6, 219–221.
Maes, A., Cremers, A., 1977. Charge density effects in ion exchange, part I—heterovalent
exchange equilibria. Journal of the Chemical Society, Faraday Transactions I 73,
1807–1814.
Chapter 12.10: Cation and Anion Exchange998
Maes, A., Rasquin, E., Cremers, A., 1982. Thermodynamic study of the influence of com-
plexation on exchange equilibria in Wyoming bentonite clay. Journal of the Chemical
Society, Faraday Transactions I 78, 2041–2049.
Maes, A., Verheyden, D., Cremers, A., 1985. Formation of highly selective cesium-exchange
sites in montmorillonite. Clays and Clay Minerals 33, 251–257.
Malcolm, R.L., Kennedy, V.C., 1969. Rate of cation exchange on clay minerals as determined
by specific ion electrode techniques. Soil Science Society of America Proceedings 33, 247–253.
Manning, B.A., Goldberg, S., 1996. Modeling arsenate competitive adsorption on kaolinite,
montmorillonite and illite. Clays and Clay Minerals 44, 609–623.
Mantin, I., 1969. Mesure des capacite
´
s d’e
´
change par l’e
´
thylene diamine et les ions complexes
de l’e
´
thyle
`
ne. Comptes Rendus de l
0
Acade
´
mie des Sciences, Paris, vol. 269, pp. 815–818.
Mantin, I., Glaeser, R., 1960. Fixation des ions cobaltihexamines par les montmorillonites
acides. Bulletin du Groupe Franc- ais des Argiles 12, 83–88.
Margulies, L., Rozen, H., Nir, S., 1988. Model for competitive adsorption of organic cations
on clays. Clays and Clay Minerals 36, b270–b276.
Martin, H., Laudelout, H., 1963. Thermodynamique de l
0
e
´
change des cations alcalins dans les
argiles. Journal de Chimie Physique, 1086–1099.
McBride, M., 1979. An interpretation of cation selectivity variations in M
+
–M
+
exchange on
clays. Clays and Clay Minerals 27, 417–422.
Meier, L., Kahr, G., 1999. Determination of cation exchange capacity (CEC) of clay minerals
using the complexes of copper (II) ion with triethylenetetramine and tetraethylenepenta-
mine. Clays and Clay Minerals 47, 386–388.
Mortland, M.M., Mellor, J.L., 1954. Conductimetric titration of soils for cation exchange
capacity. Soil Science Society of America Proceedings 18, 363–364.
Muljadi, D., Posner, A.M., Quirk, J.P., 1966a. The mechanism of phosphate adsorption by
kaolinite, gibbsite, and pseudoboehmite. Part I. The isotherms and the effect of pH on
adsorption. Journal of Soil Science 17, 212–229.
Muljadi, D., Posner, A.M., Quirk, J.P., 1966b. The mechanism of phosphate adsorption by
kaolinite, gibbsite, and pseudoboehmite. Part II. The location of the adsorption sites.
Journal of Soil Science 17, 230–237.
Muljadi, D., Posner, A.M., Quirk, J.P., 1966c. The mechanism of phosphate adsorption by
kaolinite, gibbsite, and pseudoboehmite. Part III. The effect of temperature on adsorption.
Journal of Soil Science 17, 238–247.
Nagy, N.M., Ko
´
nya, J., 1988. The interfacial processes between calcium-bentonite and zinc
ion. Colloids and Surfaces 32, 223–235.
Nir, S., Hirsch, D., Navrot, J., Banin, A., 1986. Specific adsorption of lithium, sodium,
potassium, and strontium to montmorillonite: observations and predictions. Soil Science
Society of America Journal 50, 40–45.
Nir, S., Rytwo, G., Yermiyahu, U., Margulies, L., 1994. A model for cation adsorption to
clays and membranes. Colloid and Polymer Science 272, 619–632.
Parfitt, R.L., 1978. Anion adsorption by soils and soil materials. Advances in Agronomy 30, 1–50.
