Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.


larger Mg
2+
/Al
3+
ratios (2.47–4.29) and up to 10–25% higher specific surface areas
are obtained as compared with samples prepared by classical coprecipitation
(Aramendia et al., 2002). Sol-gel materials are also more basic due to different
textural and morphological features (Prinetto et al., 2000a).
For NiAl using Ni acetylacetonate and aluminium isopropylate, the oxidic forms
(NiO–Al
2
O
3
) with a high reducibility were obtained (Jitianu et al., 2000a). The sol-
gel approach was extended to the preparation of Mg/Cr and Ni/Cr LDH. In the
latter case, some organic groups are retained on the surface (Jitianu et al., 2003).
F. Hydrothermal, Microwave, and Ultrasound Treatments
Different in situ or post-synthesis treatments were applied to the ‘as-prepared’ sam-
ples in order to control structural and textural properties. Microwaves were used
during synthesis in order to accelerate both the growing and ageing steps. Short
microwave irradiation results in a well-crystallized material compared to conven-
tional coprecipitation (Kannan and Vir Jasra, 2000; Mohmel et al., 2002). The extent
of enhancement in crystallinity depends on the nature of the trivalent metal ion
(Kannan and Vir Jasra, 2000). An increase of the specific surface area from 40 up to
240 m
2
/g is observed for synthetic hydrotalcite coprecipitated under 360 W micro-
wave irradiation (Fetter et al., 2001). In general, the surface area and porosity of the
synthetic materials are increased with the duration of microwave exposure. For
Co
6
2+
Co
2
3+
(OH)
16
(NO
3
)
2
nH
2
O micr owave-treated samples exhibit higher thermal
stability, and formation of the spinel Co
3
O
4
is delayed up to 200 1C(Zapata et al.,
2002). Charge distribution, nature of the hydrotalcite network, and the presence of
water molecules in the interlayer space lead to effective absorption of microwaves,
favouring long-range ordering.
Crystallinity of the phases can also be improved by ultrasound irradiation. Larger
LDH crystallites are observed while adsorption capacities are enhanced (Seida et al.,
2002).
Hydrothermal treatment is also used to improve the crystallinity of the com-
pounds or to increase the anion-exchange rate of low-affinity anions such as alkyl
carboxylates. Hydrothermal treatment has a strong effect on the chemical compo-
sition (Mg
2+
/Al
3+
) of synthetic hydrotalcite (Labajos et al., 1992).
G. Anion-Exchange Reactions
The anion-exchange capacity (AEC) is dependent on x. For the ideal composition of
M
II
1x
M
III
x
(OH)
2
X 0.66H
2
O, it can be calculated as
AEC ¼
x10
5
FW
ðcmol=kgÞ
13.1.4. Synthesis 1031
where the formula weight FW ¼ (M
MII
+46)+x(M
MIII
+M
X
M
MII
), and 0.66H
2
O
accounts for the total occupancy of interlayer vacant sites by water.
FW relates to full occupancy (2/3) of the interlayer crystallographic sites by water
molecules (0.66H
2
O/metal). Since AEC is dependent on x, it is constant for some
LDH materials such as LiAl
2
,Zn
2
Cr, and Cu
2
Cr, and highly tunable for some others
like Zn
x
Al (1oxo4) or Ga
x
Al (1oxo7.5) (Lopez-Salinas et al., 1997b). Table
13.1.4 gives the calculated AECs, the layer charge densities ðc :d: ¼ða
2
ffiffiffi
3
p
=2xÞ
1
Þ and
the free cross sectional area (S
free
¼ 1/c.d. (nm
2
/charge)) per layer for a series of
LDH. The usual values range from 200 to 400 cmol/kg ( ¼ meq/100 g) and show
the higher AEC of LDH in comparison to clay minerals (Parker et al., 1995; Inacio
et al., 2001; Ulibarri et al., 2001). For Mg–Al LDH with Mg/Al ¼ 3 and containing
simple anions, the AEC value is 320–360 cmol/kg. However, measured AEC values
for LDHs are often less than the values calculated from the struc tural formula,
due to contamination by carbonate anions, which have a strong affinity for LDHs.
The charge densities for LDHs range from 0.25 to 0.40 nm
2
/charge (Leroux and
Besse, 2001 ).
