Bergaya F. Handbook of Clay Science
Подождите немного. Документ загружается.

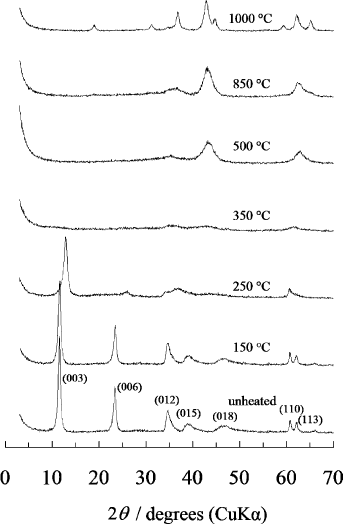
The thermal decomposition sequence of Mg-based LDH, as typified by
Mg–Al–CO
3
LDH, is well documented. The MgO phase is observed between ca.
400 and 850 1C, and has a smaller lattice constant a than pure MgO due to the
incorporation of Al
3+
ions. Above 900 1C, Al ions are released and the spinel
(MgAl
2
O
4
) is formed. Pure MgO and MgAl
2
O
4
are obtained at 1000 1C(Fig. 13.1.8)
(Miyata, 1980; Hibino et al., 1995). Mg–Ga–CO
3
LDH behave in a similar way
(Lopez-Salinas et al., 1997c). In the case of Mg–Fe–CO
3
LDH, MgFe
2
O
4
spinel
forms together with a MgO phase at 350 1C(Rouxhet and Taylor, 1969; Ferna
´
ndez
et al., 1998; Hibino and Tsunashima, 2000).
Mg-based LDH-containing oxidiz able trivalent metal ions yield oxides where the
metals occur in varied states of oxidation (Ferna
´
ndez et al., 1994 ; Labajos et al.,
1996; Labajos and Rives, 1996; Bahranowski et al., 1999). For LDH with tetravalent
metal ions, all the constituent metal ions are homogeneou sly dispersed in the oxides
that form after calci nation (Velu et al., 1997a, 1999a).
Zinc-based LDH are generally less thermally stable than their Mg-based coun-
terparts (Bertoldi et al., 1988; Fuda et al., 1993a, 1993b; Hussein et al., 1995;
Fig. 13.1.8. Changes in the XRD pattern when Mg–Al–CO
3
LDH with Mg/Al ¼ 2:1 is
heated at various temperatures. The calcinations temperatures and Miller indices are indicated
in the figure.
13.1.7. Fundamental Properties 1051
del Arco et al., 1996; Kooli et al., 1997a; Velu et al., 1997b; Pre
´
vot et al., 1998; Ennadi
et al., 2000; Crespo et al., 2001). Nickel-based LDH have thermal stabilities inter-
mediate between those of Mg- and Zn-based LDH (Clause, 1991, 1992; Trifiro
`
et al.,
1994; Barriga et al., 1996; Kannan et al., 1996; Labajos and Rives, 1996; del Arco et
al., 1999; Jitianu et al., 2000b). Cobalt-based LDH decompose at lower temperatures
than other LDH (Kannan and Swamy, 1992; Kannan et al., 1995; Xu and Zeng,
1998, 2001a; del Arco et al., 1998; Zeng and Lim, 2000; Pe
´
rez-Ramı
´
rez et al., 2001a,
2001b, 2001c, 2001d).
Other forms of LDH such as calcium-based LDH (Messersmith and Stupp, 1995),
Li–Al LDH (Herna
´
ndez et al., 1985; Hou and Kirkpatrick, 2001), and multicom-
ponent LDH (Morpurgo et al., 1996; Velu and Swamy, 1996; Alejandre et al., 1999;
Castiglioni et al., 2000; Tichit et al., 2001) show their own distingu ishing phases
during thermal decomposition.
