Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.


Figure 12-2 shows sigmoidal curves at di¤erent temperatures for the recrystalli-
zation of copper; as the temperature increases, the rate of recrystallization of copper
increases, because growth is the most important factor for copper.
At any particular temperature, the overall rate of transformation is the product of
the nucleation and growth rates. In Figure 12-3(a), the combined e¤ect of the nuclea-
tion and growth rates is shown. A maximum transformation rate may be observed at a
critical undercooling. The time required for transformation is inversely related to the
rate of transformation; Figure 12-3(b) describes the time (on a log scale) required
for the transformation. This C-shaped curve is common for many transformations in
metals, ceramics, glasses, and polymers. Notice that the time required at a temperature
corresponding to the equilibrium phase transformation would be y (i.e., the phase
transformation will not occur). This is because there is no undercooling and, hence, the
rate of homogenous nucleation is zero.
In some processes, such as the recrystallization of a cold-worked metal, we find that
the transformation rate continually decreases with decreasing temperature. In this case,
nucleation occurs easily, and di¤usion—or growth—predominates (i.e., the growth is
the rate limiting step for the transformation). The following example illustrates how the
activation energy for a solid-state phase transformation such as recrystallization can be
obtained from data related to the kinetics of the process.
Figure 12-2 The effect of temperature on recrystallization of cold-worked copper.
Figure 12-3 (a) The effect of temperature on the rate of a phase transformation is the product
of the growth rate and nucleation rate contributions, giving a maximum transformation rate at a
critical temperature. (b) Consequently, there is a minimum time (t
min
) required for the
transformation, given by the ‘‘C-curve’’.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment360
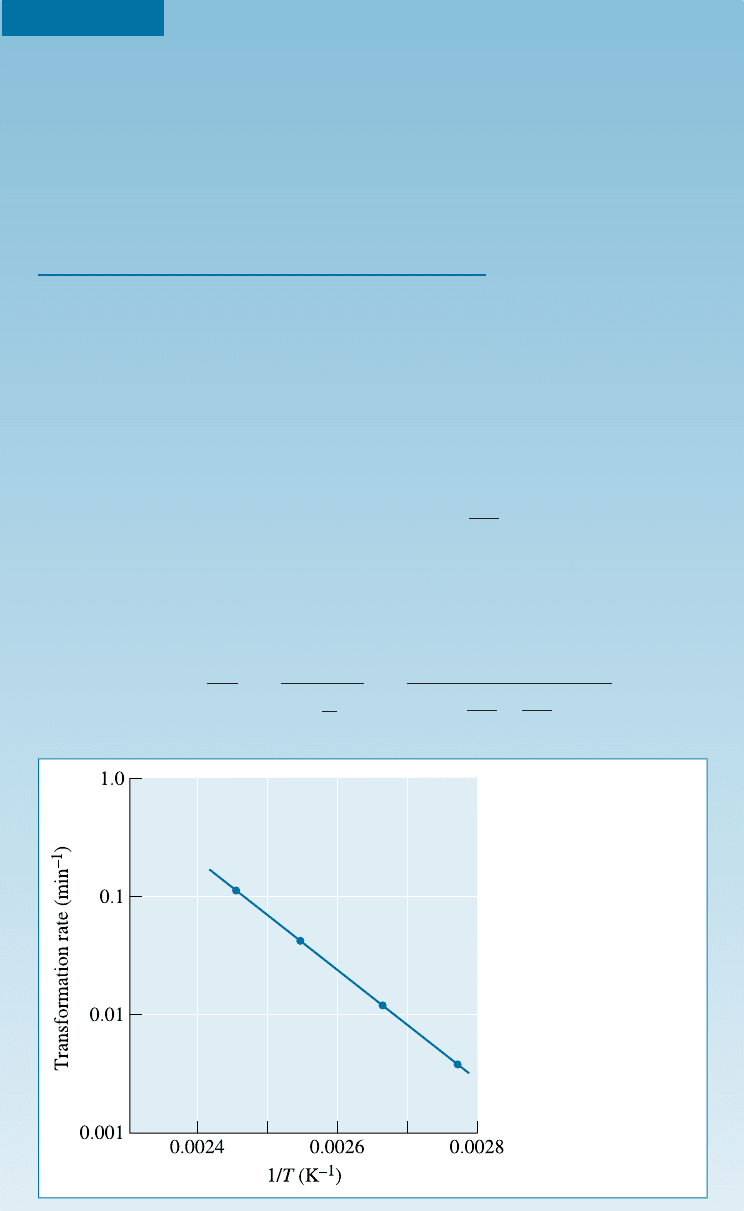
EXAMPLE 12-1 Activation Energy for the Recrystallization of Copper
Determine the activation energy for the recrystallization of copper from the
sigmoidal curves in Figure 12-2.
