Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


EXAMPLE 12-9 Heat Treatment to Generate Bainite Microstructure
Excellent combinations of hardness, strength, and toughness are obtained from
bainite. One heat treatment facility austenitized an eutectoid steel at 750
C,
quenched and held the steel at 250
C for 15 min, and finally permitted the steel
to cool to room temperature. Was the required bainitic structure produced?
SOLUTION
Let’s examine the heat treatment using Figure 12-17. After heating at 750
C,
the microstructure is 100% g. After quenching to 250
C, unstable austenite
remains for slightly more than 100 s, when fine bainite begins to grow. After
15 min, or 900 s, about 50% fine bainite has formed and the remainder of the
steel still contains unstable austenite. As we will see later, the unstable austenite
transforms to martensite when the steel is cooled to room temperature and
the final structure is a mixture of bainite and hard, brittle martensite. The heat
treatment was not successful! The heat treatment facility should have held the
steel at 250
C for at least 10
4
s, or about 3 h.
12-11 The Martensitic Reaction and Tempering
Martensite is a phase that forms as the result of a di¤usionless solid-state trans-
formation. In this transformation there is no di¤usion and, hence, it does not follow
the Avrami transformation kinetics. The growth rate in martensitic transformations
(also known as displacive or athermal transformations) is so high that nucleation be-
comes the controlling step. The phase that forms upon the quenching of steels was
named ‘‘martensite’’ by Floris Osmond in 1895 in honor of German metallurgist Adolf
Martens. Similar martensitic phase transformations occur in other systems as well.
Cobalt, for example, transforms from a FCC to a HCP crystal structure by a slight
shift in the atom locations that alters the stacking sequence of close-packed planes. Be-
cause the reaction does not depend on di¤usion, the martensite reaction is an athermal
transformation—that is, the reaction depends only on the temperature, not on the time.
The martensite reactio n often proceeds rapidly, at speeds approaching the velocity of
sound in the material.
Many other alloys such as Cu-Zn-Al and Cu-Al-Ni and Ni-Ti show martensitic
phase transformations. These transformations can also be driven by the application of
mechanical stress. Other than martensite that forms in certain type of steels, the Ni-Ti
alloy, known as nitinol (which stands for Nickel Titanium Naval Ordinance Labo-
ratory, developed by the U.S. Naval Ordinance Laboratory in the 1940s) is perhaps the
best-known example of alloys that make use of martensitic phase transformations.
These materials can remember their shape (i.e. shape memory e¤ect) and are known as
shape-memory alloys (SMAs).
Martensite in Steels In steels with less than about 0.2% C, the FCC austenite trans-
forms to a supersaturated BCC martensite structure. In higher carbon steels, the mar-
tensite reaction occurs as FCC austenite transforms to BCT (body centered tetragonal)
martensite. The relationship between the FCC austenite and the BCT martensite [Fig-
