Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


SUMMARY
V By producing a material containing two or more phases, dispersion strengthening
is obtained. In metallic materials, the boundary betwe en the phases impedes the
movement of dislocations and im proves strength. Introduction of multiple phases
may provide other benefits, including improvement of the fracture toughness of
ceramics and polymers.
V For optimum dispersion strengthening, particularly in metallic materials, a large
number of small, hard, discontinuous dispersed phase particles should form in a
soft, ductile matrix to provide the most e¤ective obstacles to dislocations. Round
dispersed phase particles minimize stress concentrations, and the final properties of
the alloy can be controlled by the relative amounts of these and the matrix.
V Intermetallic compounds, which normally are strong but brittle, are frequently in-
troduced as dispersed phases in the microstructure.
V Phase diagrams for materials containing multiple phases normally contain one or
more three-phase reactions:
9
The eutectic reaction permits liquid to solidify as an intimate mixture of two
phases. By controlling the solidification process, we can achieve a wide range of
properties. Some of the factors that can be controlled include: the grain size or
secondary dendrite arm spacings of primary microconsti tuents, the colony size of
the eutectic microconstituent, the interlamellar spacing within the eutectic mi-
croconstituent, the microstructure, or shape, of the phases within the eutectic
microconstituent, and the amount of the eutectic microconstituent that forms.
9
The eutectoid reaction causes a solid to transform to a mixture of two other
solids. As shown in the next chapter, heat treatments to control the eutectoid re-
action provide an excellent basis for dispersion strengthening of many steels and
other alloys.
GLOSSARY
Age hardening A strengthening mechanism that relies on a sequence of solid-state phase trans-
formations in generating a dispersion of ultrafine particles of a second phase. This is the same as
precipitation hardening.
Dispersion strengthening Increasing the strength of a material by forming more than one phase.
By proper control of the size, shape, amount, and individual properties of the phases, excellent
combinations of properties can be obtained.
Eutectic A three-phase, invariant reaction in which one liquid phase solidifies to produce two
solid phases.
Eutectic microconstituent A characteristic mixture of two phases formed as a result of the
eutectic reaction.
Eutectoid A three-phase, invariant reaction in which one solid phase transforms to two di¤erent
solid phases.
Hyper- A prefix indicating that the composition of an alloy is more than the composition at
which a three-phase reaction occurs.
Hypereutectic alloys An alloy composition between that of the right-hand-side end of the tie
line defining the eutectic reaction and the eutectic composition.
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams350
Hypo- A prefix indicating that the composition of an alloy is less than the composition at which
a three-phase reaction occurs.
Hypoeutectic alloy An alloy composition between that of the left-hand-side end of the tie line
defining the eutectic reaction and the eutectic composition.
Interlamellar spacing The distance between the center of a lamella or plate of one phase and the
center of the adjoining lamella or plate of the same phase.
Intermediate solid solution A nonstoichiometric intermetallic compound displaying a range of
compositions.
Intermetallic compound A compound formed of two or more metals that has its own unique
composition, structure, and properties.
Interphase interface The boundary between two phases in a microstructure. In metallic mate-
rials, this boundary resists dislocation motion and provides dispersion strengthening and precip-
itation hardening.
Isopleth A line on a phase diagram that shows constant chemical composition.
Isoplethal study Determination of reactions and microstructural changes that are expected
while studying a particular chemical composition in a system.
Lamella A thin plate of a phase that forms during certain three-phase reactions, such as the
eutectic or eutectoid.
Matrix The continuous solid phase in a complex microstructure. Solid dispersed phase particles
may form within the matrix.
Metastable miscibility gap A miscibility gap that extends below the liquidus or exists com-
pletely below the liquidus. Two liquids that are immiscible continue to exist as liquids and remain
unmixed. These systems form the basis for Vycor
TM
and Pyrex
8
glasses.
Microconstituent A phase or mixture of phases in an alloy that has a distinct appearance. Fre-
quently, we describe a microstructure in terms of the microconstituents rather than the actual
phases.
Miscibility gap A region in a phase diagram in which two phases, with essentially the same
structure, do not mix, or have no solubility in one another.
Modification Addition of alloying elements, such as sodium or strontium, which change the
microstructure of the eutectic microconstituent in aluminum-silicon alloys.
Monotectic A three-phase reaction in which one liquid transforms to a solid and a second liquid
on cooling.
Nonstoichiometric intermetallic compound A phase formed by the combination of two com-
ponents into a compound having a structure and properties di¤erent from either component. The
nonstoichiometric compound has a variable ratio of the components present in the compound
(see also Intermediate solid solution).