SOLUTION
The rate of transformation is the reciprocal of the time t required for half of
the transformation to occur. From Figure 12-2, the times required for 50%
transformation at several di¤erent temperatures can be calculated:
T (
˚
C) T (K) t (min) Rate (min
C1
)
135 408 9 0.111
119 392 22 0.045
102 375 80 0.0125
88 361 250 0.0040
The rate of transformation is an Arrhenius equation, so a plot of ln (rate)
versus 1/T (Figure 12-4 and Equation 12-4) allows us to calculate the constants
in the equation. Taking natural log of both sides of Equation 12-4:
ln(Growth rate) ¼ ln A
Q
RT
Thus, if we plot ln(Growth rate) as a function of 1=T, we expect a straight line
that has a slope of Q =R .
Slope ¼
Q
R
¼
2
6
6
6
4
D lnðrateÞ
D
1
T
3
7
7
7
5
¼
½ðlnð0:111Þlnð0:004ÞÞ
1
408
1
361
Figure 12-4
Arrhenius plot of
transformation rate
versus reciprocal
temperature for
recrystallization of
copper (for Example
12-1).
12-1 Nucle ation and Growth in Solid-State Reactions 361
Q=R ¼ 10;414
Q ¼ 20;693
cal
mol
8 0:111 ¼ A exp
20;693 cal=mol
1:987
cal
deg mol
ð408 degÞ
0
B
@
1
C
A
A ¼ 0:111=8:21 10
12
¼ 1:351 10
10
s
1
8 rate ¼ 1:351 10
10
exp
20;693
RT
In this particular example, the rate at which the reaction occurs increases as
the temperature increases, indicating that the reaction may be dominated by
di¤usion.
12-2 Alloys Strengthened by Exceeding the Solubility Limit
In Chapter 11, we learned that lead-tin (Pb-Sn) alloys containing about 2 to 19% Sn can