Effect of Interlayer Anions
The thermal decomposition of LDH into the corresponding oxides is moderately
affected by the nature of the interlayer anions. For example, DTA indicates that the
nitrate forms of Mg–Al LDH are thermally more stable when the layer charge x
( ¼ Al/(Mg+Al)) increases (Xu and Zeng, 2001b). By contrast, the carbonate forms
show a decrease in decomposition temperature when x increases. The thermal be-
haviour of the nitrate forms is attributed to the arrange ment of the interlayer nitrate
ions which changes from ‘flat-lying’ to ‘stick-lying’ as the number of nitrate anions
increases (Xu and Zeng, 2001c). Dehydroxylation is apparently retarded when the
nitrate group adopts the ‘stick-lying’ conformation, being alternately attached to top
and bottom hydroxyl sheets of contiguous layers.
LDH, containing certain organic anions, can change their basal spacings on mild
heating. Expansion or contraction may occur during loss of interlayer water. Thus,
the basal spacing of Li–Al LDH with intercalated lon g-chain fatty acids
(CH
3
(CH
2
)
n
COOH, n ¼ 10, 12, and 14) expands on heating to 100 1C, whereas
that of the analogous Mg–Al forms contracts (Borja and Dutta, 1992). Terephtha-
late and benzoate intercalates of Mg–Al LDH also show changes in basal spacing
due to a change in orientation of the anions from vertical to horizontal or to in-
terstratification (Vucelic e t al., 1995; Kooli et al., 1996).
Contraction of basal spacings also occurs when anions are grafted to the hy-
droxide layer s. Grafting can enhance thermal stability (Pre
´
vot et al., 1998). The
higher thermal stability of LDH sulphate relative to the carbonate and hydroxide
forms may be explained in terms of the grafting of sulphate anions to the hydroxide
layers (Constantino and Pinnavaia, 1995).
Calcination/Hydration Cycles and Reconstruction
The thermal behaviour of Mg–Al–CO
3
LDH is characterized by three major steps
(Fig. 13.1.9)(Miyata, 1980; Sato et al., 1986a, Labajos et al., 1992; Pesic et al., 1 992;
Rey et al., 1992; MacKenzie et al., 1993; Hibino et al., 1995; Hudson et al., 1995;
Chapter 13.1: Layered Double Hydroxides1052
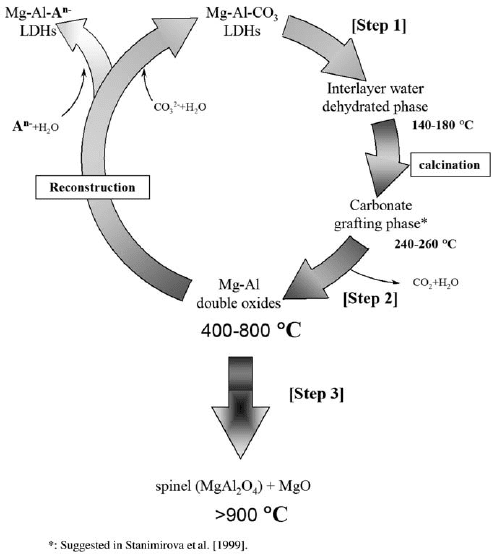
Tichit et al., 1998). Up to 250 1C, interlayer water is lost (step 1). At 250–500 1C, the
layer structure collapses, and the LDH converts into a mixed-oxide MgO-like phase
(step 2). The mixed oxide can reconstruct into the (original) LDH by exposure to an
aqueous solution (see Section 13.1.4.D). At ca. 900 1C the mixed oxide decomposes
to MgO and MgAl
2
O
4
spinel (step 3). The foregoing is a simplified outline of the
thermal behaviour of LDH.
In reality, the processes involved in steps 1–3 are more complicated than described.