Peritectic A three-phase reaction in which a solid and a liquid combine to produce a second
solid on cooling.
Peritectoid A three-phase reaction in which two solids combine to form a third solid on cooling.
Precipitate A solid phase that forms from the original matrix phase when the solubility limit is
exceeded.
Glossary 351
Precipitation hardening A strengthening mechanism that relies on a sequence of solid-state
phase transformations in generating a dispersion of ultrafine precipitates of a second phase
(Chapter 12). This is the same as age hardening. It is a form of dispersion strengthening.
Primary microconstituent The microconstituent that forms before the start of a three-phase
reaction.
Solvus A solubility curve that separates a single-solid phase region from a two-solid phase
region in the phase diagram.
Stoichiometric intermetallic compound A phase formed by the combination of two components
into a compound having a structure and properties di¤erent from either component. The stoi-
chiometric intermetallic compound has a fixed ratio of the components present in the compound.
PROBLEMS
3
Section 11-1 Principles and Examples of
Dispersion Strengthening
11-1 What are the requirements of a matrix and precip-
itate for dispersion strengthening to be e¤ective?
Section 11-2 Intermetallic Compounds
11-2 What is an intermetallic compound? How is it
di¤erent from other compounds? For example,
other than the obvious di¤erence in composition
how is TiAl di¤erent from, for example, Al
2
O
3
?
11-3 Explain clearly the two di¤erent ways in which
intermetallic compounds can be used.
11-4 What are some of the major problems in the uti-
lization of intermetallics for high-temperature
applications?
Section 11-3 Phase Diagrams Containing Three-
Phase Reactions
11-5 Define the terms eutectic, eutectoid, peritectic,
peritectoid, and monotectic reactions.
11-6 What is an invariant reaction? Show that for a
two-component system the number of degrees of
freedom for an invariant reaction is zero.
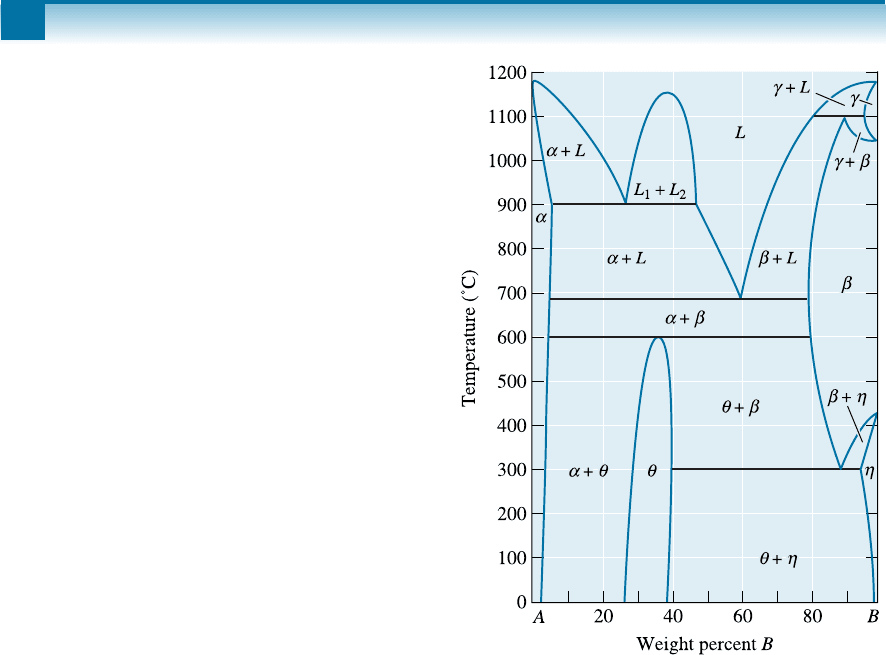
11-7 A hypothetical phase diagram is shown in Figure
11-25.
(a) Are there any intermetallic compounds pres-
ent? If so, identify them and determine
whether they are stoichiometric or non-
stoichiometric.
(b) Identify the solid solutions present in the
system. Is either material A or B allotropic?
Explain.
(c) Identify the three-phase reactions by writing
down the temperature, the reaction in equa-
tion form, the composition of each phase in
the reaction, and the name of the reaction.
11-8 The Cu-Zn phase diagram is shown in Figure
11-26.
Figure 11-25 Hypothetical phase diagram (for
Problem 11-7).