Miyata (1983) reported the ion-exchange isotherms at 25 1C of [Mg–Al] for a
series of monovalent and divalent anions. All isotherms display a sigmoid shape
arising from a continuous mixing of the anions in the LDH.
The powder XRD study during exchange of various systems [M
II
–M
III
–X/Y]
(Miyata, 1983), and more particularly of [Zn–Cr–Cl/X] and [Zn–Al–Cl/X] (X ¼ F
,
Br
,I
), does not show a continuous variation of basal spacings with increasing
molar fraction of the index anion. Non-misci bility of the different anions in LDH
and the short-lived coexistence of two LDH phases are observed. Only a few
examples are known of CO
2
3
=NO
3
and CO
2
3
=SO
2
4
mixed intercalates in synthetic
hydrotalcites, while mixed anion compositions are found in natural minerals. Pref-
erential anion intercalation is often observed because of the co-intercalation process
is thermodynamically unfavourable.
Table 13.1.4. Anion-exchange capacity values for some synthetic LDH
[M
II
–M
III
] x Formula
weight
AEC
(cmol/kg)
a (nm) Charge density
(charge/nm
2
)
Equivalent
surface area
(nm
2
/charge)
[Mg–Al–Cl] 0.20 77.92 256.7 0.3060 2.47 0.405
0.25 79.83 313.2 0.3054 3.09 0.323
0.33 82.88 398.2 0.3042 4.24 0.236
[Zn–Al–Cl] 0.20 110.80 180.5 0.309 2.49 0.401
0.25 110.65 225.9 0.308 3.13 0.319
0.33 110.41 298.9 0.307 4.16 0.240
[Mg–Al–CO
3
] 0.33 81.08 407.0 0.3042 4.24 0.236
[Mg–Al–NO
3
] 0.33 91.64 360.1 0.3042 4.24 0.236
[Li–Al-Cl] 0.33 78.12 422.4 0.3070 4.16 0.240
Chapter 13.1: Layered Double Hydroxides1032
From a thermodynami c point of view, exchange in LDH depends mainly on the
electrostatic interactions between positively charged hydroxylated sheets and the
exchanging anions, and to a small extent on the free energy involved in the changes
of hydration (Israeli et al., 2000). Another important feature is that the equilib rium
constant increases when the radius of the bare anion decreases. Exchange is therefore
favoured for ingoing anions with a high charge density. By calculating the equilib-
rium constant of various exchange reactions, Miyata (1983) were able to list the
order of affinity of LDH for monovalent anions
OH
4F
4Cl
4Br
4NO3
4I
and divalent anions
CO
2
3
4C
10
H
4
N
2
O
8
S
2
4SO
2
4
These results confirm the strong affinity for carbonate anions and underline the
strong need to prepare these compounds under a CO
2
-free atmosphere. For
[Cu–Al–X/Y] systems, Yamaoka et al. (1998) determined a similar selectivity se-
quence for monovalent anions, while for divalent oxoanions the following order was
proposed:
HPO
2
4
; HAsO
2
4
; 4CrO
2
4
4SO
2
4
4MoO
2
4
The selectivity for divalent anions is higher than for mon ovalent anions. Accord -
ing to these results, nitrate- and chloride-containing LDHs appear to be among the
best precursors for exchange reactions.
For Ni
1x
M
x
(OH)
2
(CO
3
)
x/2
n(H
2
O) (M ¼ Co, Fe), a strong dependence of basal
spacings on the size and charge of the intercalated ions is observed. The spacing
increases in the following order:
CO
2
3
oNO
3
oSO
2
4
oClO
4
oOAc
These hydroxides exhibit a selectivity for the anions in the sequence
CO
2
3
SO
2
4
4Cl
4ClO
4
4NO
3
4OAc
Recent interest in using anionic clays for environmental remediation led to the re-
investigation of their anion-exchange properties. Examining these properties over a
wide concentration range of the incoming anions allows surface and bulk processes
to be differentiated, and the rate of exchange at surface sites to be quantified. Inacio
et al. (2001) and Ulibarri et al. (2001) showed that both the type of interlayer anion
and the crystallinity of the LDH strongly affect AEC. In adsorption experiments
conducted under identical conditions [Mg–Al–CO
3
] does not adsorb dodecylsul-
phate whereas, [Mg–Al–Cl] adsorbs it completely. Molecular dynamics and ion
13.1.4. Synthesis 1033
diffusion studies at surfaces and interfaces (Kalinichev et al., 2002) show that the
structure and surface composition of LDH control the fluid structure in the inter-
layer space, as well as the effective diffusion coefficients of surface-adsorbed species,
their surface lifetimes, and their rotational and translational dynamics.