Peech, M., 1945. Determination of exchangeable cations and exchange capacity of soils. Soil
Science 59, 25.
Pleysier, J., Janssens, J., Cremers, A., 1986. A clay suspension stability end point titration
method for measuring cation exchange capacity of soils. Soil Science Society of America
Journal 50, 887–891.
References 999
Pleysier, J., Juo, A.S.R., 1980. A single extraction method using silver-thiourea for measuring
exchangeable cations and effective CEC in soils with variable charges. Soil Science 129,
205–211.
Quirk, J.P., 2001. The significance of the threshold and turbidity concentrations in relation to
sodicity and microstructure. Australian Journal of Soil Research 39, 1185–1217.
Rao, K.P.C., Krishna Murti, G.S.R., 1987. Influence of noncrystalline material on phosphate
adsorption by kaolin and bentonite clays. In: Schultz, L.G., van Olphen, H., Mumpton,
F.A. (Eds.), Proceedings of the International Clay Conference, Denver 1985. The Clay
Minerals Society, Bloomington, IN, pp. 179–185.
Reichenbach, H., Graf von, 1973. Exchange equilibria of interlayer cations in different particle
size fractions of biotite and phlogopite. In: Serratosa, J.M. (Ed.), Proceedings of the Inter-
national Clay Conference, Madrid 1972. Division de Ciencias, CSIC, Madrid, pp. 457–466.
Reichenbach, H., Graf von, Rich, C.I., 1969. Potassium release from muscovite as influenced
by particle size. Clays and Clay Minerals 17, 23–29.
Rhodes, C.N., Brown, D.R., 1994. Rapid determination of the cation exchange capacity of
clays using Co(II). Clay Minerals 29, 799–801.
Rytwo, G., Banin, A., Nir, S., 1996. Exchange reactions in the Ca–Mg–Na- montmorillonite
system. Clays and Clay Minerals 44, 276–285.
Rytwo, G., Serban, C., Nir, S., Margulies, L., 1991. Use of methylene blue and crystal violet
for the determination of exchangeable cations in montmorillonite. Clays and Clay Minerals
39, 551–555.
Sawhney, B.L., 1972. Selective sorption and fixation of cations by clay minerals: a review.
Clays and Clay Minerals 20, 93–100.
Schlegel, M.L., Charlet, L., Manceau, A., 1999b. Sorption of metal ions on clay minerals II.
Mechanism of Co sorption on hectorite at high and low ionic strength and impact on the
sorbent stability. Journal of Colloid and Interface Science 220, 392–405.
Schlegel, M.L., Manceau, A., Chateigner, D., Charlet, L., 1999a. Sorption of metal ions on
clay minerals I. Polarized EXAFS evidence for the adsorption of Co on the edges of
hectorite particles. Journal of Colloid and Interface Science 215, 140–158.
Schofield, R.K., Samson, H.R., 1954. Flocculation of kaolinite due to the attraction of op-
positely charge crystal faces. Discussions of the Faraday Society 18, 135–145.
Schwertmann, U., 1962. Die selektive Kationenadsorption der Tonfraktion einiger Bo
¨
den aus
Sedimenten. Zeitschrift fu
¨
r Pflanzenerna
¨
hrung und Bodenkunde 97, 9–25.
Scott, A.D., Ismail, F.T., Locaties, R.R., 1973. Changes in interlayer potassium exchange-
ability induced by heating micas. In: Serratosa, J.M. (Ed.), Proceedings of the International
Clay Conference, Madrid, 1972. Division de Ciencias, CSIC, Madrid, pp. 467–479.
Searle, P.L., 1986. The measurement of soil cation exchange properties using the single
extraction, silver thiourea method: an evaluation using a range of New Zealand soils.
Australian Journal of Soil Research 24, 193–200.
Shainberg, I., Alperovitch, N.I., Keren, R., 1987. Charge density and Na–K–Ca exchange on
smectites. Clays and Clay Minerals 35, 68–73.
Siantar, D., Fripiat, J.J., 1995. Lead retention and complexation in a magnesium smectite
(hectorite). Journal of Colloid and Interface Science 169, 400–407.