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams352
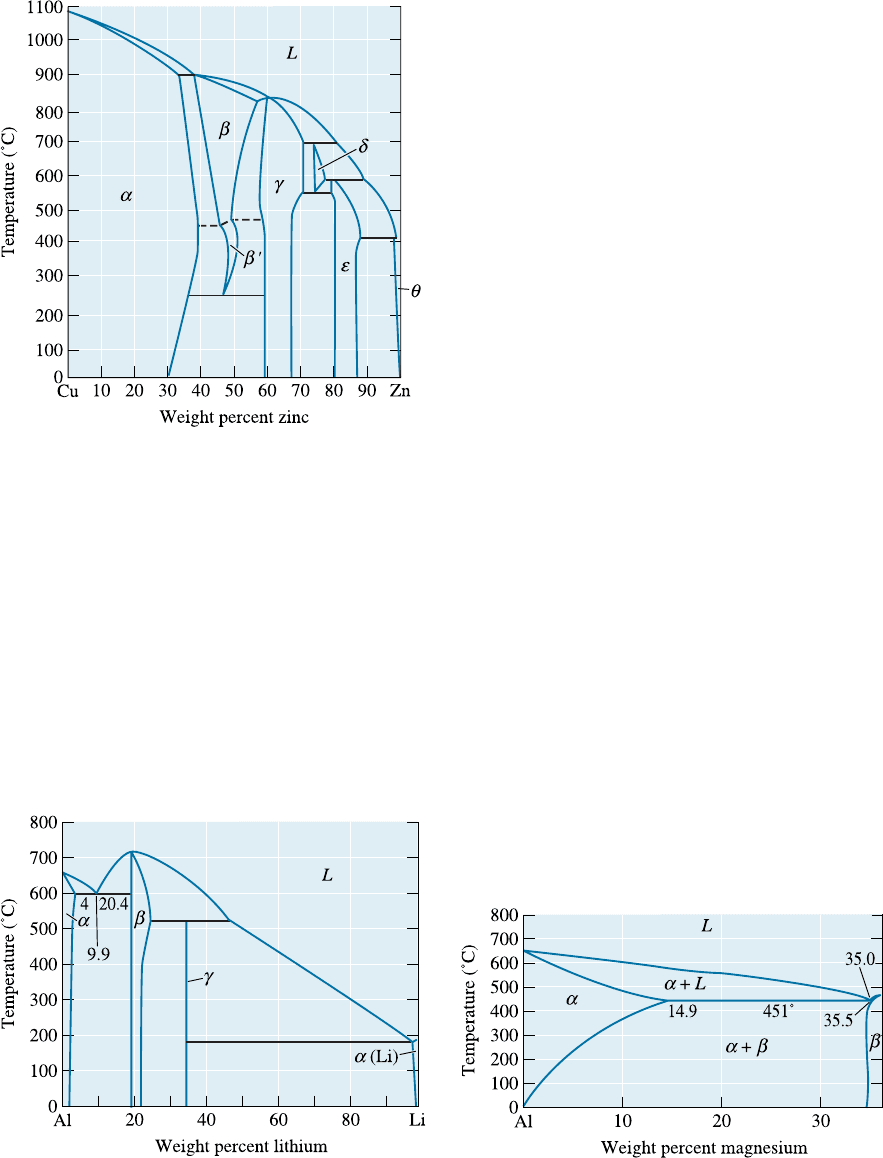
(a) Are any intermetallic compounds present?
If so, identify them and determine whether
they are stoichiometric or nonstoichiometric.
(b) Identify the solid solutions present in the
system.
(c) Identify the three-phase reactions by writing
down the temperature, the reaction in equa-
tion form, and the name of the reaction.
11-9 The Al-Li phase diagram is shown in Figure
11-27.
(a) Are any intermetallic compounds present? If
so, identify them and determine whether they
are stoichiometric or nonstoichiometric. De-
termine the formula for each compound.
(b) Identify the three-phase reactions by writing
down the temperature, the reaction in equa-
tion form, the composition of each phase in
the reaction, and the name of the reaction.
11-10 An intermetallic compound is found for 38 wt%
Sn in the Cu-Sn phase diagram. Determine the
formula for the compound.
11-11 An intermetallic compound is found for 10 wt%
Si in the Cu-Si phase diagram. Determine the
formula for the compound.
Section 11-4 The Eutectic Phase Diagram
11-12 Consider a Pb-15% Sn alloy. During solid-
ification, determine
(a) the composition of the first solid to form,
(b) the liquidus temperature, solidus temper-
ature, solvus temperature, and freezing
range of the alloy,
(c) the amounts and compositions of each
phase at 260
C,
(d) the amounts and compositions of each
phase at 183
C, and
(e) the amounts and compositions of each
phase at 25
C.
11-13 Consider an Al-12% Mg alloy (Figure 11-28).