Loss of interlayer water in step 1 is concomitant with the appearance of tetrahedrally
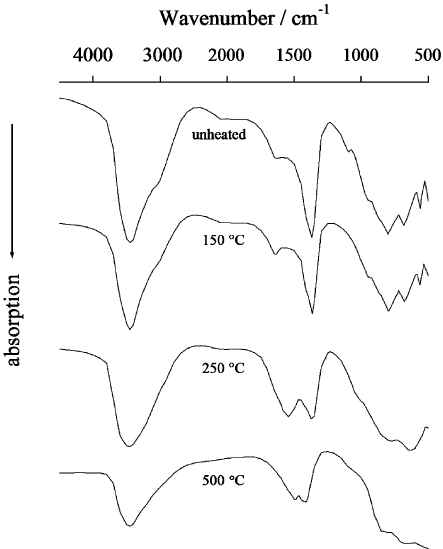
coordinated Al (Bellotto et al., 1996b). The metaphases that form are characterized
by a slight contraction of basal spacing, the absence of (0 0 6) reflection (see
Fig. 13.1.8), and the splitting of the carbonate u
3
band (at 1360 cm
1
) into two bands
at 1330 and 1540 cm
1
(see Fig. 13.1.10)(Rey et al., 1992; Hibino et al., 1995; Bellotto
et al., 1996b; Kanezaki, 1998a, 1998b, 1998c; Tichit et al., 1998; Rocha et al., 1999;
Millange et al., 2000; Pe
´
rez-Ramı
´
rez et al., 2001d), due probably to grafting of car-
bonate anions to the hydroxide layers (Stanimirova et al., 1999). Formation of tet-
rahedrally coordinated Al was also observed during step 2 (MacKenzie et al., 1993;
Fig. 13.1.9. Decomposition of Mg–Al–CO
3
LDHs by calcinations and reconstruction from
calcined LDHs in aqueous solutions.
13.1.7. Fundamental Properties 1053
Be
´
res et al., 1997; Tichit et al., 1998; Rocha et al., 1999). However, the local location
of Al(IV) is uncertain (Rebours et al., 1994; Shen et al., 1998), and this Al(IV)
disappears after reconstruction (Rey et al., 1992; Parker et al., 1995). A variety of
anions can be intercalated following calcination at ca. 500 1C (step 2) and subsequent
reconstruction (Fig. 13.1.9), (Chibwe and Jones, 1989a, 1989b; Dimotakis and Pin-
navaia, 1990; Cavani et al., 1991; Narita et al., 1991; Newman et al., 1998, 2001)
although some carbonate anions remain in the calcined materials (e.g., Clause, 1991;
Hibino et al., 1995; Kloprogge et al., 2001). A decrease in anion sorption capacity
(Parker et al., 1995) or a change in crystal symmetry (Stanimirova et al., 2001) may
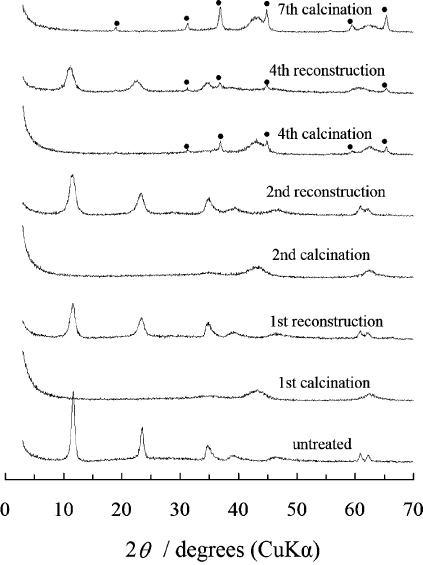
also occur in the first calcination/reconstruction cycle, while a MgAl
2
O
4
spinel phase
may appear in further cycles (see Fig. 13.1.11)(Hibino and Tsunashima, 1998).
Mixed oxides obtained from LDH often serve as catalysts. Reconstruction leads
to a redu ction in catalyti c activity. In addition, undesirable oxide phases can appear
upon repeated cycles of calcination and reconstruction (Alcaraz et al., 1998; Hibino
and Tsunashima, 1998; Marchi and Apesteguı
´
a, 1998; Pe
´
rez-Ramı
´
rez et al., 2001a).
The ease of reconstruction depends on the nature of the constituent metal cations
Fig. 13.1.10. Changes in the IR spectrum when Mg–Al–CO
3
LDH with Mg/Al ¼ 2:1 is
heated at various temperatures. The calcinations temperatures are indicated on the spectra.
Chapter 13.1: Layered Double Hydroxides1054
(Sato et al., 1988; Kooli et al., 1997a; Tichit et al., 2002a). For example, mixed oxides
from Ni-based LDH are much more difficult to reconstruct than those from Mg- or
Zn-based LDHs. Mixed oxides from Mg-based LDH can be reconstructed in an
aqueous solution at room temperature. With Zn-based LDH, some ZnO-like phase
remains under the same mild conditions. Ni-based LDH require hy drothermal
treatment for reconstruction, whereas thermal treatment gives rise to crystalline ZnO
in the case of Zn-based LDH.