ure 12-21(a)] shows that carbon atoms in the 1/2, 0, 0 type of interstitial sites in the
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment380
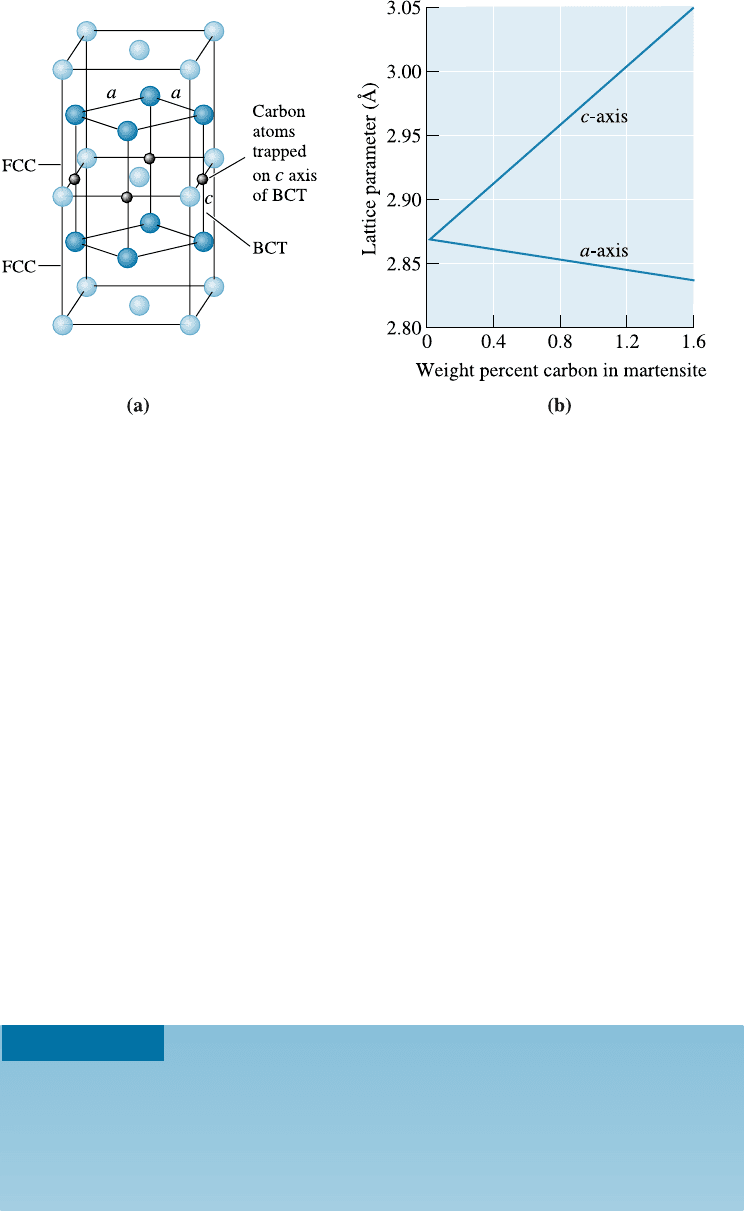
FCC cell can be trapped during the transformation to the body-centered structure,
causing the tetragonal structure to be produced. As the carbon content of the steel
increases, a greater number of carbon atoms are trapped in these sites, thereby increas-
ing the di¤erence between the a- and c-axes of the martensite [Figure 12-21(b)].
The steel must be quenched, or rapidly cooled, from the stable austenite region to
prevent the formation of pearlite, bainite, or primary microconsti tuents. The martensite
reaction begins in an eutectoid steel when austenite cools below 220
C, the marten-
site start (M
s
) temperature (Figure 12-17). The amount of martensite increases as
the temperature decreases. When the temperature passes below the martensite finish
temperature (M
f
), the steel should contain 100% martensite. At any intermediate
temperature, the amount of martensite does not change as the time at that temperature
increases.
Owing to the conservation of mass, the composition of martensite must be the same
as that of the austenite from which it forms. There is no long-range di¤usion during the
transformation that can change the composition. Thus, in iron-carbon alloys, the initial
austenite composition and the final martensite composition are the same. The following
example illustrates how heat treatment is used to produce a dual phase steel.
Figure 12-21 (a) The unit cell of BCT martensite is related to the FCC austenite unit cell.
(b) As the percentage of carbon increases, more interstitial sites are filled by the carbon atoms
and the tetragonal structure of the martensite becomes more pronounced.
EXAMPLE 12-10
Design of a Heat Treatment for a Dual Phase Steel
Unusual combinations of properties can be obtained by producing a steel
whose microstructure contains 50% ferrite and 50% martensite; the martensite
provides strength and the ferrite provides ductility and toughness. Design a
heat treatment to produce a dual phase steel in which the composition of the
martensite is 0.60% C.
12-11 The Martensitic Reaction and Tempering 381
SOLUTION
To obtain a mixture of ferrite and martensite, we need to heat-treat a hypo-
eutectoid steel into the a þ g region of the phase diagram. The steel is then
quenched, permitting the g portion of the structure to transform to martensite.
The heat treatment temperature is fixed by the requirement that the mar-
tensite contain 0.60% C. From the solubility line between the g and the a þ g
regions, we find that 0.60% C is obtained in austenite when the temperature is
about 750
C. To produce 50% martensite, we need to select a steel that gives
50% austenite when the steel is held at 750
C. If the carbon content of the steel
is x, then:
% g ¼
ðx 0:02Þ
ð0:60 0:02Þ
100 ¼ 50 or x ¼ 0:31% C
Our final design is:
1. Select a hypoeutectoid steel containing 0.31% C.
2. Heat the steel to 750
C and hold (perhaps for 1 h, depending on the thick-
ness of the part) to produce a structure containing 50% ferrite and 50%
austenite, with 0.60% C in the austenite.