During solidification, determine
(a) the composition of the first solid to form,
(b) the liquidus temperature, solidus temper-
ature, solvus temperature, and freezing
range of the alloy,
(c) the amounts and compositions of each
phase at 525
C,
(d) the amounts and compositions of each
phase at 450
C, and
(e) the amounts and compositions of each
phase at 25
C.
Figure 11-26 Binary phase diagram for the copper-
zinc system (for Problem 11-8).
Figure 11-27 The aluminum-lithium phase diagram
(for Problem 11-9).
Figure 11-28 Portion of the aluminum-magnesium
phase diagram (for Problems 11-13).
Problems 353
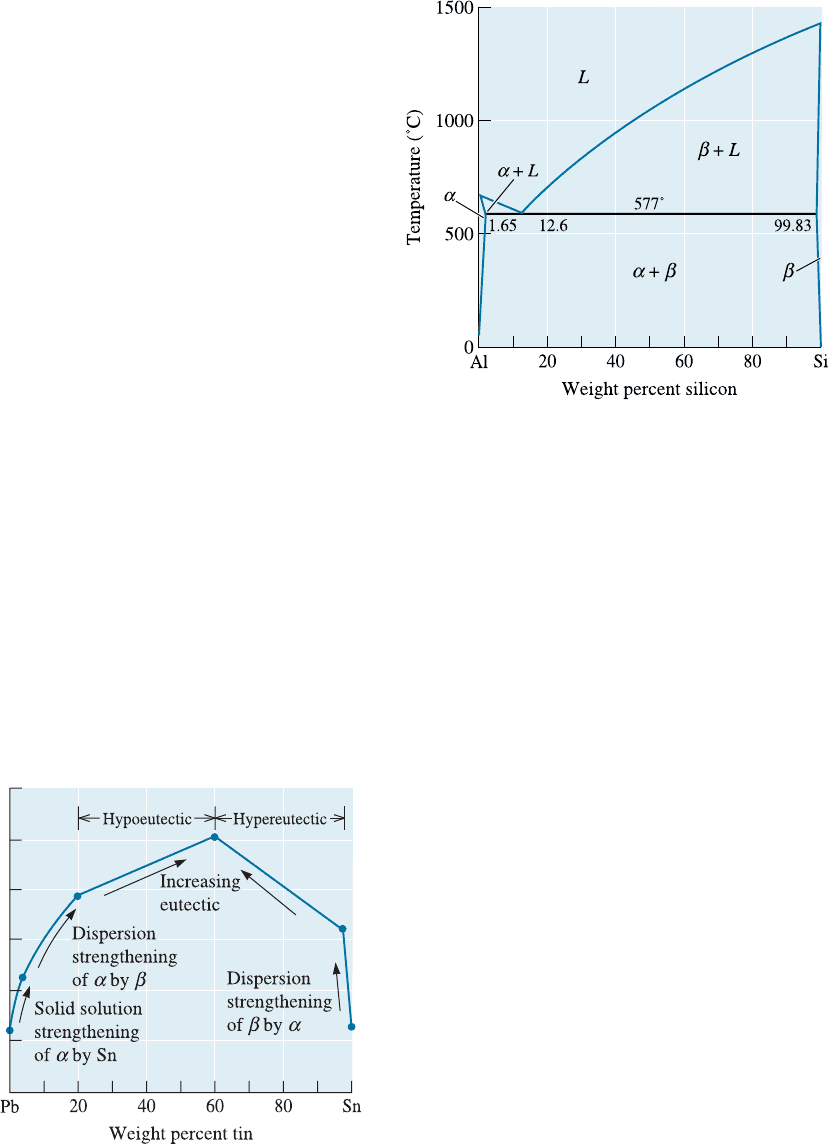
11-14 Consider a Pb-35% Sn alloy. Determine
(a) if the alloy is hypoeutectic or hypereutectic,
(b) the composition of the first solid to form
during solidification,
(c) the amounts and compositions of each
phase at 184
C,
(d) the amounts and compositions of each
phase at 182
C,
(e) the amounts and compositions of each mi-
croconstituent at 182
C, and
(f) the amounts and compositions of each
phase at 25
C.
11-15 Consider a Pb-70% Sn alloy. Determine
(a) if the alloy is hypoeutectic or hypereutectic,
(b) the composition of the first solid to form
during solidification,
(c) the amounts and compositions of each
phase at 184
C,
(d) the amounts and compositions of each
phase at 182
C,
(e) the amounts and compositions of each mi-
croconstituent at 182
C, and
(f) the amounts and compositions of each
phase at 25
.