be dispersion-strengthened because the solubility of tin in lead is exceeded.
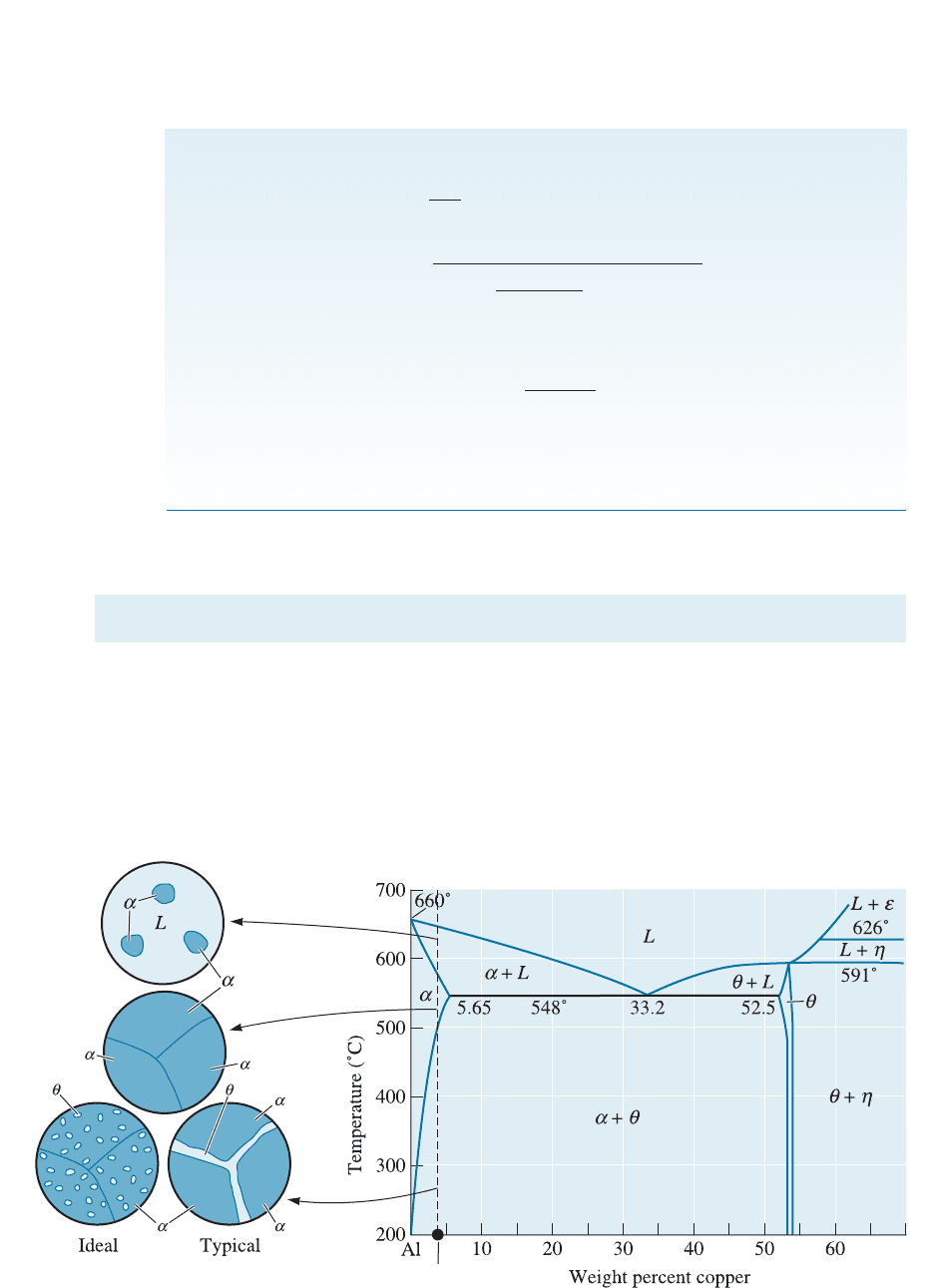
A similar situation occurs in aluminum-copper alloys. For example, the Al-4% Cu
alloy (shown in Figure 12-5) is 100% a above 500
C. The a phase is a solid solut ion
of aluminum containing copper up to 5.65 wt%. On cooling below the solvus tempera-
4
Figure 12-5 The aluminum-copper phase diagram and the microstructures that may develop
during cooling of an Al-4% Cu alloy.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment362
ture, a second phase, y, precipitates. The y phase, which is the hard, brittle intermetallic
compound CuAl
2
, provides dispersion strengthening. Applying the lever rule to the
phase diagram shown in Figure 12-5, we can show that at 200
C and below, in a 4% Cu
alloy, only about 7.5% of the final structure is y. We must control the precipitation of
the second phase to satisfy the requirements of good dispersion strengthening.
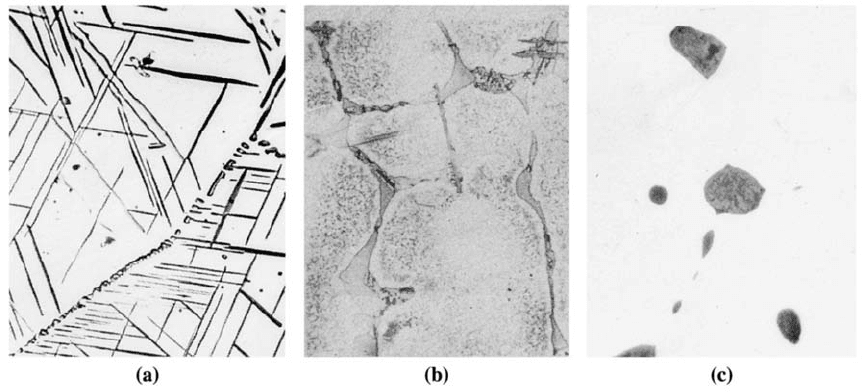
Widmanstðtten Structure The second phase may grow so that certain crystallographic
planes and directions in the precipitate are parallel to preferred planes and directions in
the matrix, creating a basket-weave pattern known as the Widmanstðtten structure. This
growth mechanism minimizes strain and surface energies and permits faster growth
rates. Widmanstðtten growth produces a characteristic appearance for the precipitate.