Experimental adsorption isotherms, usually interpreted in terms of the classical
Langmuir or Freundlich models (Inacio et al., 2000), can yield adsorption capacity
coefficients. For amphoteric anions such as glyphosate (N-(phosphonome-
thyl)glycine) two different adsorbent/adsorbate interactions are identified: electro-
static adsorption and ligand exchange. Adsorption is limited to the external surface,
and the distribution coefficients (K
d
) depend on the pH of the solution (Sanchez
Martin et al., 1999). In a study of internal versus external uptake of anions, Boclair
et al. (2001) showed ferrocyanide does not displace interlayer carbonate from syn-
thetic hydrotalcite but is adsorbed on the outside of the particles. Anion uptake here
is controlled by specific hydrogen bonding requirements and not by charge density
alone, a feature that can be used to control whether uptake will be both internal and
external, or only external.
H. Synthesis of Polyoxometallate-LDH
Direct methods are not suitable for the preparation of oxoanion- and polyoxomet-
allate (POM)-containing LDH because of their ab ility to incorporate or precipitate
metal cations. Anion-exchange reactions are alternatively employed, requiring tight
control of solution pH (Don Wang et al., 1995; Rives and Ulibarri., 1999). Indeed,
the pH of exchange must be compatible with the domain of stabili ty of the hydroxy
layer and the anion to be intercalated. For example, complete exchange of chloride
by V
10
O
6
28
in [Zn–Al–Cl], [Zn–Cr–Cl], and [Ni–Al–Cl] using ½NH
4
6
½V
10
O
6
28
6H
2
O
must be carried out at pH ¼ 4.5 (Kwon et al., 1988; Doeuff et al., 1989; Malherbe
et al., 1997). At higher pH, carbonate intercalation may occu r preventing complete
exchange. Direct exchange of decavanadate in [Zn
2
–Al] can be facilitated by ultra-
sound treatment (Kooli et al., 1997b ). It is noteworthy that such pH co nditions are
not compatible with the existence of the basic LDH matrix ([Mg–Al], [Ca–Al]).
Silicate species in [Zn–Cr] and [Zn–Al] are intercalated either by anion exchange or
coprecipitation (Schu
¨
tz and Biloen, 1987; Depe
`
ge et al., 1996). The pH of the so-
lution must then be higher than 9.0 in order to prevent precipitation of undesirable
amorphous silicates and metal hydroxides.
Pillaring ions may also be intercalated through a reconstruction process (Miyata
and Hirose, 1978; Drezdon, 1988; Chibwe and Jones, 1989b; Dimotakis and
Pinnavaia, 1990; Ulibarri et al., 1994; Pinnavaia, 1995; Hibino and Tsunashima,
1997; Nijs et al., 1999).
An alternative method involves exchange in expanded, organic anion-containing
LDH (Drezdon, 1988; Dimotakis and Pinnavaia, 1990; Malherbe et al., 1997,
1998). Drezdon et al. (1988) were able to exchange decavanadate in [Mg–Al-ter-
ephtalate]. Terephtalate anions were previously introduced in the structure in order
Chapter 13.1: Layered Double Hydroxides1034
to increase the basal spacing up to 1.4 nm and facilitate the diffusion of V
10
O
6
28
under acidification (pH ¼ 5.0). LDH with nonanoic, adipic, p-toluenesulphonic, and
squaric acids in their anionic forms were also used as intermediate exchangers
(Dimotakis and Pinnavaia, 1990). References to POM intercalation in LDH are
given in Table 13.1.5.
Pillaring is also accomplished by the grafting of ethylene glycol or anions
(chromate, dichromate, phosphate, phosphonate, or sulphonate) to the hydroxide
layers (Depe
`
ge et al., 1994; Costantino et al., 1997; Khaldi et al., 1998; Guimara
˜
es
et al., 2000; Malherbe and Besse, 2000; Pre
´
vot et al., 2001). Grafting reduces the
basal spacing.
Owing to the relatively high-layer charge density of LDH, the intercalated anions
are sometimes close packed in the interlayer space.