11-16 Calculate the total % b and the % eutectic micro-
constituent at room temperature for the following
lead-tin alloys: 10% Sn, 20% Sn, 50% Sn, 60% Sn,
80% Sn, and 95% Sn. Using Figure 11-18, plot the
strength of the alloys versus the % b and the %
eutectic and explain your graphs.
11-17 Consider an Al-4% Si alloy. (See Figure 11-19.)
Determine
(a) if the alloy is hypoeutectic or hypereutectic,
(b) the composition of the first solid to form
during solidification,
(c) the amounts and compositions of each
phase at 578
C,
(d) the amounts and compositions of each phase
at 576
C, the amounts and compositions of
each microconstituent at 576
C, and
(e) the amounts and compositions of each
phase at 25
C.
11-18 Consider an Al-25% Si alloy. (See Figure 11-19.)
Determine
(a) if the alloy is hypoeutectic or hypereutectic,
(b) the composition of the first solid to form
during solidification,
(c) the amounts and compositions of each
phase at 578
C,
(d) the amounts and compositions of each
phase at 576
C,
(e) the amounts and compositions of each mi-
croconstituent at 576
C, and
(f) the amounts and compositions of each
phase at 25
C.
11-19 A Pb-Sn alloy contains 45% a and 55% b at
100
C. Determine the composition of the alloy.
Is the alloy hypoeutectic or hypereutectic?
11-20 An Al-Si alloy contains 85% a and 15% b at
500
C. Determine the composition of the alloy.
Is the alloy hypoeutectic or hypereutectic?
10
20
30
40
50
60
Tensile strength (MPa)
0
Figure 11-18 (Repeated for Problem 11-16)
The effect of the composition and strengthening
mechanism on the tensile strength of lead-tin alloys.
Figure 11-19 (Repeated for Problems 11-17 and
11-18) The aluminum-silicon phase diagram.
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams354
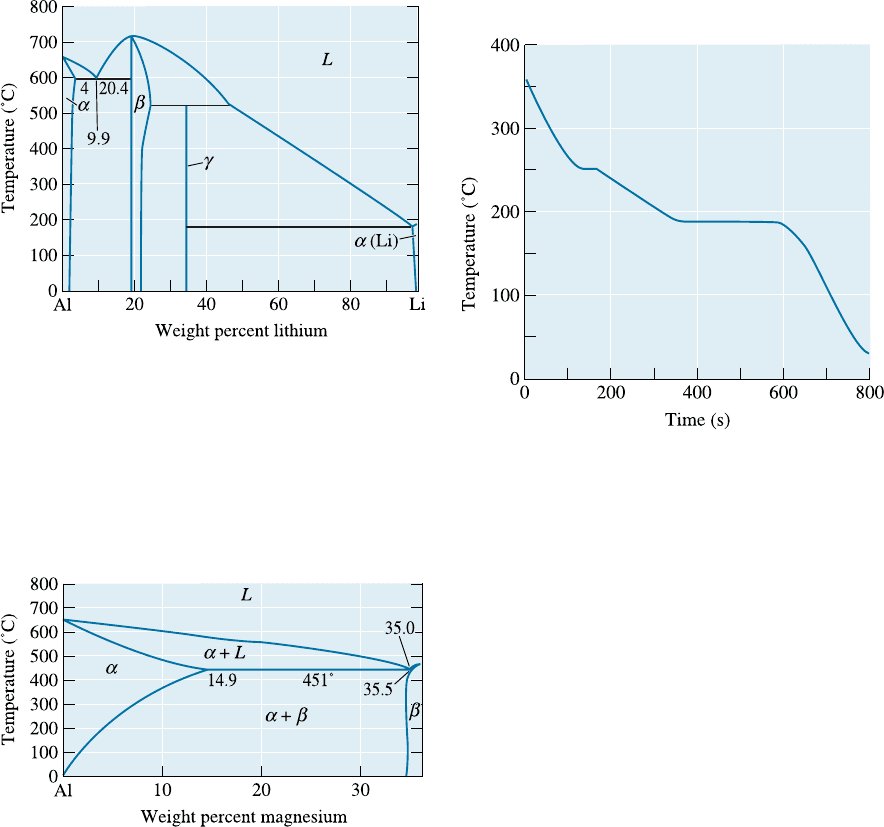
11-21 A Pb-Sn alloy contains 23% primary a and 77%
eutectic microconstituent. Determine the com-
position of the alloy.
11-22 An Al-Si alloy contains 15% primary b and 85%
eutectic microconstituent. Determine the com-
position of the alloy.
11-23 Observation of a microstructure shows that
there is 28% eutectic and 72% primary b in an
Al-Li alloy (Figure 11-27).