When a needle-like shape is produced [Figure 12-6(a)], the Widmanstðtten precipitate
may encourage the nucleation of cracks, thus reducing the ductility of the material.
However, some of these structures make it more di‰cult for cracks, once formed, to
propagate, therefore providing good fracture toughness. Certain titanium alloys and
ceramics obtain toughness in this way.
Interfacial Energy Relationships We expect the precipitate to have a spherical shape
in order to minimize surface energy. However, when the precipitate forms at an inter-
face, the precipitate shape is also influenced by the interfacial energy (g
pm
) of the boun-
dary between the matrix ðmÞ grains and the precipitate ðpÞ.
If the precipitate phase completely wets the grain (similar to how water wets glass)
then the dih edral angle is zero and the second phase would grow as a continuous layer
along the grain boundaries of the matrix phase. If the dihedral angle is small, the pre-
cipitate may be continuous. If the precipitate is also hard and brittle, the thin film that
surrounds the matrix grains causes the alloy to be very brittle [Figure 12-6(b)].
On the other hand, discontinuous and even spherical precipitates form when the
dihedral angle is large [Figure 12-6(c)]. This occurs if the precipitate phase does not wet
the matrix.
Figure 12-6 (a) Widmanstðtten needles in a Cu-Ti alloy (420). (From ASM Handbook,
Vol. 9, Metallography and Microstructure (1985), ASM International, Materials Park,
OH 44073.) (b) Continuous y precipitate in an Al-4% Cu alloy, caused by slow cooling (500).
(c) Precipitates of lead at grain boundaries in copper (500).
12-2 Alloys Strengthened by Exceeding the Solubility Limit 363

Coherent Precipitate Even if we produce a uniform distribution of discontinuous
precipitate, the precipitate may not significantly disrupt the surrounding matrix struc-
ture. Consequently, the precipitate blocks slip only if it lies directly in the path of the
dislocation.
But when a coherent precipitate forms, the planes of atoms in the crystal structure
of the precipitate are related to—or even continuous with—the planes in the crystal
structure of the matrix. Now a widespread disruption of the matrix crystal structure is
created, and the movement of a dislocation is impeded even if the dislocation merely
passes near the coherent precipitate. A special heat treatment, such as age hardening,
may produce the coherent precipitate.
12-3 Age or Precipitation Hardening
Age hardening,orprecipitation hardening, is produced by a sequence of phase trans-
formations that leads to a uniform dispersion of nano-sized, coherent precipitates in a
softer, more ductile matrix. The inadvertent occurrence of this process may have helped
the Wright broth ers, who, on December 17, 1903, made the first controllable airplane
flight that changed the world forever. Recently, Dr. Frank Gayle and co-workers at the
National Institute of Standards and Technology (NIST) have shown that the alumi-
num alloy used by the Wright brothers for making the engine of the first airplane ever
flown picked up copper from the casting mold. The age hardening occurred in-
advertently as the mold remained hot during the casting process.
12-4 Applications of Age-Hardened Alloys
Before we examine the details of the mechanisms of phase transformations that are
needed for age hardening to occur, let’s examine some of the applications of this tech-
nique. A major advantage of precipitation hardening is that it can be used to increase
the yield strength of many metallic materials via relatively simple heat treatme nts and
without creating significant changes in density. Thus, the strength-to-density ratio of an
alloy can be improved substantially using age hardening. For example, the yield
strength of an aluminum alloy can be increased from about 138 MPa to 414 MPa as a
result of age hardening.
Nickel-based super alloys (alloys based on Ni, Cr, Al, Ti, Mo, and C) are pre-
cipitation hardened by precipitation of a Ni
3
Al-like g
0
phase that is rich in Al and Ti.
Similarly, titanium alloys (e.g., Ti-6Al-4V), stainless steels, Be-Cu and many steels are
precipitation hardened and used for a variety of applications.
New sheet-steel formulations are designed such that precipitation hardening occurs
in the material while the paint on the chassis is being ‘‘baked’’ or cured (@100
C).
These bake-hardenable steels are just one example of steels that take advantage of the
strengthening e¤ect provided by age-hardening mechanisms.