Staunton, S., Rouboud, M., 1997. Adsorption of
137
Cs on montmorillonite and illite: effect of
charge compensating cation, ionic strength, concentration of Cs, K and fulvic acid. Clays
and Clay Minerals 45, 251–260.
Chapter 12.10: Cation and Anion Exchange1000
Strawn, D., Sparks, D.L., 1999. The use of XAFS to distinguish between inner- and outer-
sphere lead adsorption complexes on montmorillonite. Journal of Colloid and Interface
Science 216, 257–269.
Theng, B.K.G., Greenland, D.J., Quirk, J.P., 1967. Adsorption of alkylammonium cations by
montmorillonite. Clay Minerals 7, 1–17.
Theng, B.K.G., Russell, M., Churchman, G.J., Parfitt, R.L., 1982. Surface properties of
allophane, halloysite, and imogolite. Clays and Clay Minerals 30, 143–149.
Thomson, H.S., 1850. On the adsorbent power of soils. Journal of the Royal Agricultural
Society of England 11, 68–74.
Tournassat, C., Ferrage, E., Poinsignon, C., Charlet, L., 2004. The titration of clay minerals
II. Structure-based model and implications for clay reactivity. Journal of Colloid and
Interface Science 273, 234–246.
van Bemmelen, J.M., 1888. U
¨
ber die Absorptionsverbindungen und das Absorptions-
vermo
¨
gen der Ackererde. Landwirtschaftliche Versuchsstation 35, 69–136.
van Olphen, H., 1951a. A tentative method for determination of the base exchange capacity of
small samples of clay minerals. Clay Minerals Bulletin 1, 169–170.
van Olphen, H., 1951b. Rheological phenomena of clay sols in connection with the charge
distribution on the micelles (sic!). Discussions of the Faraday Society 11, 82–84.
van Olphen, H., 1977. An Introduction to Clay Colloid Chemistry, 2nd edition. Wiley, New York.
van Olphen, H., Fripiat, J.J., 1979. Data Handbook for Clay Materials and other Non-
metallic Minerals. Pergamon Press, Oxford.
Verburg, K., Baveye, P., 1994. Hysteresis in the binary exchange of cations on 2:1 clay
minerals: a critical review. Clays and Clay Minerals 42, 207–220.
Violante, A., Pigna, M., 2002. Competitive sorption of arsenate and phosphate on different
clay minerals and soils. Soil Science Society of America Journal 66, 1788–1796.
Way, J.T., 1852. On the power of soils to absorp (sic!) manure. Journal of the Royal Ag-
ricultural Society of England 13, 123–143.
Weiss, A., 1958a. U
¨
ber das Kationenaustauschvermo
¨
gen der Tonminerale. I. Vergleich der Un-
tersuchungsmethoden. Zeitschrift fu
¨
r Anorganische und Allgemeine Chemie 297, 232–256.
Weiss, A., 1958b. U
¨
ber das Kationenaustauschvermo
¨
gen der Tonminerale. II. Kationenaus-
tausch bei den Mineralen der Glimmer-, Vermiculit- und Montmorillonitgruppe. Zeitschrift
fu
¨
r Anorganische und Allgemeine Chemie 297, 257–286.
Weiss, A., 1958c. U
¨
ber a
¨
quimolaren Kationenaustausch bei niedrig geladenen Ion-
enaustauschern. Kolloid-Zeitschrift 158, 22–28.
Weiss, A., Mehler, A., Koch, G., Hofmann, U., 1956. U
¨
ber das Anionenaustauschvermo
¨
gen
der Tonminerale. Zeitschrift fu
¨
r Anorganische und Allgemeine Chemie 284, 247–271.
Wendelbo, R., Rosenqvist, I.T., 1987. Effects of anion adsorption on mechanical properties of
clay–water sytems. In: Schultz, L.G., van Olphen, H., Mumpton, F.A. (Eds.), Proceedings
of the International Clay Conference, Denver 1985. The Clay Minerals Society, Bloom-
ington, IN, pp. 422–426.