(a) Determine the composition of the alloy and
whether it is hypoeutectic or hypereutectic.
(b) How much a and b are in the eutectic mi-
croconstituent?
11-24 Write the eutectic reaction that occurs, includ-
ing the compositions of the three phases in
equilibrium, and calculate the amount of a and
b in the eutectic microconstituent in the Mg-Al
system (Figure 11-28).
11-25 Calculate the total amount of a and b and the
amount of each microconstituent in a Pb-50%
Sn alloy at 182
C. What fraction of the total a
in the alloy is contained in the eutectic micro-
constituent?
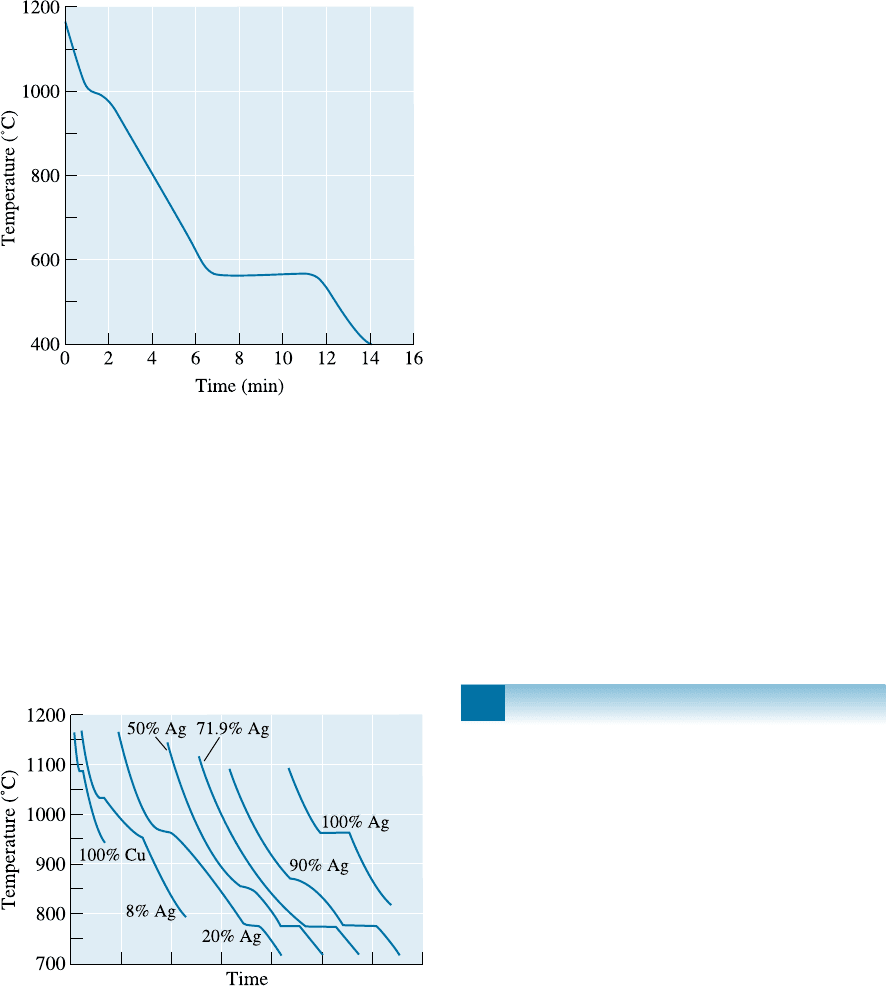
11-26 Figure 11-29 shows a cooling curve for a Pb-Sn
alloy. Determine
(a) the pouring temperature,
(b) the superheat,
(c) the liquidus temperature,
(d) the eutectic temperature,
(e) the freezing range,
(f) the local solidification time,
(g) the total solidification time, and
(h) the composition of the alloy.
11-27 Figure 11-30 shows a cooling curve for an Al-Si
alloy. Determine
(a) the pouring temperature,
(b) the superheat,
(c) the liquidus temperature,
(d) the eutectic temperature,
(e) the freezing range,
(f) the local solidification time,
(g) the total solidification time, and
(h) the composition of the alloy.
11-28 Draw the cooling curves, including appropriate
temperatures, expected for the following Al-Si
alloys:
(a) Al-4% Si,
(b) Al-12.6% Si,
Figure 11-27 (Repeated for Problem 11-23) The
aluminum-lithium phase diagram.
Figure 11-28 Portion of the aluminum-magnesium
phase diagram (for Problems 11-24).
Figure 11-29 Cooling curve for a Pb-Sn alloy (for
Problem 11-26).
Problems 355
(c) Al-25% Si, and
(d) Al-65% Si.