A limitation associated with this mechanism is that age-hardened alloys can be used
over a limited range of temperatures. At higher temperatures, the precipitates formed
initially begin to grow and eventually dissolve if the temperatures are high enough
(Section 12-8). This is where alloys in which dispersion strengthening is achieved by
using a second phase that is insoluble are more e¤ective than age-hardened alloys.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment364
12-5 Microstructural Evolution in Age or Precipitation Hardening
How do precipitates form in precipitation hardening? How do they grow or age? Can
the precipitates grow too much, or overage, so that they can not provide maximum
dispersion strengthening? Answers to these questions can be found by following the
microstructural evolution in the sequence of phase transformations that are necessary
for age hardening.
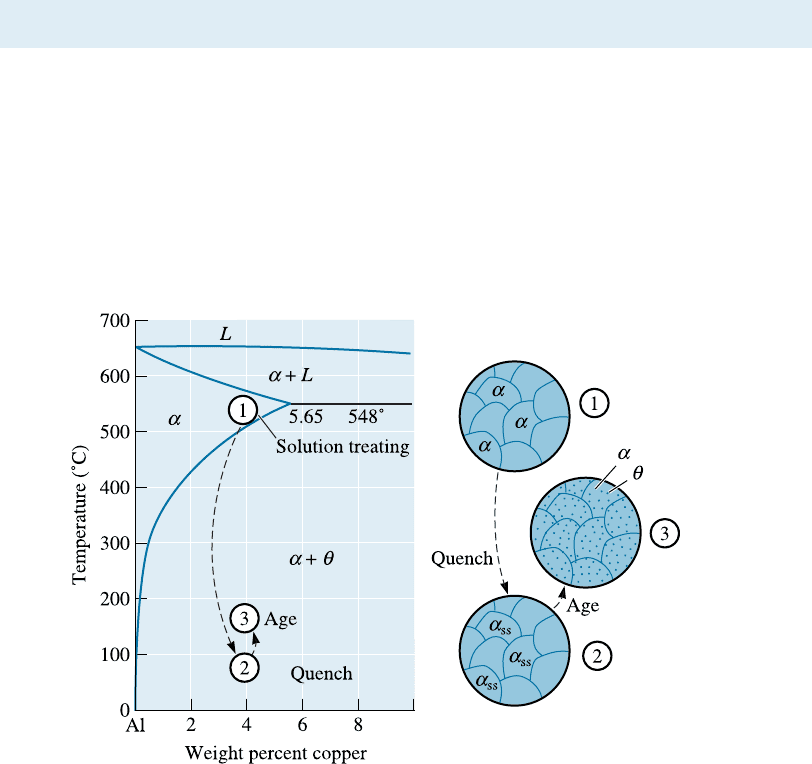
Let’s use Al-Cu as an archetypal system to illustrate these ideas. The Al-4% Cu
alloy is a classical example of an age-hardenable alloy. There are three steps in the age-
hardening heat treatment (Figure 12-7).
Step 1: Solution Treatment In the solution treatment, the alloy is first heated above
the solvus temperature and held until a homogeneous solid solution a is produced. This
step dissolves the y phase precipitate and reduces any microchemical segregation pres-
ent in the original alloy.
We could heat the alloy to just below the solidus temperature and increase the
rate of homogenization. However, the presence of a nonequilibrium eutectic micro-
constituent may cause melting (hot shortness, Chapter 10). Thus, the Al-4% Cu alloy
is solution-treated between 500
C and 548
C, that is, between the solvus and the eu-
tectic temperatures.
Step 2: Quench After solution treatment, the alloy, which contains only a in its
structure, is rapidly cooled, or quenched. The atoms do not have time to di¤use to
potential nucleation sites, so the y does not form. After the quench, the structure is a
supersaturated solid solution ða
ss
Þ containing excess copper, and it is not an equilibrium
structure. It is a metastable structure. This situation is e¤ecti vely the same as under-
Figure 12-7 The aluminum-rich end of the aluminum-copper phase diagram showing the three
steps in the age-hardening heat treatment and the microstructures that are produced.
12-5 M icrostructural Evolution in Age or Precipitation Hardening 365
cooling of water, molten metals, and silicate glasses (Chapter 9). The only di¤erence is
we are dealing with materials in their solid state.