3. Quench the steel to room temperature. The austenite transforms to marten-
site, also containing 0.60% C.
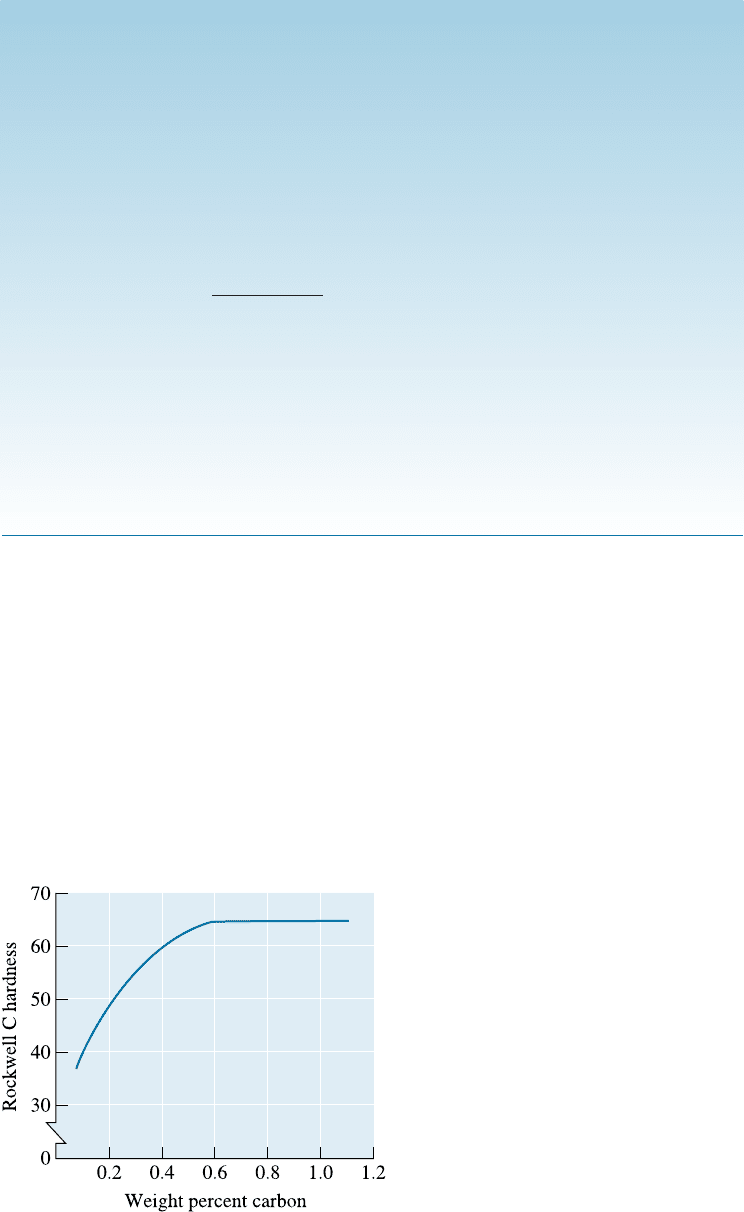
Figure 12-22
The effect of carbon content on the
hardness of martensite in steels.
Properties of Steel Martensite Martensite in steels is very hard and brittle, just like
ceramics. The BCT crystal structure has no close-packed slip planes in which dis-
locations can easily move. The martensite is highly supersaturated with carbon, since
iron normally contains less than 0.0218% C at room temperature, and martensite con-
tains the amount of carbon present in the steel. Finally, martensite has a fine grain size
and an even finer substructure within the grains.
The structure and properties of steel martensites depend on the carbon content
of the alloy (Figure 12-22). When the carbon content is low, the martensite grows in
a ‘‘lath’’ shape, composed of bundles of flat, narrow plates that grow side by side
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment382
[Figure 12-23(a)]. This martensite is not very hard. At a higher carbon content, plate
martensite grows, in which flat, narrow plates grow individually rather than as bundles
[Figure 12-23(b)]. The hardness is much greater in the higher carbon, plate martensite
structure, partly due to the greater distortion, or large c/a ratio, of the crystal structure.