Xu, S., Harsh, J.B., 1992. Alkali cation selectivity and surface charge of 2.1 clay minerals.
Clays and Clay Minerals 40, 567–574.
References 1001
Handbook of Clay Science
Edited by F. Bergaya, B.K.G. Theng and G. Lagaly
Developments in Clay Science, Vol. 1
r 2006 Elsevier Ltd. All rights reserved.
1003
Chapter 12.11
THERMAL ANALYSIS
F. ROUQUEROL, J. ROUQUEROL AND P.L. LLEWELLYN
Laboratoire MADIREL, UMR CNRS-Universite
´
de Provence Centre de St Je
´
ro
ˆ
me,
13397 Marseille Cedex 20, France
12.11.1. BACKGROUND AND DEVELOPMENT
Clays were among the first materials to be studied by thermal analysis following
the development of this technique toward the end of the 19th century (Le Chatelier,
1887). In general terms, thermal analysis embraces any technique where a physical
property of the material in question is assessed as a functi on of temperature. This is
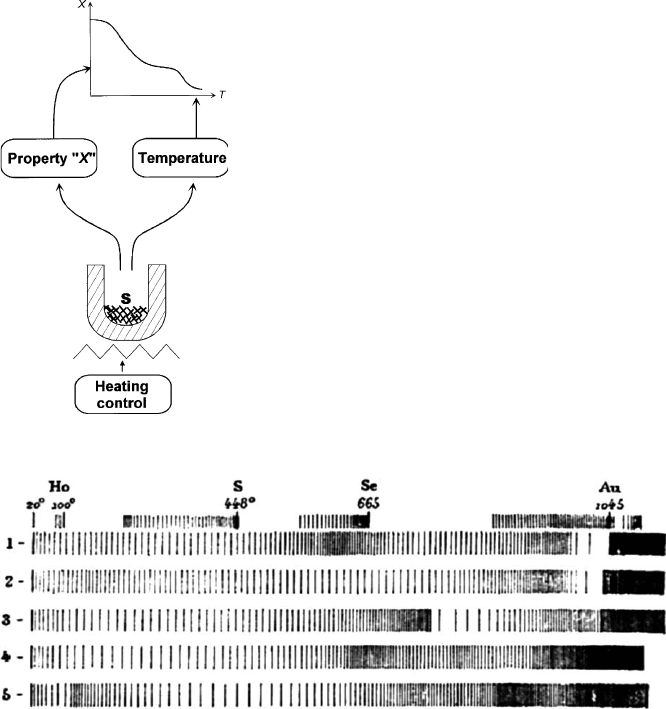
illustrated in Fig. 12.11.1 where property ‘‘X’’ (e.g., mass, temperature difference,
length, flow of evolved gas) is plotted against temperature.
In his famous work, entitled ‘‘About the action of heat on clays’’, Henri Le
Chatelier (1887), a professor at the School of Mines in Paris, applied two of his newly
invented thermal analytical methods to the study of clays. The first invention was the
Pt/(Pt,10%Rh) thermocouple (still a standard, more than a century later), while the
second was a special photographic recording method. In this method the temperature
reached by the sample (proportional to the distance from the origin of the recording)
and the actual rate of heating (as influenced by self-cooling of the sample) are si-
multaneously recorded. This is deduced from the appearance of a spark, at 2-second
intervals, in the form of a small bar on a photographic plate (Fig. 12.11.2). When an
endothermic phenomenon occurs, the actual heating rate of the sample decreases
causing the density of the bars to increase. Thus, the physical property assessed by
Le Chatelier (1887) was the self-cooling of the sample vs its temperature. This was the
first automatic recording of a thermal analysis experiment on clays.