Step 3: Age Finally, the supersaturated solid solution ( a
ss
) is heated at a temperature
below the solvus temperature. At this aging temperature, atoms di¤use only short dis-
tances. Because the a
ss
phase is metastable, the extra copper atoms di¤use to numerous
nucleation sites and precipitates grow. Eventually, if we hold the alloy for a su‰cient
time at the aging temperature, the equilibrium a þ y structure is produced. Note that
even though the structure that is formed has two equilibrium phases (i.e., a þ y), the
morphology of the phases is di¤erent from the structure that would have been obtained
by the slow cooling of this alloy (Figure 12-5). When we go through the three steps de-
scribed previously, we produce the y phase in the form of ultrafine uniformly dispersed
second-phase precipitate particles. This is what we need for e¤ective precipitation
strengthening.
The following two examples illustrate the e¤ect of quenching on the composition of
phases and a design for an age-hardening treatment.
EXAMPLE 12-2
Composition of Al-4% Cu Alloy Phases
Compare the composition of the a solid solution in the Al-4% Cu alloy at room
temperature when the alloy cools under equilibrium conditions with that when
the alloy is quenched.
SOLUTION
From Figure 12-5, a tie line can be drawn at room temperature. The composi-
tion of the a determined from the tie line is about 0.02% Cu. However, the
composition of the a after quenching is still 4% Cu. Since a contains more than
the equilibrium copper content, the a is supersaturated with copper.
EXAMPLE 12-3
Design of an Age-Hardening Treatment
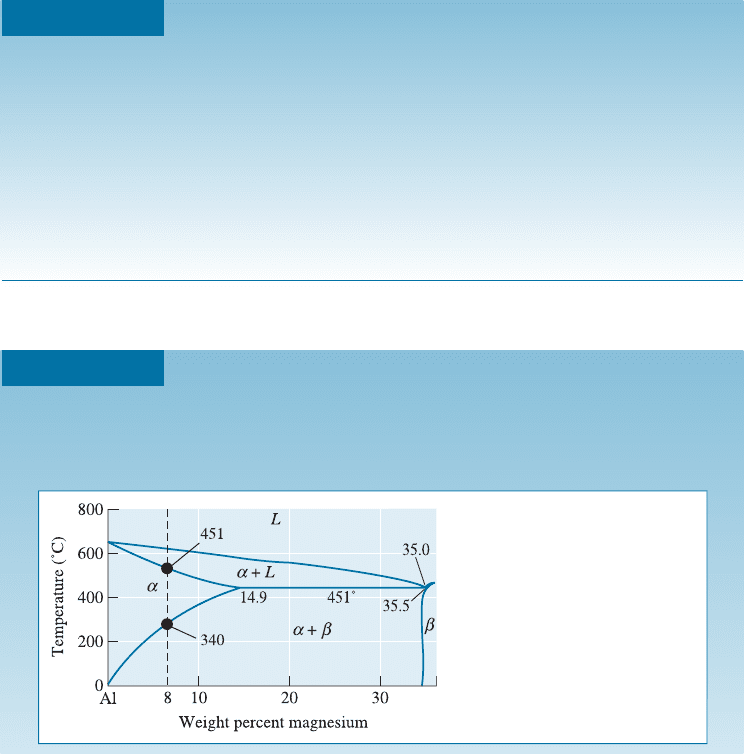
A portion of a magnesium-aluminum phase diagram is shown in Figure 12-8.
Suppose a Mg-8% Al alloy is responsive to an age-hardening heat treatment.
Design a heat treatment for the alloy.
Figure 12-8
Portion of the aluminum-
magnesium phase diagram.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment366

SOLUTION
Step 1: Solution-trea t at a temperature between the solvus and the eutectic to
avoid hot shortness. Thus, heat between 340
C and 451
C (Figure 12-8).
Step 2: Quench to room temperature fast enough to prevent the precipitate
phase b from forming.
Step 3: Age at a temperature below the solvus, that is, below 340
C, to form a
fine dispersion of b phase.
Nonequilibrium Precipitates during Aging During aging of aluminum-copper alloys,
a continuous series of other precursor precipitate phases forms prior to the formation of
the equilibrium y phase. This is fairly common in precipitation-hardened alloys. The
simplified diagram in Figure 12-7 does not show these intermediate phases. At the start
of aging, the copper atoms concentrate on f100g planes in the a matrix and produce
very thin precipitates called Guinier-Preston (GP) zones. As aging continues, more
copper atoms di¤use to the precipitate and the GP-I zones thicken into thin disks, or
GP-II zones. With continued di¤usion, the precipitates develop a greater degree of
order and are called y
0
. Finally, the stable y precipitate is produced.