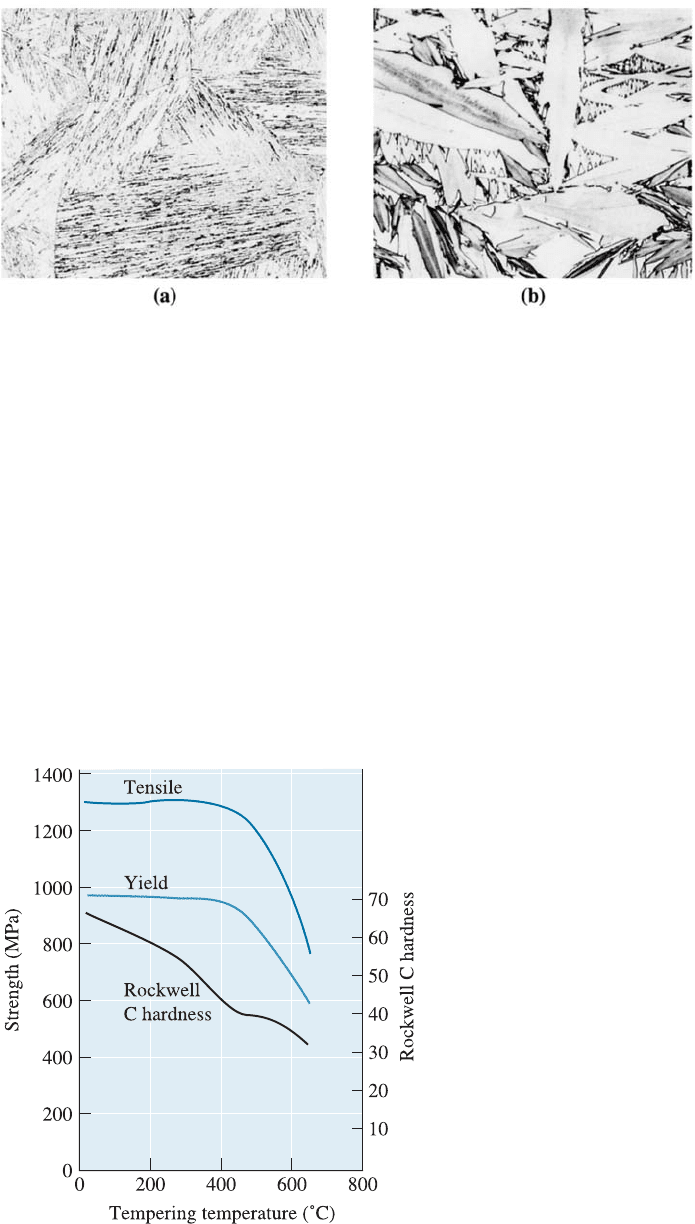
Tempering of Steel Martensite Martensite is not an equilibrium phase. This is why it
does not appear on the Fe-Fe
3
C phase diagram (Figure 12-11). When martensite in a
steel is heated below the eutectoid temperature, the thermodynamically stable a and
Fe
3
C phases precipitate. This process is called tempering. The decomposition of
martensite in steels causes the strength and hardness of the steel to decrease while the
ductility and impact properties are improved (Figure 12-24). Note that the term tem-
pering here is di¤erent from the term we used for tempering of silicate glasses. In both
Figure 12-23 (a) Lath martensite in low-carbon steel (80). (b) Plate martensite in high-
carbon steel (400). (From ASM Handbook, Vol. 8, (1973), ASM International, Materials
Park, OH 44073.)
Figure 12-24
Effect of tempering temperature on
the properties of an eutectoid steel.
12-11 The Martensitic Reaction and Tempering 383

tempering of glasses and tempering of steels, however, the key result is an increase in
the toughness of the material.
At low tempering temperatures, the martensite may form two transition phases—
a lower carbon martensite and a very fine nonequilibrium e-carbide, or Fe
2:4
C. The steel
is still strong, brittle, and perhaps even harder than before tempering. At higher tem-
peratures, the stable a and Fe
3
C form and the steel becomes softer and more ductile.
If the steel is tempered just below the eutectoid temperature, the Fe
3
C becomes very
coarse and the dispersion-strengthening e¤ect is greatly reduced. By selecting the
appropriate tempering temperature, a wide range of properties can be obtained. The
product of the tempering process is a microconstituent called tempered martensite
(Figure 12-25).