Equally interesting were the reasons that Le Chatelier put forward to justify his
choice of clays for the experiment: ‘‘the hyd rated aluminium silicates (clays, kaolins,
etc y) y generally form mixtures too complex for chemical analysis to furnish any
precise data on their structure. I have thought that in studying the temperature of
dehydration of these substances, one could perhaps begin to characterise a small
number of chemical species and to distinguish the presence of each of these in
various mixtures. If one rapidly heats a small quantity of clay, there is produced, at
DOI: 10.1016/S1572-4352(05)01037-8
the moment of dehydration, a deceleration in the temperature rise (elevation); this
point of arrest can be used to establish a distinction among the various hydrated
aluminium silicates. The comparison of observations carried out on a very large
number of clays, shows that the complexity of these substances is much less than one
would have originally been led to believe’’ (quoted from the full English translation
by Wendlandt and Collins (1976). From these very first experiments Le Chatelier
(1887) concluded: ‘‘The clays could now be classified, according to their pyrogeneous
decomposition, in five distinct categor ies’’.
Fig. 12.11.1. Principle of thermal analysis in general (Rouquerol, 2003).
Fig. 12.11.2. Original thermal analysis traces of Le Chatelier for (1) halloysite, (2) allophane,
(3) kaolin, (4) pyrophillite, (5) montmorillonite. The short traces on top were used for tem-
perature calibration after the boiling point of water (Ho), sulphur (S) or selenium (Se) or after
the melting point of gold (Au).
Chapter 12.11: Thermal Analysis1004
The next step, taken at a much later date, was to relate the thermal behaviour of
clays to their crystalline structure. Caille
`
re and He
´
nin (1948) discussed the temperatures
of dehydration (an endothermic phenomenon), dehydroxylation (also endothermic)
and recrystallisation (exothermic) in relation to the structures and chemical compo-
sitions of a large set of phyllosilicates and fibrous clay minerals. Given the marked
effect of impurities on the differential thermal analysis (DTA) patterns, Caille
`
re
and He
´
nin (1948) further suggested that DTA may provide a simple means of tracing
the origin of a natural sample. It was not until the late 1950s, however, that DTA
became an important tool for clay mineral identification. Structural studies, indicating
the location of water and hydroxyls (as decribed below for simple phyllosilicates)
provided insight into the dehydration behaviour of clay minerals (Mackenzie, 1970).
Fo
¨
ldvari (1991) gave a comprehensive description of all types of water in minerals
(and especially clay minerals) that can be assessed by thermal analysis. A first great
distinction can be made between the water that occurs (i) as molecular water, and (ii)
as hydroxyls capable of cond ensing into water during thermal analysis. ‘Molecular
water’ can exist as interlayer water (in smectites, this water is stabilized by the
presence of exchangeable cations), as zeolitic water (in mordenite and sepiolite) in
cavities or channels, as water adsorbed on the external surface of clay minerals, or as
water formed by capillary condensation in large pores (in chrysotile). Most of these
types of water can be removed by heating at temperatures o100 1C and the initial
amount largely depends on ambient conditions of humidity and temperature. Hy-
droxyls are part of the clay structure and cannot be released without causing more or
less irreversible structural modifications. Hydroxyls make up what is often called
‘constitutional water’. This term, however, may lead to misunderstanding because
hydroxyls can only become water through condensation. In phyllosilicates, these
hydroxyls are usually bound to the structure by ionic or covalent bonds. Since
hydroxyls have a broad bond energy distribution (due to the different locations in
the structure), and their diffusion between sheets or in channels can be hindered in
different ways, hydroxyls are liberated over a very broad temperature range (between
150 and 1000 1C). This phenomenon is precisely what makes the thermal analysis of
clay minerals so interesting. At the same time, water and hydroxyls can serve as
probes to differentiate clay miner als.
Before discussing some results on the thermal analysis of clay minerals, we will briefly
describe the main techniques available and the two basic modes of operation, namely,
‘temperature-controlled thermal analysis’ and ‘sample-controlled thermal analysis’.