An excellent example of a metal cation-dependent reconstruction was reported by
Alcaraz et al. (1998), who were able to solve the problem of deactivation (induced by
rehydration of the oxide catalysts back to LDH) by using Ni
2+
instead of Mg
2+
for
the synthesis of the LDH precursor.
Layered Double Oxides and Pre-spinel Phases
Oxides obtained by decomposition of LDH form a single-phase rock salt-type solid
solution before segregating into M(II)O and spinel phases when the temperature is
Fig. 13.1.11. Changes in the XRD pattern when Mg–Al–CO
3
LDH with Mg/Al ¼ 2:1 is
subjected to repeated calcinations/reconstruction. Diffraction peaks corresponding to spinel
are labeled with a solid circle.
13.1.7. Fundamental Properties 1055
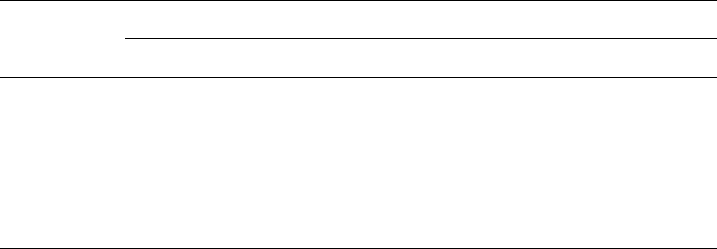
further increased. There is no doubt that the mixed oxides retain some structural
homogeneity. Models for local ordering or local segregation in the calcined materials
were proposed. One such model involves formation of a layered double oxide (LDO)
(Hudson et al., 1995; Carlino et al., 1996; Carlino, 1997). Like LDH, LDO can
intercalate guest anions.
Another model involves formation of a pre-spinel or quasi-amorphou s spinel-type
phase. Calcination of Ni–Al LDH between 350 and 800 1C gives rise to the following
local phases: (i) a NiO phase; (ii) a spinel-type phase; and (iii) an alumina-type phase
(Clause et al., 1992; Trifiro
`
et al., 1994; Jitianu et al., 2000b; Pe
´
rez-Ramı
´
rez et al.,
2001d).
When Ni–Fe LDH are calcined at 450 1C, an amorphous phase forms with a
structure related to inverse spinels such as NiFe
2
O
4
or g-Fe
2
O
3
(del Arco et al., 1999).
C. Surface and Colloidal Properties
Surface Characterization
The surface properties of LDH influence catalytic reactivity, colloidal stability, ad-
sorption, and intercalation. The specific surface area and AEC of LDH were de-
scribed in Sections 13.1.6.B and 13.1.4.G, respectively.
Zeta potentials were measured for Mg–Al–CO
3
/NO
3
LDH as a function of pH or
electrolyte concentration (Table 13.1.7). At a fixed salt concentration the isoelectric
point (IEP) decreases sharply for divalent anion (Chaˆ telet et al., 1996). Divalent
anions also reduce particle mobility (Lagaly et al., 2001a). These results show that
divalent anions are more strongly adsorbed by LDH than monovalent anions.
The zero point of charge (z.p.c.) (see Chapter 5) ranges from 12.0 to 12.3 for
Mg–Al LDH and from 10.3 to 10.8 for Mg–Fe LDH (Han et al., 1998; Hou et al.,
2001). The amount of charge may be calculated from potentiometric titration.
Table 13.1.7. Isoelectric point for LDH in various electrolytes
Electrolyte IEP
pH at 0.01 M salt concentration
a
Salt concentration (mmol/L) at pH 7
b
Water 11.1 —
NaCl >11.1 >100
NaSCN — 87
Na
2
SO
4
7.6 3
Na
2
CO
3
7.8 3
Na
2
CrO
4
6.7 —
Na
2
HPO
4
— 0.7
a
Values from Chaˆ telet et al. (1996).
b
Values from Lagaly et al. (2001a).