Martensite in Other Systems The characteristics of the martensite reaction are di¤er-
ent in other alloy systems. For example, martensite can form in iron-based alloys that
contain little or no carbon by a transformation of the FCC crystal structure to a BCC
crystal structure. In certain high-manganese steels and stainless steels, the FCC struc-
ture changes to a HCP crystal structure during the martensite transformation. In addi-
tion, the martensitic reaction occurs during the transformation of many polymorphic
ceramic materials, including ZrO
2
, and even in some crystalline polymers. Thus the
terms martensitic reaction and martensite are rather generic. In the context of steel
properties, microstructure, and heat treatment, the term ‘‘martensite’’ refers to the hard
and brittle bct phase obtained upon the quenching of steels.
The properties of martensite in other alloys are also di¤erent from the properties
of steel martensite. In titanium alloys, BCC titanium transforms to a HCP martensite
structure during quenching. However, the titanium martensite is softer and weaker than
the original structure. The martensite that forms in other alloys can also be tempered.
The martensite produced in titanium alloys can be reheated to permit the precipitation
of a second phase. Unlike the case of steel, however, the tempering process increases,
rather than decreases, the strength of the titanium alloy.
SUMMARY
V Solid-state phase transformations, which have a profound e¤ect on the structure
and properties of a material, can be controlled by proper heat treatments. These
heat treatments are designed to provide an optimum distribution of two or more
phases in the microstructure. Dispersion strengthening permits a wide variety of
structures and properties to be obtained.
Figure 12-25
Tempered martensite in steel (500). (From
ASM Handbook, Vol. 9, Metallography and
Microstructure (1985), ASM International
Materials Park, OH 44073.)
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment384

V Age hardening, or precipitation hardening, is one powerful method for controlling
the optimum dispersion strengthening in many metallic alloys. In age hardening,
a very fine widely dispersed coherent precipitate is allowed to precipitate by a heat
treatment that includes (a) solution treating to produce a single-phase solid sol-
ution, (b) quenching to retain that single phase, and (c) aging to permit a precip-
itate to form. In order for age hardening to occur, the phase diagram must show
decreasing solubility of the solute in the solvent as the temperature decreases.
V The most widely used eutectoid reaction occurs in producing steels from iron-
carbon alloys: Either pearlite or bainite can be produced as a result of the eutectoid
reaction in steel. In addition, primary ferrite or primary cementite may be present,
depending on the carbon content of the alloy. The trick is to formulate a micro-
structure that consists of a right mix of metal-like phases that are tough and
ceramic-like phases that are hard and brittle.
V Factors that influence the mechanical properties of the microconstituent produced
by the eutectoid reaction include (a) the composition of the alloy (amount of
eutectoid microconstituent), (b) the grain size of the original solid, the eutectoid
microconstituent, and any primary microconstituents, (c) the fineness of the struc-
ture within the eutectoid microconstituent (interlamellar spacing), (d) the cooling
rate during the phase transformation, and (e) the temperature at which the trans-
formation occurs (the amount of undercooling).
V A martensitic reaction occurs with no long-range di¤usion. Again, the best known
example occurs in steels:
The amount of martensite that forms depends on the temperature of the
transformation (an athermal reaction).
Martensite is very hard and brittle, with the hardness determined primarily
by the carbon content.
The amount and composition of the martensite are the same as the austenite
from which it forms.
V Martensite can be tempered. During tempering, a dispersion-strengthened structure
is produced. In steels, tempering reduces the strength and hardness but improves
the ductility and toughness.
GLOSSARY
Age hardening A special dispersion-strengthening heat treatment. By solution treatment,
quenching, and aging, a coherent precipitate forms that provides a substantial strengthening
e¤ect. Also known as precipitation hardening.
Artificial aging Reheating a solution-treated and quenched alloy to a temperature below the
solvus in order to provide the thermal energy required for a precipitate to form.
Athermal transformation When the amount of the transformation depends only on the temper-
ature, not on the time (same as martensitic transformation or displacive transformation).
Austenite The name given to the FCC crystal structure of iron.
Avrami relationship Describes the fraction of a transformation that occurs as a function of time.
This describes most solid-state transformations that involve di¤usion, thus martensitic trans-
formations are not described.
Bainite A two-phase microconstituent, containing ferrite and cementite, that forms in steels that
are isothermally transformed at relatively low temperatures.
Glossary 385
Cementite The hard, brittle ceramic-like compound Fe
3
C that, when properly dispersed, pro-
vides the strengthening in steels.