The nonequilibrium precipitates—GP-I, GP-II, and y
0
—are coherent precipitates.
The strength of the alloy increases with aging time as these coherent phases grow in size
during the initial stages of the heat treatment. When these coherent precipitates are
present, the alloy is in the aged condition. This important development in the micro-
structure evolution of precipitation-h ardened alloys is the reason the time for heat
treatment during aging is very important. Since the changes in the structure occur at a
nano-scale, no change is seen in the structure of the alloy at a micro-s cale. This is one
reason why the mechanisms of precipitation hardening were established more firmly
only in the 1950s, (when electron microscopes were starting to be used), even though
the phenomenon was discovered earlier.
When the stable noncoherent y phase precipitates, the strength of the alloy begins
to decrease. Now the alloy is in the overaged condition. The y phase still provides some
dispersion strengthening, but with increasing time, the y phase grows larger and even
the simple dispersion-strengthening e¤ect diminishes.
12-6 Effects of Aging Temperature and Time
The properties of an age-hardenable alloy depend on both aging temperature and aging
time (Figure 12-9). At 260
C, di¤usion in the Al-4% Cu alloy is rapid, and precipitates
quickly form. The strength reaches a maximum after less than 0.1 h (6 minutes) ex-
posure. Overaging occurs if the alloy is held for longer than 0.1 h.
At 190
C, which is a typical aging temperature for many aluminum alloys, a longer
time is required to produce the optimum strength. However, there are benefits to using
the lower temperature. First, the maximum strength increases as the aging temperature
decreases. Second, the alloy maintains its maximum strength over a longer period of
time. Third, the properties are more uniform. If the alloy is aged for only 10 min at
260
C, the surface of the part reaches the proper temperature and strengthens, but the
center remain s cool and ages only slightly. The example that follows illustrates the e¤ect
of aging heat treatment time on the strength of aluminum alloys.
12-6 Effects of Aging Temperature and Time 367
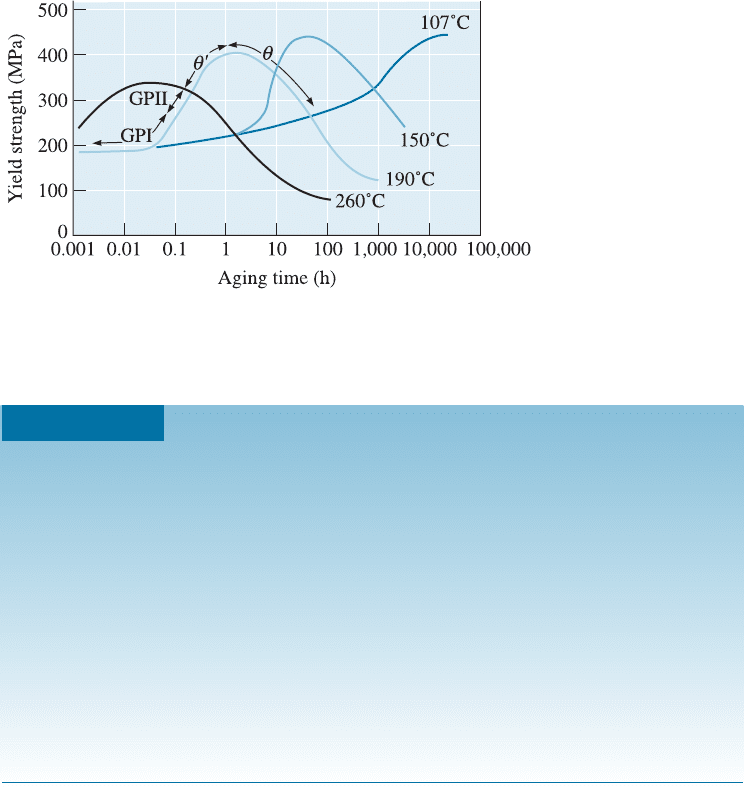
Figure 12-9 The effect of aging temperature and time on the yield strength of an Al-4% Cu
alloy.
EXAMPLE 12-4 Effect of Aging Heat Treatment Time on the Strength of
Aluminum Alloys
The operator of a furnace left for his hour lunch break without removing the
Al-4% Cu alloy from the furnace used for the aging treatment. Compare the
e¤ect on the yield strength of the extra hour of aging for the aging temper-
atures of 190
C and 260
C.