I. Synthesis of Organo-LDH
Both direct precipitation and anion-exchange reactions were extensively used for the
intercalation of organic anions in LDH (Meyn et al., 1990; Bonnet et al., 1997; Nijs
et al., 1998; Pre
´
vot et al., 1998). Urea hydrolysis appears to be a good method to
prepare organo-LDH of good crystallinity (Trujillano et al., 2002).
Amino acid-intercalated LDH were obtained by coprecipitation (Aisawa et al.,
2001). The degree of intercalation is strongly depen dent on pH due to the amp-
hoteric character of amino acids. Recently, anion-exchange reaction as a soft chem-
istry process was intensively studied for intercalation of anions of pharmaceutical
interest such as salicylate, citrate, glutamate, and aspartate (Tronto et al., 2001).
The preferential intercalation of organic anions in [Zn–Al] (in the order naph-
thalene-2,6-disulphonate>terephthalatebanthraquinone-2,6-disulphonate) was in-
terpreted in terms of the molecular recognition ability of the LDH structure (Kuk
and Huh, 1998).
Table 13.1.5. References for POM–LDH
LDH POM anions References
[Mg
2
–Al]
V
10
O
6
28
Drezdon (1988)
[Mg
2
–Al]
V
10
O
6
28
Dimotakis and Pinnavaia (1990)
[Mg
2
–Al]
SiW
9
V
3
O
7
40
Weber et al. (1993)
[Zn
2
–Al]
V
10
O
6
28
Kooli and Jones (1995)
[Zn
2
–Al] SiW
11
O
39
Co(H
2
O)
6
Hu et al. (1997)
[Zn
2
–Al] a-[SiV
3
W
9
O
40
]
7
a-[BV(IV)W
11
O
40
]
7
a-[PV
3
W
9
O
40
]
6
a-[H
2
W
12
O
40
]
6
Kwon and Pinnavaia (1992)
[Zn
2
–Al] SiW
11
O
39
, SiW
11
O
39
Mn(H
2
O) Guo et al. (2002)
13.1.4. Synthesis 1035
When direct intercalation and standard exchange reactions are unsuccessful, al-
ternative methods can be used. For instance, Dimotakis et al. (1990) reacted syn-
thetic meixnerite, [Mg
3
Al(OH)
8
]OH 2H
2
O, with the free acid form of the desired
anion in the presence of glycerol as a swelling agent. Reaction with nonanoic, adipic,
p-toluenesulphonic, and squaric acids gives the corresponding intercalates in quan-
titative yield as single crystalline phases with basal spacings of 2.02, 1.44, 1.74, and
1.01 nm. The reaction of the basic Mg–Al mixed oxides obtained by calcination of
the hydrotalcite with molten organic acids leads to the reconstruction of the LDH
with intercalated organic anions (Carlino et al., 1996).
A new method of anion exch ange was proposed by Crepaldi et al. (2000b).An
anionic surfactant intercalated LDH is contacted with a solution containing the
anion to be exchanged and a cationic surfactant. The anion exchange is favoured by
the formation of a salt between the anionic and cationic surfactants, which are easily
separated from the clay aqueous suspension with an organic solvent.
J. Synthesis of LDH-Based Nanocomposites
There are several possible strategies to incorporate a polymer into LDH (Scho
¨
llhorn,
1996) (see also Chapter 10.3): (i) intercalation of the monomer and subsequent in situ
polymerization, (ii) direct intercalation of extended polymer chains via exchange
reactions (in the case of low molecular-weight species) or via coprecipitation, (iii)
transformation of the host material into a colloidal dispersion and subsequent re-
stacking in presence of the polymer, and (iv) reconst ruction of the LDH structure in
the presence of the polymer (Table 13.1.6).
In situ polymerization is highly suitable and was achieved by incorporation of
various monomers, such as aniline, aminocaproic acid, or styrene sulphonate. The
process is limited by two factors (Leroux and Besse, 2001; Leroux et al., 2001b): (i)
monomer-to-monomer distance when the monomer is strongly anchored (or grafted)
to the host matrix, i.e., its degree of freedom must somehow be in agreement with the
layer charge; and (ii) the polymerization conditions (temperature, pH, or redox
reaction) must leave the layer structure intact.