Chapter 13.1: Layered Double Hydroxides1056
However, the separate contribution of surface adsorption and ion exchange to z.p.c.
values is difficult to assess.
Surface Modification
Hydrophobization of LDH by exchange with organic anions (surfactants) is an
important procedure for obtaining new types of adsorbents. Thus, intercalation of
dodecyl sulphate or dode cyl benzenesulphonate into Ca–Al LDH yields hydropho-
bic LDH capable of adsorbing organic solvents, such as n-heptane, benzene, toluene,
and n-propanol. Thes e surfactant-modified LDH show interlayer swelling in n-hep-
tane (De
´
ka
´
ny et al., 1997). Hydrophobic LDH can also be obtained by intercalation
of myristic acid, or esterificat ion of sebacoyl chloride with surface hydroxyl groups
on the interlayer surface (Jakupca and Dutta, 1995; Morioka et al., 1995; Esumi and
Yamamoto, 1998). In studying the adsorption of surfactants, Pavan et al.
(1998–2000) found that the interlayer carbonate anions of LDH are not replaced
by surfactants through simp le ion exchange. However, some carbonate anions can be
exchanged by organic acids from the melt (Carlino and Hudson, 1994, 1995; Carlino
et al., 1996; Carlino, 1997).
Extraction of interlayer dodecyl sulphate by a cationic surfactant ( N-cetyl-N,N,
N-trimethylammonium bromide) leads to fast anion exchange, the amount of do-
decyl sulphate exchanged being proportional to the amount of cationic surfactant
added (Crepaldi et al., 1999, 2000b). New hybrid films of LDH and surfactants were
prepared by the Langmuir–Blodgett (LB) method to obtain electrodes for sensors
(He et al., 2001). Finally, modification of the interlayer surface with polymerized
silicate was reported, offering a way of synthesizing new catalysts (Yun et al., 1995;
Depe
`
ge et al., 1996).
Colloidal Dispersions and Delamination
The term ‘colloidal solution of LDH’ is somewhat ambiguous since it can be in-
terpreted in two ways: a suspension of LDH particles, or a dispersion of exfoliated
single layers of LDH (i.e., delaminated LDH). For example, the turbid supernatant
after intense washing with water, or treatment with ultrasound, consists of a sus-
pension of LDH particles. Such suspensions were used to measure electrophoretic
mobility (Lagaly et al., 2001a) and rheological properties (Albiston et al., 1996).
Colloidal suspensions of LDH were also used as solid emulsion stabilizers (Abend
et al., 1998; Neuha
¨
usler et al., 1999; Thieme et al., 1999; Lagaly et al., 2001b).
Oil–water emulsions, stabilized by heterocoagulates of bentonite and LDH, are
useful for cosmetic or pharmaceutical formulations.
A dispersion of delaminated LDH is totally different from a suspension of par-
ticles. Delamination, which can be thought of as infinite swelling, results in colloidal
solutions that are very stable. Several methods for delaminating LDH were reported:
treatment of Zn–Al–dodecyl sulphate LDH in alcohols under reflux at 120 1C
(Adachi-Pagano et al., 2000), simple mixin g of Mg–Al–glycine LDH and formamide
(Hibino and Jones, 2001), mixing water and Mg–Al –alkoxide LDH prepared in
13.1.7. Fundamental Properties 1057
alcohols (Gardner et al., 2001), and mixing Mg–Al–dodecyl sulphate LDH and
acrylate monomers at 70 1C(O’Leary et al., 2002). When colloidal dispersions of
delaminated LDH are gently dried, well-ordered LDH are recovered (Hibino and
Jones, 2001; Leroux et al., 2001a). On the other hand, when such dispersions are
freeze-dried, the recovered materials are X-ray amorphous (Leroux et al., 2001a).
Nanocomposites of LDH and polymers were obtained from colloidal dispersions of
delaminated LDH and polymers (Leroux and Besse, 2001).