Coherent precipitate A precipitate whose crystal structure and atomic arrangement have a
continuous relationship with the matrix from which the precipitate is formed. The formation of
coherent precipitate provides excellent disruption of the atomic arrangement in the matrix and
provides excellent strengthening.
Displacive transformation A phase transformation that occurs via small displacements of atoms
or ions and without di¤usion. Same as athermal or martensitic transformation.
Ferrite The name given to the BCC crystal structure of iron that can occur as a or d. This is not
to be confused with ceramic ferrites which are magnetic materials.
Guinier-Preston (GP) zones Tiny clusters of atoms that precipitate from the matrix in the early
stages of the age-hardening process. Although the GP zones are coherent with the matrix, they
are too small to provide optimum strengthening.
Interfacial energy The energy associated with the boundary between two phases.
Isothermal transformation When the amount of a transformation at a particular temperature
depends on the time permitted for the transformation.
Martensite A metastable phase formed in steel and other materials by a di¤usionless, athermal
transformation.
Martensitic transformation A phase transformation that occurs without di¤usion. Same as
athermal or displacive transformation. These occur in steels, Ni-Ti and many ceramic materials.
Natural aging When a coherent precipitate forms from a solution-treated and quenched age-
hardenable alloy at room temperature, providing optimum strengthening.
Pearlite A two-phase lamellar microconstituent, containing ferrite and cementite, that forms in
steels cooled in a normal fashion or isothermally transformed at relatively high temperatures.
Shape-memory alloys (SMAs) Certain materials which develop microstructures that, after being
deformed, can return the material to its initial shape when heated (e.g. Ni-Ti alloys).
Solution treatment The first step in the age-hardening heat treatment. The alloy is heated above
the solvus temperature to dissolve any second phase and to produce a homogeneous single-phase
structure.
Strain energy The energy required to permit a precipitate to fit into the surrounding matrix
during nucleation and growth of the precipitate.
Supersaturated solid solution The solid solution formed when a material is rapidly cooled from
a high-temperature single-phase region to a low-temperature two-phase region without the second
phase precipitating. Because the quenched phase contains more alloying element than the sol-
ubility limit, it is supersaturated in that element.
Tempering A low-temperature heat treatment used to reduce the hardness of martensite by
permitting the martensite to begin to decompose to the equilibrium phases. This leads to in-
creased toughness.
TTT diagram The time-temperature-transformation diagram describes the time required at any
temperature for a phase transformation to begin and end. The TTT diagram assumes that the
temperature is constant during the transformation.
Widmanstðtten structure The precipitation of a second phase from the matrix when there is a
fixed crystallographic relationship between the precipitate and matrix crystal structures. Often
needle-like or plate-like structures form in the Widmanstðtten structure.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment386
PROBLEMS
3
Section 12-1 Nucleation and Growth in Solid-
State Reactions
12-1 How is the equation for nucleation of a phase in
the solid state di¤erent from that for a liquid to
solid transformation?
12-2 Determine the constants c and n in Equation 12-2
that describe the rate of crystallization of poly-
propylene at 140
C. (See Figure 12-26.)
Section 12-2 Alloys Strengthened By Exceeding
the Solubility Limit
12-3 What are the di¤erent ways by which a second
phase can be made to precipitate in a two-phase
microstructure?
12-4 Explain, when cooled slowly, why it is that the
second phase in Al-4% Cu alloys nucleates and
grows along the grain boundaries. Is this usu-
ally desirable?
12-5 What properties of the precipitate phase are
needed for precipitation hardening? Why?
Section 12-3 Age or Precipitation Hardening
12-6 What is the principle of precipitation hardening?
12-7 What is a supersaturated solution? How do we
obtain supersaturated solutions during precip-
itation hardening? Why is the formation of a
supersaturated solution necessary?
12-8 Suppose that age hardening is possible in the
Al-Mg system. (See Figure 12-8.)
(a) Recommend an artificial age-hardening heat
treatment for each of the following alloys,
and
(b) compare the amount of the b precipitate that
forms from your treatment of each alloy.
(i) Al-4% Mg (ii) Al-6% Mg
(iii) Al-12% Mg
(c) Testing of the alloys after the heat treatment
reveals that little strengthening occurs as a
result of the heat treatment. Which of the re-
quirements for age hardening is likely not
satisfied?