Acrylate-[Mg
2
Al] LDH hybrid material, obtained by exchange of Cl
or NO
3
can
be polymerized at 80 1C(Tanaka et al., 1989). Carbonate LDH phase does not react
with acrylate anions. Acrylic acid, intercalated into an iron-substituted nickel LDH
material, can be polymerized by potassium persulphate (Rey et al., 1999).
Challier and Slade (1994) were the first to intercalate conjugated polymers into
the LDH framework. Terephthalate and hexacyanoferrate-exchanged [Cu
2
Cr]
LDH phases are used as host matrices for the oxidative polymerization of aniline.
An alternative method consists of intercalation of a soluble anionic monomer
such as aniline-2-sulphonate or metanilic acid (3-aminobenzene sulphonic acid—
H
2
NC
6
H
4
SO
3
H). These monomers can polymerize under less drastic conditions
than aniline, giving rise to a relatively well-ordered system (Moujahid et al.,
2002b). Poly(a,b aspartate) was intercalated into [Mg
3
Al] LDH by in situ thermal
Chapter 13.1: Layered Double Hydroxides1036

polycondensation or by direct intercalation as a co-solute in the basic reaction so-
lution (Whilton et al., 1997).
Generally, the presence of polymer not only affects the crystallinity but also the
dimension and morphology of the host material but enhances the thermal stability of
the LDH.
K. LDH with Intercalated Nanoparticles
In order to prepare mesoporous materials or multifunctional catalysts, inter calation
of oxide nanoparticles was performed in different ways. Manganese oxide nano-
particles were intercalated into Mg–Al LDH by ion exchange of interlayer nitrate
with permanganate anion, followed by reduction with organic reagents (
D(+)glu-
cose, ethan ol,
L(+)ascorbic acid) or by photodecomposition (Villegas et al., 2002).
The requirement by industry for materials with a high surface area stimulated
research into developing of nano-sized LDH. Nano-size Co
II
Al-hydrotalcite-like
particles (5–7 nm) on g-Al
2
O
3
as support were synthesized by Xu et al. (2001) and
Trainor et al. (2000). g-Al
2
O
3
also acts as the source of Al
3+
ions. Zhao et al. (2002)
synthesized LDH [Mg
1x
Al
x
(OH)
2
](CO
3
)
x/2
yH
2
O(x ¼ 1:723:3) by separating the
nucleation and ageing steps. The key features of this method are a very rapid mixing
Table 13.1.6. LDH–polymer nanocomposites
LDH–polymer Pathway References
LiAl–PANI a Isupov et al. (2001)
CuCr–PANI a Moujahid et al. (2002b)
CuAl–PANI a Challier and Slade (1994)
CaAl–PVA a Messersmith and Stupp (1995)
MgAl–poly(a,b-aspartate) a, b1 Whilton et al. (1997)
MgAl–PSS b2 Oriakhi et al. (1997)
ZnAl–PSS b2 Oriakhi et al. (1997)
a, b2 Moujahid et al. (2002a)
MgAl–PS a Shouldice et al. (1995)
MgAl–PA, PVS b2 Wilson et al. (1999)
ZnAl-PA, PVS b2 Oriakhi et al. (1996)
CoAl–PVS b2 Oriakhi et al. (1996)
CaAl–PA, PVS, PSS b2 Oriakhi et al. (1996)
MgAl–PA a Tanaka et al. (1989)
NiFe–PA b1 Rey et al. (1999)
ZnAl–PSS a, b1, c Leroux and Besse (2001)
Note: PANI, poly(aniline); PVA, poly(vinylalcohol); PSS, poly(styrene sulphonate); PVS, poly(vinyl sul-
phonate); PA, poly(acrylic acid). Pathways: a, in situ polymerization; b1, polymer incorporation; b2,
polymer coprecipitation; c, restacking.
13.1.4. Synthesis 1037
and nucleation process in a colloid mill followed by separated ageing. Uniform
crystals with a narrow range of diameters are obtained.
L. Grafting Reactions
After grafting, the interlayer anions are not exchanged with other anions, and are
then stabilized in the interlayer space (Costantino et al., 1997). Grafting also occurs
in other combinations of LDH and guest anions, such as Cu–Cr LDH/CrO
4
2
and
Cr
2
O
7
2
, Zn–Al LDH/ethylene glycol, and Zn–Al and Cu–Cr LDH/organ ic anion
(Depe
`
ge et al., 1994; Const antino and Pinnavaia, 1995; Khaldi et al., 1998; Pre
´
vot
et al., 1998; Guimara
˜
es et al., 2000; Malherbe and Besse, 2000; Pre
´
vot et al., 2001).