Surface Properties of Calcined Materials
Thermal decomposition of the LDH structure increases the specific surface area
(Table 13.1.8)(Kannan and Swamy, 1992; Labajos et al., 1992; Barriga et al., 1996;
Kannan et al., 1996; Marchi and Apesteguı
´
a, 1998; Shen et al., 1998; Hussein et al.,
2001; Tichit et al., 2001; Olsbye et al., 2002). Formation of oxide phases and sin-
tering of particles at high temperature cause a marked de crease in specific surface
area. Nitrogen adsorption–desorption isotherms of the calcined materials are clas-
sified as type IIb rather than type IV. Type IIb isotherms are characteristic of ag-
gregates of plate-like particles, classically found with clay minerals (Tichit et al.,
2001).
The acidity and basicity of calcined Mg–Al–CO
3
LDH were evaluat ed by micro-
calorimetric adsorption of NH
3
and CO
2
. Acidity decreases in the order
g-Al
2
O
3
>LDH calcined at 400 1C>LDH calcined at 600 1 CELDH calcined at
800 1C, while basicity follows the order MgO>LDH calcined at 600 1C>LDH cal-
cined at 800 1C>LDH calcined at 400 1C. IR spectroscopy indicates that the acid/
base sites on the surface of calcined LDH are mainly of the Lewis type (Shen et al.,
1998). Weak Lewis acid sites appear to be associated with Al
3+
ions, while basicity is
related to isolated surface hydroxyl groups (del Arco et al., 1993). Calcined Mg–Al
LDH contain surface sites of low (OH
group), medium (Mg–O pairs), and strong
(O
2
anions) basicity. The relative contribution of low- and medium-strength basic
sites to the total basicity increases with increasing Al
3+
content (Di Cosimo et al.,
1998).
13.1.8. INDUSTRIAL AND ENVIRONMENTAL APPLICATIONS:
CONVENTIONAL AND NOVEL
A. Catalytic Applications
Mixed oxides obtained by calcination of LDH can serve as solid-base catalysts for (i)
polymerization of alkene oxides; (ii) aldol condensation of aldehydes and ketones;
(iii) methane or hydrocarbon steam reforming; (iv) methanation; (v) synthesis of
methanol and higher alcohols; (vi) hydrocarbon (Fischer–Tropsch) synthesis; and
(vii) hydrolysis of nitriles (Cavani et al., 1991; Vaccari, 1998, 1999b). The thermal
decomposition of LDH, containing transition metal ions, was intensively studied
Chapter 13.1: Layered Double Hydroxides1058

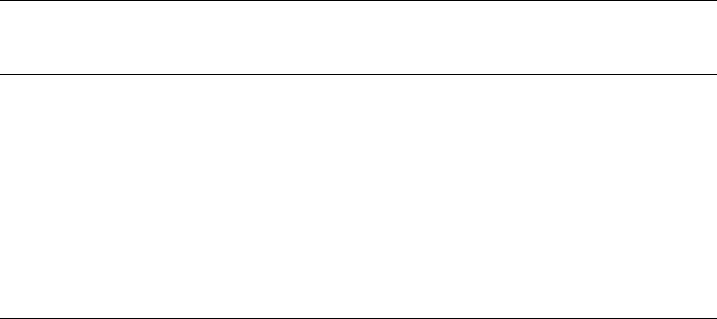
Table 13.1.8. Specific surface area of calcined LDH
Composition
a
Treatment for
original LDH
Degassing
temperature
(1C)
Calcination
temperature
(1C)
Specific
surface area
(m
2
/g)
References
Co/Al ¼ 2/1 Aging, 65 1C,
24 h
120 Not calcined 47 Kannan and
Swamy (1992)200 270
Mg/Al ¼ 2/1 None Not described 750 ca. 200 Labajos et al.
(1992)1100 ca. 50
Ni/Mn ¼ 2/1 None Not described Not calcined 70 Barriga et al.
(1996)125 80
450 58
700 15
1000 o1
Ni/Mn ¼ 3/1 Not calcined 70
125 72
450 56
700 17
1000 4
Ni/Al ¼ 2/1 Aging, 65 1C,
24 h
Not described Not calcined 92 Kannan et al.