12-9 An Al-2.5% Cu alloy is solution-treated,
quenched, and overaged at 230
C to produce a
stable microstructure. If the y precipitates as
spheres with a diameter of 9000 A and a density
of 4.26 g/cm
3
, determine the number of precip-
itate particles per cm
3
.
Section 12-4 Applications of Age-Hardened
Alloys
12-10 Why is precipitation hardening an attractive
mechanism of strengthening for aircraft mate-
rials?
12-11 Why are most precipitation-hardened alloys
suitable only for relatively low-temperature ap-
plications?
Section 12-5 Microstructural Evolution in Age or
Precipitation Hardening
12-12 Explain the three basic steps encountered dur-
ing precipitation hardening.
Section 12-6 Effects of Aging Temperature and
Time
12-13 What is aging? Why is this step needed in pre-
cipitation hardening?
12-14 What do the terms ‘‘natural aging’’ and ‘‘artifi-
cial aging’’ mean?
Figure 12-26 The effect of temperature on the
crystallization of polypropylene (for Problem 12-2).
Figure 12-8 (Repeated for Problem 12-8) Portion of
the aluminum-magnesium phase diagram.
Problems 387
12-15 In the plane flown by the Wright brothers, how
was the alloy precipitation strengthened?
Section 12-7 Requirements for Age Hardening
12-16 Can all alloy compositions be strengthened
using precipitation hardening? Can we use this
mechanism for the strengthening of ceramics,
glasses, or polymers?
12-17 A conductive copper wire is to be made. Would
you choose precipitation hardening as a way of
strengthening this wire? Explain.
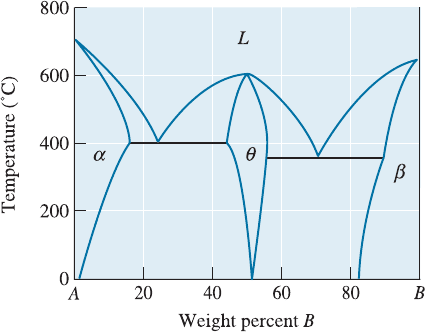
12-18 Figure 12-27 shows a hypothetical phase dia-
gram. Determine whether each of the following
alloys might be good candidates for age hard-
ening, and explain your answer. For those
alloys that might be good candidates, describe
the heat treatment required, including recom-
mended temperatures.
(a) A-10% B (b) A-20% B (c) A-55% B
(d) A-87% B (e) A-95% B
Section 12-8 Use of Age-Hardenable Alloys at
High Temperatures
12-19 Why is it that certain aluminum (not nickel-
based) alloys strengthened using age hardening
can lose their strength on welding?
12-20 Would you choose a precipitation-hardened
alloy to make an aluminum alloy baseball bat?
Section 12-9 The Eutectoid Reaction
12-21 Write down the eutectoid reaction in Fe-Fe
3
C
system.
12-22 Compare and contrast eutectic and eutectoid
reactions.
12-23 Define the following terms: ferrite, austenite,
pearlite, and cementite.
12-24 The pearlite microstructure is similar to a
ceramic-metal nanocomposite. True or False.
Comment.
12-25 What is the di¤erence between a microconstitu-
ent and a phase?
12-26 For an Fe-0.35% C alloy, determine
(a) the temperature at which austenite first be-
gins to transform on cooling,
(b) the primary microconstituent that forms,
(c) the composition and amount of each phase
present at 728
C,
(d) the composition and amount of each phase
present at 726
C, and
(e) the composition and amount of each mi-
croconstituent present at 726
C.
12-27 For an Fe-1.15% C alloy, determine
(a) the temperature at which austenite first
begins to transform on cooling,
(b) the primary microconstituent that forms,
(c) the composition and amount of each phase
present at 728
C,
(d) the composition and amount of each phase
present at 726
C, and
(e) the composition and amount of each mi-
croconstituent present at 726
C.
12-28 A steel contains 8% cementite and 92% ferrite at
room temperature. Estimate the carbon content
of the steel. Is the steel hypoeutectoid or hyper-
eutectoid?
12-29 A steel contains 18% cementite and 82% ferrite
at room temperature. Estimate the carbon con-
tent of the steel. Is the steel hypoeutectoid or
hypereutectoid?
12-30 A steel contains 18% pearlite and 82% primary
ferrite at room temperature. Estimate the carbon
content of the steel. Is the steel hypoeutectoid or
hypereutectoid?