13.1.5. STRUCTURE
A. General
The first structural studies were performed by Allmann (1968) and Ingram and
Taylor (1967) , using monocrystals of sjo
¨
grenite and pyroaurite. The former mineral
crystallizes in a hexagonal symmetry with the space group P6
3
/mmc with lattice
parameters a ¼ 0:316 nm and c ¼ 1:566 nm (twice the basal spacing). The stacking
sequence of the layers is of the type 2H (–AB–BA–AB–). The cell has Z ¼ 1=4 and
the formula is Mg
6
Fe
2
(OH)
16
CO
3
4H
2
O. The pyroaurite crystallizes in rho-
mbohedral symmetry, space group R-3 m, with the cell parameters a ¼ 0:311 nm
and c ¼ 2:341 nm. The stacking sequence is of the 3R type (–AB–BC–CA–AB–). The
crystal structure of shigaite [Mn
2
Al(OH)
6
]
3
(SO
4
)
2
Na(H
2
O)
6
6H
2
O is rhombohedral,
space group R-3, with cell parameters a ¼ 0:9512 nm and c ¼ 3:3:74 nm (Cooper and
Hawthorne, 1996).
The stacking sequences 3R and 2H are often found. Natural Mg
6
Al
2
(OH)
16-
CO
3
4H
2
O may crystallize in 2H
1
(manasseite) or 3R
1
(hydrotalcite) structural po-
lytypes. 1H is observed for the hydrated sulphate phase (Ennadi et al., 1994).
Recently, a polytype 3R
2
(–AB–CA–BC–AB–) is reported for a MgAl LDH
prepared by hydrothermal treatment of alumi nium oxide and magnesium oxide
(Newman and Jones, 2002). Many synthetic LDH such as Zn
2
Al(OH)
6
Cl 2H
2
O
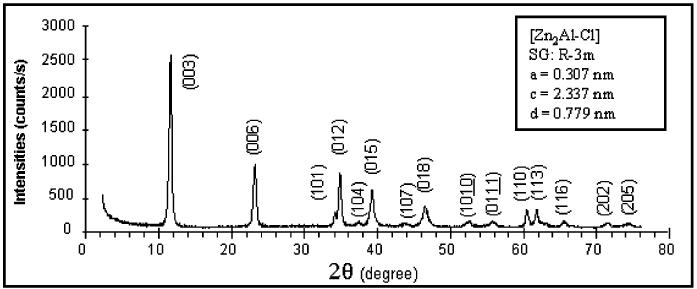
(Fig. 13.1. 3 ) crystallize in the 3R structural polytype.
More complexed stacking is observed in LiAl
2
(OH)
7
2H
2
O(Thiel et al., 1993).
On the basis of a Rietveld refinement, the authors showed that the cations within
each [LiAl
2
(OH)
6
]
+
layer are ordered but their relative positions in successive layers
are random. Indexing in a hexagonal cell indicates a C-centred monoclinic cell
with b ¼ a
ffiffiffi
3
p
and cos b ¼a=3c. A 12-layer model (w ith up to 54 layers) is also
proposed.
In the case of hydrocalumite types (Taylor, 1973; Kirkpatrick et al., 1999;
Kalinichev et al., 2000), the highest symmetry of the layers [Ca
2
Al(OH)
6
]
+
is P-3, the
Chapter 13.1: Layered Double Hydroxides1038
water molecules being linked to Ca
2+
ions. The nature of the interlayer anions
determines the space group. For large anions, the space group is R-3, while for
small anions (such as Cl
) it is C2/c. Ca
2
Al(OH)
6
NO
3
2H
2
O shows a structural
transition from P-3c1 to R-3c induced by grafting of NO
3
anions (Meyn et al.,
1990).
B. Cation and Anion Ordering
The question of order within the LDH sheets (Vucelic et al., 1995, 1997) is important
for catalytic applications where large surface areas combined with a high degree of
metal dispersion are required.