(1996)450 170
Ni/Al ¼ 2.5/1 Not calcined 124
450 146
Ni/Al ¼ 3/1 Not calcined 83
450 137
Ni/Al ¼ 3.5/1 Not calcined 121
450 92
(Cu+Co+Zn)/
Al ¼ 3/1
60 1C during
coprecipitation
400 400 203 Marchi and
Apesteguı
´
a
(1998)
500 163
600 79
Mg/Al ¼ 3/1 None Not described 400 195 Shen et al.
(1998)600 132
800 114
Mg/Al ¼ 4/1 Aging, 70 1C,
18 h
Not described Not calcined 68.0 Hussein et al.
(2001)500 201.9
Co/Al ¼ 3/1 None 200 Not calcined — Tichit et al.
(2001)300 166
500 145
Co/Mg/Al ¼ 2/1/1 Not calcined 174
300 230
500 149
Co/Ni/Mg/Al ¼ 1.5/
0.5/1/1
Not calcined 17
300 209
500 163
Co/Ni/Mg/Al ¼ 1/1/
1/1
Not calcined 20
300 235
500 199
Co/Ni/Mg/Al ¼ 0.5/
1.5/1/1
Not calcined 30
300 254
500 204
Ni/Mg/Al ¼ 2/1/1 None 200 Not calcined 20 Tichit et al.
(2001)300 251
(continued on next page)
13.1.8. Industrial and Environmental Applications: Conventional and Novel 1059
because the products are potentially useful as catalyst precursors. The transition
metals include nickel (Clause , 1991, 1992; Trifiro
`
et al., 1994; Alcaraz et al., 1998; del
Arco et al., 1999; Jitianu et al., 2000b), cobalt (del Arco et al., 1998; Pe
´
rez-Ramı
´
rez
et al., 2001a, 2001d; Tichit et al., 2001), iron (Raja and Santhanalakshmi, 1996; Ge
et al., 2001), an d copper (Morpurgo et al., 1996; Velu and Swamy, 1996; Marchi and
Apesteguı
´
a, 1998 ; Castiglioni et al., 2000). The reactivity of LDH itself (as a solid-
base catalyst) was investigated using 2-methyl-3-butyn-2-ol as a probe (Constantino
and Pinnavaia, 1995).
An emerging trend is the application of LDH-derived catalysts in green chemistry.
Solid-base catalysts are environment friendly, as compared with their liquid counter-
parts, because their waste products are easier to dispose than the huge volumes of
liquid alkali waste (Ono and Baba, 1997). Solid bases from LDH were used in mer-
captan oxidation for sweetening (Alcaraz et al., 1998), formation of C–C bonds
(Choudary et al., 2000, 2001b), and condensation of citral and ketones (Roelofs et al.,
2001). Environment friendly technologies using LDH were also developed for the
production of hydrogen from vegetable oils (Marquevich et al., 2001), the simulta-
neous production of hydrogen and nanocarbon by decomposition of methane
(Li et al., 2000), and the high-rate synthesis of amine N-oxides (Choudary et al.,
2001a). Velu et al. (1999b, 2000b) investigated Cu–Zn–Al–Zr LDH as precursors of
catalysts for steam reforming of methanol to produce CO-free hydrogen for fuel cells.
Another environmental application of LDH-derived catalysts involves decom-
posing gaseous air pollut ants and greenhouse gases. The performance of Cu–Mg–Al
mixed oxides (obtained by calcination of LDH) in the selective catalytic reduction of
NO by NH
3
is comparable to that of a commercial V
2
O
5
–WO
3
/TiO
2
catalyst
Table 13.1.8 (Continued )
Composition
a
Treatment for
original LDH
Degassing
temperature
(1C)
Calcination
temperature
(1C)
Specific
surface area
(m
2
/g)
References
500 192
Co/Ni/Al ¼ 1.5/
1.5/1
Not calcined 13
300 201
500 144
Co/Ni/Mg/Al ¼ 1.2/
1.2/0.6/1
Not calcined 15
300 234
500 127
Co/Ni/Mg/Al ¼ 0.8/
10.8/1.4/1
Not calcined 96
300 296
500 270
Mg/Al ¼ 3/1 60 1C during
coprecipitation
Not described 800 162 Olsbye et al.
(2002)
a
Compensating anions are carbonate or nitrate.
Chapter 13.1: Layered Double Hydroxides1060