12-31 A steel contains 94% pearlite and 6% primary
cementite at room temperature. Estimate the
carbon content of the steel. Is the steel hypo-
eutectoid or hypereutectoid?
12-32 A steel contains 55% a and 45% g at 750
C.
Estimate the carbon content of the steel.
12-33 A steel contains 96% g and 4% Fe
3
C at 800
C.
Estimate the carbon content of the steel.
12-34 A steel is heated until 40% austenite, with a
carbon content of 0.5%, forms. Estimate the
Figure 12-27 Hypothetical phase diagram (for
Problem 12-18).
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment388
temperature and the overall carbon content of
the steel.
12-35 A steel is heated until 85% austenite, with a
carbon content of 1.05%, forms. Estimate the
temperature and the overall carbon content of
the steel.
Section 12-10 Controlling the Eutectoid
Reaction
12-36 Why are the distances between lamellae formed
in an eutectoid reaction typically separated by
distances smaller than those formed in eutectic
reactions?
12-37 Compare the interlamellar spacing and the yield
strength when an eutectoid steel is isothermally
transformed to pearlite at
(a) 700
C, and
(b) 600
C.
12-38 Why is it that a eutectoid steel exhibits di¤erent
yield strengths and % elongations, depending
upon if it was cooled slowly or relatively fast?
12-39 What is a TTT diagram?
12-40 Sketch and label clearly di¤erent parts of a TTT
diagram for a plain-carbon steel with 0.77%
carbon.
12-41 On the TTT diagram what is the di¤erence be-
tween the g and g
u
phases?
12-42 How is it that bainite and pearlite do not appear
in the Fe-Fe
3
C diagram? Are these phases or
microconstituents?
12-43 Why is it that we cannot make use of TTT dia-
grams for describing heat treatment profiles in
which samples are getting cooled over a period
of time (i.e., why are TTT diagrams suitable for
only following isothermal transformations)?
12-44 What is bainite? Why do steels containing bain-
ite exhibit higher levels of toughness?
12-45 An isothermally transformed eutectoid steel is
found to have a yield strength of 410 MPa. Es-
timate
(a) the transformation temperature, and
(b) the interlamellar spacing in the pearlite.
12-46 Determine the required transformation temper-
ature and microconstituent if an eutectoid steel
is to have the following hardness values:
(a) HRC 38 (b) HRC 42
(c) HRC 48 (d) HRC 52
12-47 Describe the hardness and microstructure in an
eutectoid steel that has been heated to 800
C for
1 h, quenched to 350
C and held for 750 s, and
finally quenched to room temperature.
12-48 Describe the hardness and microstructure in an
eutectoid steel that has been heated to 800
C,
quenched to 650
C, held for 500 s, and finally
quenched to room temperature.
12-49 Describe the hardness and microstructure in an
eutectoid steel that has been heated to 800
C,
quenched to 300
C and held for 10 s, and finally
quenched to room temperature.
12-50 Describe the hardness and microstructure in an
eutectoid steel that has been heated to 800
C,
quenched to 300
C and held for 10 s, quenched
to room temperature, and then reheated to
400
C before finally cooling to room temper-
ature again.
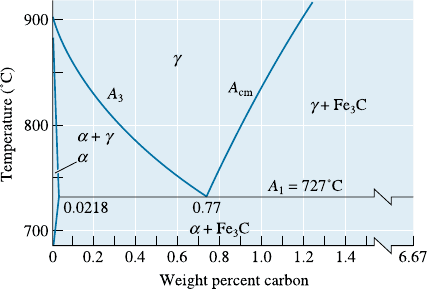
12-51 A steel containing 0.3% C is heated to various
temperatures above the eutectoid temperature,
held for 1 h, and then quenched to room tem-
perature. Using Figure 12-28, determine the
amount, composition, and hardness of any
martensite that forms when the heating temper-
ature is:
(a) 728
C (b) 750
C
(c) 790
C (d) 850
C
Section 12-11 The Martensitic Reaction and
Tempering
12-52 What is the di¤erence between solid-state phase
transformations such as the eutectoid reaction
and the martensitic phase transformation?
12-53 What is the di¤erence between isothermal and
athermal transformations?
Figure 12-28 The eutectoid portion of the Fe-Fe
3
C
phase diagram (for Problems 12-51, 12-56, 12-57,
and 12-58).
Problems 389