Structural cation ordering is indicated by the presence of additional diffraction
peaks related to superlattices as observed in pyroaurite (Mg
2,3
Fe–NO
3
)(Vucel ic et
al., 1997), and (LiAl
2
–OH) (Thiel et al., 1993; Besserguenev et al., 1997). For the
latter compound, the ordering of the cations is explained by migration of Li
+
ions
into the empty octahedra sites. Apart from these few cases, there is a general lack of
cation ordering because of the large difference in size between M
II
and M
III
cations
(Bellotto et al., 1996). This applies to many LDH with Al
3+
as trivalent cations. Yet,
despite the lack of long-range ordering, local ordering is generally observed by XAS
spectroscopy (see below and also Chapter 12.3). Cation ordering requires particular
structural conditions. Thus, a M(II)/M(III) ratio of 2 implies that each divalent
cation is surrounded by 3 M(II) and 3 M(III) and that the trivalent cations are
surrounded by 6 M(II) (Hofmeister and von Platen, 1992). A change in this ratio also
induces a change in the theoretical local order.
The interlayer anions may also show a regular distribution, exemplified by the
highly ordered two-dimensional superlattice present in (Zn
2
Al2SO
2
4
)(Bookin
et al., 1993).
Fig. 13.1.3. X-ray diffraction pattern of Zn
2
Al(OH)
6
Cl 2H
2
O.
13.1.5. Structure 1039
C. Stacking Phenomena
Staging
Most of the articles on the intercalation chemistry of LDH describe the fully
exchanged materials, while only a few report on mixed ion-exchanged forms.
Mendiboure and Scho
¨
llhorn (1987) investigated the competitive intercalation of
ClO
4
and NO
3
into [ZnCr] LDH. The two phases coexist, implying that the for-
mation of phases with mixed anions in the interlayer space is energetically unfa-
vourable. Theoretical models would suggest that regular stacking, referred to as
‘staging’, as common ly observed in graphite, does not occur in LDH due to the
rigidity of the layers (Schon et al., 1988). However, using in situ time-resolved XRD,
Fogg et al. (1998) demonstrated that [LiAl
2
(OH)
6
Cl 2H
2
O] can form second-stage
intermediates, i.e., every seco nd layer is filled by dicarboxylate anions (succinate,
tartrate, adipate, fumarate, maleate,
L-malate, phthalate, and terephthalate). This
technique was also used by O’Hare et al. (2000) to investigate the intercalation
chemistry of layer materials. Kaneyoshi and Jones (1998) observed layer interstrat-
ification during the interlayer exchange of terephthalate with chloride and nitrate
anions. This is ascribed to two orientations of terephthalate anions, vertical and
horizontal, which can be controlled by varying the layer charge density and the
extent of drying. On the other hand, Iyi et al. (2002), studying the direct synthesis of
mixed hydroxide/azobenzene intercalates, assume a segregation of the hydrophobic
organic anions and the hydrophilic inorganic anions, similar to that reported for
staged fluorohectorites (Ijdo and Pannavaia, 1998). Colloidal layered nanoparticles
are formed in solution that subsequently transform into staging structures when
dried. These few cases show that staging in LDH can occur either during the ex-
change process, or by direct synthesis, and is associated with different interlayer
contents or different orientations of the same molecule.
Random Stacking
Synthetic LDH commonly show faults in the stacking of successive layers as a result
of fine intergrowth of the rhombohedral with hexagonal polytypes. This random
stacking sequence is expected on the basis of the equal layer–interlayer topology of
the two polytypes. In a turbostratically disordered phase, the layers are randomly
orientated about the c-axis, thus eliminating all (hkl) reflect ions. The only reflections
seen are the (0 0 l) and the two-dimensional (hk) bands. In particular, stacking faults
are indicated by the broadness and the asymmetry of the (01 l) reflections. Although
routinely observed in synthetic materials, only a few studies refer to the influence of
preparation techniques, composition, and nature of the layer–interlayer bonds, on
layer-stacking faults (Pausch et al., 1986 ; Clause et al., 1992). XRD measurements on
a series of NiAl2CO
2
3
LDH powder samples by Solin et al. (1996) and Hines et al.
(1997) provided evidence for a (
p
3
p
3)R301 honeycomb superlattice ordering in
the octahedral sheet only for x ¼ 0:33 but not for other compositions. The anionic
layer also forms a commensurable in-plane structure (
p
13
p
13)R13.91 at x ¼ 0: 33
Chapter 13.1: Layered Double Hydroxides1040