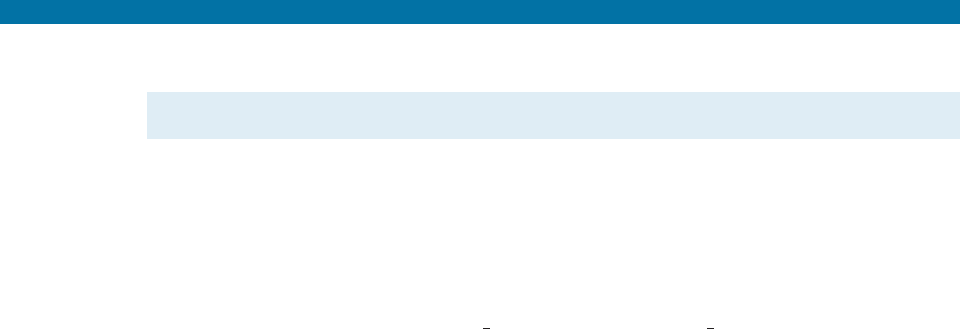
11-29 Cooling curves are obtained for a series of Cu-
Ag alloys (Figure 11-31). Use this data to pro-
duce the Cu-Ag phase diagram. The maximum
solubility of Ag in Cu is 7.9% and the maximum
solubility of Cu in Ag is 8.8%. The solubilities
at room temperature are near zero.
Section 11-5 Strength of Eutectic Alloys
11-30 In regards to eutectic alloys, what does the term
‘‘modification’’ mean? How does it help prop-
erties of the alloy?
11-31 For the Pb-Sn system, explain why the tensile
strength is maximum at the eutectic composition.
11-32 Does the shape of the proeutectic phase have
an e¤ect on the strength of eutectic alloys?
Explain.
Section 11-6 Eutectics and Materials
Processing
11-33 Explain why Pb-Sn alloys are used for soldering.
11-34 Refractories used in steel making include silica
brick that contain very small levels of alumina
(Al
2
O
3
). The eutectic temperature in this system
is about 1587
C. Silica melts at about 1725
C.
Explain what will happen to the load bearing
capacity of the bricks if a small amount of alu-
mina gets incorporated into the silica bricks.
Section 11-7 Nonequilibrium Freezing in the
Eutectic System
11-35 What is hot shortness? How does it a¤ect the
temperature at which eutectic alloys can be used?
Design Problems
g
11-36 Design a processing method that permits a Pb-
15% Sn alloy solidified under nonequilibrium
conditions to be hot worked.
11-37 Design a eutectic di¤usion bonding process to
join aluminum to silicon. Describe the changes
in microstructure at the interface during the
bonding process.
11-38 Design an Al-Si brazing alloy and process that
will be successful in joining an Al-Mn alloy that
has a liquidus of 659
C and a solidus of 656
C.
Brazing, like soldering, involves introducing a
liquid filler metal into a joint without melting
the metals that are to be joined.
Figure 11-30 Cooling curve for an Al-Si alloy
(for Problem 11-27).
Figure 11-31 Cooling curves for a series of Cu-Ag
alloys (for Problem 11-29).
C H A P TE R 1 1 Dispersion Strengthening and Eutectic Phase Diagrams356

12
Dispersion Strengthening by
Phase Transformations and
Heat Treatment
Have You Ever Wondered?
9 Who invented and flew the first controllable airplane?
9 How do engineers strengthen aluminum alloys used in aircrafts?
9 Why do some steels become very hard upon quenching from high temperatures?
9 What alloys are used to make orthodontic braces?
9 Would it be possible to further enhance the strength and, hence, the dent resistance of sheet
steels after the car chassis is made?
In Chapter 11, we examined in detail how
second-phase particles can increase the strength
of metallic materials. In this chapter, we further
discuss dispe rsion strengthening as we describe
a variety of solid-state transformation processes
including precipitation or age hardening and
the eutectoid reaction. We also examine how
nonequilibrium phase transformations in parti-
cular, the martensitic reaction can provide
strengthening.
As we discuss these strengthening mecha-
nisms, keep in mind the characteristics that pro-
357
duce the most desirable dispersion strengthening
as discussed in Chapter 11:
9
The matrix should be relatively soft and
ductile and the precipitate, or second
phase, should be strong;
9
the precipitate particles should be round
and discontinuous;
9
the second-phase particles should be small
and numerous; and
9
in general, the more precipitate phase we
have, the stronger the alloy will be.
As in Chapter 11, we will concentrate on how
phase transformations influence the strength of
the materials and how heat treatments can influ-
ence other properties. Since we will be dealing
with solid-state phase transformations, we will
begin with a discussion on the nucleation and
growth of second-phase particles in solid-state
phase transformations.