12.11.2. TEMPERATURE-CONTROLLED (CONVENTIONAL) THERMAL
ANALYSIS
More than 95% of the thermal analysis work on clay minerals is carried out using
techniques where the temperature programme of the furnace (or, sometimes, the
sample) is selected and imposed by the experimenter. This ‘temperature-controlled’ or
12.11.2. Temperature-Controlled (Conventional) Thermal Analysis 1005
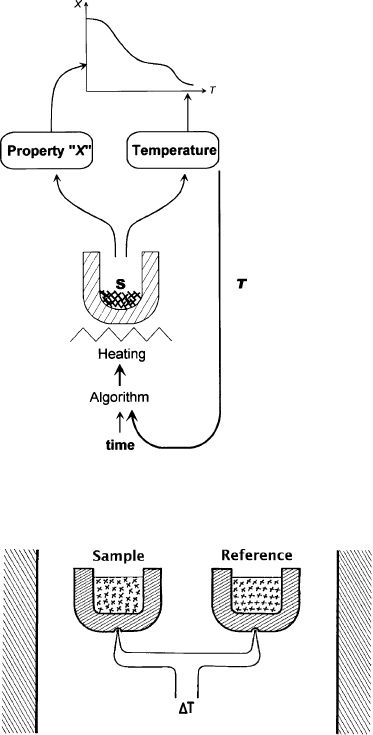
‘conventional’ thermal analysis is schematically shown in Fig. 12.11.3. Here the heat-
ing control loop makes use of the temperature of the sample or furnace (together with
the measurement of elapsed time, in order to get a temperature programme). More
often than not the programme is linear, that is, a constant heating rate is imposed.
In the case of clay minerals, the property ‘‘X’’ refers, first of all, to the temper-
ature difference (DT) between one poin t in/close to the sample and another point in/
close to an inert reference ( Fig. 12.11.4).
Following Le Chatelier (1887), such an experiment measures the self-cooling or self-
heating of the sample but with the added benefit of having a permanent comparison
Fig. 12.11.3. Principle of conventional or ‘‘temperature-controlled’’ thermal analysis
(Rouquerol, 2003).
Fig. 12.11.4. Principle of DTA.
Chapter 12.11: Thermal Analysis1006
between the ‘sample temperature’ and the ‘reference temperature’. This arrangement
was due to Roberts-Austen (1899), a chemist at the Royal Mint, London, and later
named ‘Differential Thermal Analysis’ (DTA). The reference temperature would be
similar to that of the sample if it did not undergo any thermal transformation. Since
the temperature difference is usually smaller than 10 K, this can be the full scale of the
recorder and small thermal events may be detected with great sensitivity. These events
can be endothermic (usually corresponding to water losses) or exothermic (usually
reflecting structural rearrangements). Let us stress that maximum sensitivity, in DTA,
is obtained at maximum heating rates (typically between 5 and 20 K/min).
For quantitative thermal analysis, ‘Differential Scanning Calorimetry’ (DSC) is by
principle superior to DTA since the former method not only determines the temper-
ature of transformation but also the corresponding heat. Nevertheless, DSC has two
drawbacks: (i) the upper temperature (typically between 600 and 850 1C) is lower than
that obtainable by DTA (X1000 1C); and (ii) the energy of dehydroxylation is not a
constant (being dependent on the type of bond), and hence, no simple relationship
exists between the amount of heat absorbed and the amount of water released.
The latter limitation is overcome by ‘Thermogravimetry’ (TG) where the quantity
measured is the sample mass. Here the mass of water evolved and thus its amount is
precisely measured irrespective of bonding mode. TG also allows slow heating rates
to be selected but unlike the situation with DTA or DSC, these conditions do not
diminish the sensitivity or accuracy of the experiment.
At first sight the combination of DTA with TG, avail able in a number of modern
instruments, is attractive. However, good critical judgment is required for interpre-
ting the results. This is because, the two techniques may not reveal the same thing
although the measurements are done sim ultaneously. For example, a mass loss (due
to the release of water vapour from the upper part of the sample) can be detected few
minutes before the self-cooling wave reached the DTA thermocouple in the bottom
of the crucible. Further, the best sample arrangement for thermogravimetry is a thin
layer of powder, whereas the best one for DTA is a heap around or above the
thermocouple. Thus, both experiments cannot simultaneously be at their best.