SOLUTION
At 190
C, the peak strength of 400 MPa occurs at 2 h (Figure 12-9). After 3 h,
the strength is essentially the same.
At 260
C, the peak strength of 340 MPa occurs at 0.06 h. However, after 1 h,
the strength decreases to 250 MPa.
Thus, the higher aging temperature gives lower peak strength and makes
the strength more sensitive to aging time.
Aging at either 190
C or 260
C is called artificial aging, because the alloy is heated
to produce precipitation. Some solution-treated and quenched alloys age at room tem-
perature; this is called natural aging. Natural aging requires long times—often several
days—to reach maximum strength. However, the peak strength is higher than that
obtained in artificial aging, and no overaging occurs.
An interesting observation made by Dr. Gayle and coworkers of NIST is a striking
example of the di¤erence between natural aging and artificial aging. Dr. Gayle and
coworkers analyzed the aluminum alloy of the engine used in the Wright brothers’ air-
plane. They found two interesting things. First, they found that the original alloy had
undergone precipitation hardening as a result of being held in the mold for a period of
time and at a temperature that was su‰cient to cause precipitation hardening. Second,
since when the research was done, from 1903 until about 1993 (almost ninety years), the
alloy had continued to age naturally! This could be seen from two di¤erent size dis-
tributions for the precipitate particles using transmission electron microscopy. In some
aluminum alloys (designated as T4) used to make tapered poles or fasteners, it may be
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment368

necessary to refrigerate the alloy to avoid natural aging at room temperature. If not, the
alloy would age at room temperature, become harder and not be workable!
12-7 Requirements for Age Hardening
Not all alloys are age hardenable. Four conditions must be satisfied for an alloy to have
an age-hardening response during heat treatment:
1. The alloy system must display decreasing solid solubility with decreasing tem-
perature. In other words, the alloy must form a single phase on heating above the solvus
line, then enter a two-phase region on cooling.
2. The matrix should be relatively soft and ductile, and the precipitate should be
hard and brittle. In most age hardenable alloys, the precipitate is a hard, brittle inter-
metallic compound.
3. The alloy must be quenchable. Some alloys cannot be cooled rapidly enough to
suppress the formation of the precipitate. Quenching may, however, introduce residual
stresses that cause distortion of the part (Chapter 8). To minimize residual stresses,
aluminum alloys are quenched in hot water, at about 80
C.
4. A coherent precipitate must form.
As men tioned before in Section 12-4, a number of important alloys, including cer-
tain stainless steels and alloys based on aluminum, magnesium, titanium, nickel, chro-
mium, iron, and copper, meet these conditions and are age-hardenable.
12-8 Use of Age-Hardenable Alloys at High Temperatures
Based on our previous discussion, we would not select an age-hardened Al-4% Cu alloy
for use at high temperatures. At service temperatures ranging from 100
C to 500
C, the
alloy overages and loses its strength. Above 500
C, the second phase redissolves in the
matrix and we do not even obtain dispersion strengthenin g. In general, the aluminum
age-hardenable alloys are best suited for service near room temperature. However,
some magnesium alloys may maintain their strength to about 250
C and certain nickel
superalloys resist overaging at 1000
C.
We may also have problems when welding age-hardenable alloys (Figure 12-10).
During welding, the metal adjacent to the weld is heated. The heat-a¤ected zone (HAZ)
contains two principle regions. The lower temperature zone near the una¤ected base
metal is exposed to temperatures just below the solvus and may overage. The higher
temperature zone is solution-treated, eliminating the e¤ects of age hardening. If the
solution-treated zone cools slowly, stable y may form at the grain boundaries, embrit-
tling the weld area. Very fast welding processes such as electron-beam welding, com-
plete reheat treatment of the area after welding, or welding the alloy in the solution-
treated condition improve the quality of the weld (Chapter 9). Welding of nickel-based
superalloys strengthened by precipitation hardening does not pose such problems since
the precipitation process is sluggish and the welding process simply acts as a solution
and quenching treatment. The process of friction stir welding has also been recently
applied to welding of Al and Al-Li alloys for aerospace and aircraft applications.
12-8 Use of Age-Hardenable Alloys at High Temperatures 369