12-1 Nucleation and Growth in Solid-State Reactions
In Chapter 9, we discussed nucleation of a solid nucleus from a melt. We also discussed
the concepts of supersaturation, undercooling, and homogeneous and heterogeneous
nucleation. Let’s now see how these concepts apply to solid-state phase transformations
such as the eutectoid reaction. In order for a precipitate of phase b to form from a solid
matrix of phase a, both nucleation and growth must occur. The total change in free
energy required for nucleation of a spherical solid precipitate from the matrix is:
DG ¼
4
3
pr
3
DG
vða!bÞ
þ 4pr
2
s
ab
þ
4
3
pr
3
e ð 12-1Þ
The first two terms include the free energy change per unit volume (DG
v
), and the en-
ergy change needed to create the unit area of the interface (s
ab
), just as in solidification.
However, the third term takes into account the strain energy per unit volume (e), the
energy required to permit a precipitate to fit into the surrounding matrix during the
nucleation and growth of the precipitate, introduced when the precipitate forms in a
solid, rigid matrix. The precipitate does not occupy the same volume that is displaced,
so additional energy is required to accommodate the precipitate in the matrix.
Nucleation As in solidification, nucleation occurs most easily on surfaces already
present in the structure, thereby minimizing the surface energy term. Thus, the precip-
itates heterogeneously nucleate most easily at grain boundaries and other defects.
Growth Growth of the precipitates normally occurs by long-range di¤usion and redis-
tribution of atoms. Di¤using atoms must be detached from their original locations
(perhaps at lattice points in a solid solution), move through the surrounding material
to the nucleus, and be incorporated into the crystal structure of the precipitate. In some
cases, the di¤using atoms might be so tightly bonded within an existing phase that the
detachment process limits the rate of growth. In other cases, attaching the di¤using
atoms to the precipitate—perhaps because of the lattice strain—limits growth. This
result sometimes leads to the formation of precipitates that have a special relationship
to the matrix structure that minimizes the strain at the interface between the parent
phase and the precipitate particles. In most cases, however, the controlling factor is the
di¤usion step.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment358

Kinetics The overall rate, or kinetics, of a transformation depends on both nucleation
and growth. If more nuclei are present at a particular temperature, growth occurs from
a larger number of sites and the phase transformation is completed in a shorter period
of time. At higher temperatures, the di¤usion coe‰cient is higher, growth rates are
higher, and again we expect the transformation to be completed in a shorter time, as-
suming an equal number of nuclei.
The rate of transformation is given by the Avrami equation (Equation 12-2), with
the fraction of the transformation, f , related to time, t,by
f ¼ 1 expðct
n
Þð12-2Þ
where c and n are constants for a particular temperature. This Avrami relationship,
shown in Figure 12-1, produces a sigmoidal, or S-shaped, curve. This equation can de-
scribe most solid-state phase transformations. An incubation time, t
0
, during which no
observable transformation occurs, is the time required for nucleation to occur. Initially,
the transformation occurs slowly as nuclei form.
The incubation period is followed by rapid growth as atoms di¤use to the growing
precipitate. Near the end of the transformation, the rate again slows as the source of
atoms available to di¤use to the growing precipitate is depleted. The transformation is
50% complete in time t; the rate of transformation is often given by the reciprocal of t:
Rate ¼ 1=t ð12-3Þ
Effect of Temperature In many phase transformations, the material undercools below
the temperature at which the phase transformation occurs under equilibrium condi-
tions. Recall from Chapter 9 the undercooling of water and other liquids and other
supersaturation phenomena. Because both nucleation and growth are temperature-
dependent, the rate of phase transformation depends on the undercooling (DT). The
rate of nucleation is low for small undercoolings (since the therm odynamic driving
force is low) and increases for larger undercoolings as the thermodynamic driving force
increases at least up to a certain point (since di¤usion becomes slower as temperature
decreases). At the same time, the growth rate of the new phase decreases continuously,
because of slower di¤usion, as the undercooling increases. The growth rate follows an
Arrhenius relationship (recall, Equation 5-1):
Growth rate ¼ A exp
Q
RT
ð12-4Þ
where Q is the activation energy (in this case for the phase transformation), R is the gas
constant, T is the temperature, and A is a constant.
Figure 12-1
Sigmoidal curve showing the rate
of transformation of FCC iron at a
constant temperature. The incuba-
tion time t
0
and the time t for 50%
transformation are also shown.
12-1 Nucle ation and Growth in Solid-State Reactions 359