A fourth thermal analysis technique, called ‘Thermodilatometry’, is well suited for
investigating the sintering and firing behaviour of clay minerals. Here the quantity
‘‘X’’ refers to the length or volume of the sample. Indeed, therm ogravimetry is not
very useful above 800 1C because the residual water constitutes only a few per cent of
the initial quantity. On the other hand, thermodilatometry can readily detect the
dimensional changes due to sintering that occur at these temperatures.
For a given sample the temperature at which specific thermal phenomena
occur (as detected by DTA, DSC or TG) is well defined and reproducible, provided
the experimental procedure is very carefully defined. This means that any change
in sample mass/shape/size, crucible cover, location of the two thermocouples,
atmosphere (sample preconditioning), or heating rate is able to modify the resulting
DTA, DSC or TG curve. For this reason, one cannot speak of ‘standard’ temper-
atures for the various DTA peaks or TG steps. Nor can a DTA or TG curve be
12.11.2. Temperature-Controlled (Conventional) Thermal Analysis 1007
reproduced by someone else if the above experimental variables are not specified. On
the other hand, highly reproducible and well-resolved DTA or TG patterns, can be
obtained speedil y (often within o1 h) if the same experimental procedure is always
used. The latter point is sufficient reason for using these thermal analytical tech-
niques to compare, screen, and check natural clays and clay minerals. We should
also add that although the peak/step temperatures are strongly dependent on he ating
rate (temperature shifts as high as 100 K are observed when the heating rate drops
from 10 to 0.5 K/min), the general shape of a DTA or TG curve, with its peaks or
steps, is quite specific to, and hence, can serve as a fingerprint of a given clay.
Another notew orthy feature of thermal analysis is that it provides a continuous trace
of the temperature-induced transformation. By contrast, spectroscopic techniques
commonly used to assess and interpret structural and surface modifications provide
a discontinu ous analysis of selected states of the sample.
12.11.3. SAMPLE-CONTROLLED THERMAL ANALYSIS
The limitations of DTA, DSC and TG, arising from the large dependence of the
recordings on experimental details, also apply to most other conventional, temper-
ature-controlled thermal analysis techniques. The main problem stems from the
existence of (i) temperature gradients enhanced by the self-cooling of the sample on
dehydration, and (ii) pressure gradients produced within the sample by the release of
water vapour. Since these two types of gradients are directly related to the rate of
thermolysis, it is desirable to control and limit this rate.
The left-hand scheme in Fig. 12.11.5 shows the simplest and most straightforward
manner to achieve this end. By exclusively using the physical quantity ‘‘X’’ (sample
mass, length, enthalpy, gas evolved etc.), which is directly related to the rate or extent
of thermal transformation, heating of the sample is controlled in such a way as to
keep the reaction rate at the desired level. The procedure is therefore, referred to as
‘Controlled transformation Rate Thermal Analysis’ (CRTA).
The right-hand scheme in Fig. 12.11.5 represents the generalised principle of what
is now called ‘Sample-Controlled Thermal Analysis’ (SCTA) which includes CRTA,
the most ancient and most developed form. SCTA also opens the possibility of using
a more complex heating control device, where the temperature (T) of the sample can
play a part. In any SCTA experiment, the ‘X loop’ (i.e., the use of a feedback from
the sample) is compulsory whereas the ‘T-loop’ is only supplementary and optional.
As we shall illustrate by a few examples later on, the SCTA approach proved to be
extremely rewarding for analytical purposes (enhanced resolution and separation due to
the control of temperature and pressure gradients), preparative purposes (homogeneity
of the final product) and kinetic purposes (sample temperature and its rate of trans-
formation can be accurately measured, while the latter parameter can be modulated).
This principle of SCTA was successfully applied to ‘Evolved Gas Detection’
(Rouquerol, 1964), ‘Thermogravimetry’ (Erdey et al., 1965) and ‘Thermodilatometry’
Chapter 12.11: Thermal Analysis1008
