Askeland D.R., Fulay P.P. Essentials of Materials Science & Engineering
Подождите немного. Документ загружается.

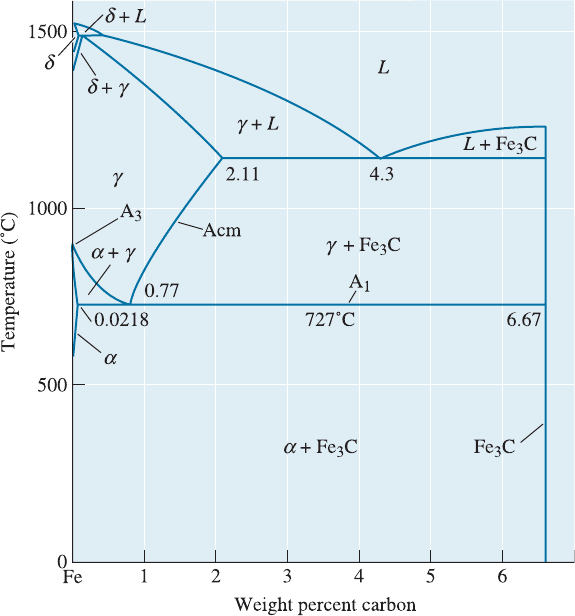
12-9 The Eutectoid Reaction
In Chapter 11, we defined the eutectoid as a solid-state reaction in which one solid
phase ðS
1
Þ transforms to two other solid phases (S
2
and S
3
):
S
1
! S
2
þ S
3
ð12-5Þ
As an example of how we can use the eutectoid reaction to contro l the microstructure
and properties of an alloy, let’s examine the technologically important portion of the
iron-iron carbide (Fe-Fe
3
C) phase diagram (Figure 12-11), which is the basis for steels
and cast irons. The formation of the two solid phases (a and Fe
3
C) permits us to obtain
dispersion strengthening. The ability to control the occurrence of the eutectoid reaction
(this includes either making it happen, slowing it down, or avoiding it all together) is
probably the most important step in the thermomechanical processing of steels. On the
Fe-Fe
3
C diagram, the eutectoid temperature is known as the A
1
temperature. The
boundary between austenite (g) and the two-phase field consisting of ferrite (a) and
austenite is known as the A
3
. The boundary between austenite (g) and the two-phase
field consisting of cementite (Fe
3
C) and austenite is known as the A
cm
.
We are normally not interested in the carbon-rich end of the Fe-C phase diagram
and this is why we examine the Fe-Fe
3
C diagram as part of the Fe-C binary phase
diagram.
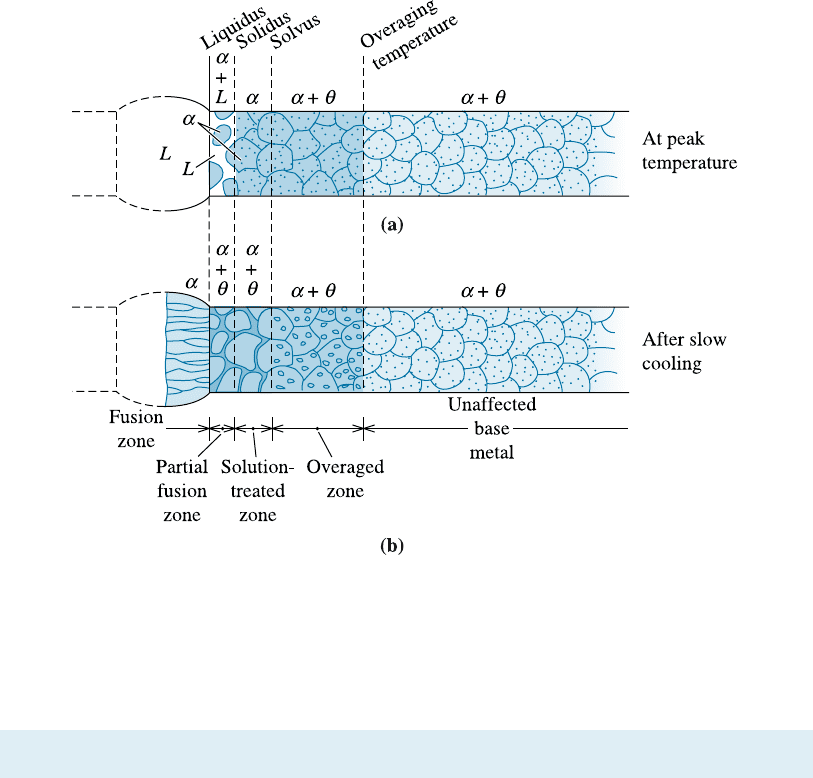
Figure 12-10 Microstructural changes that occur in age-hardened alloys during fusion welding:
(a) microstructure in the weld at the peak temperature, and (b) microstructure in the weld after
slowly cooling to room temperature.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment370
Solid Solutions Iron goes through two allotropic transformations (Chapter 3) during
heating or cooling. Immediately after solidification, iron forms a BCC structure called
d-ferrite. On further cooling, the iron transforms to a FCC structure called gamma ðgÞ
or austenite. Finally, iron transforms back to the BCC structure at lower temperatures;
this structure is called alpha ðaÞ or ferrite. Both of the ferrites (a and d) and the aus-
tenite are solid solutions of interstitial carbon atoms in iron (Example 4-3). Normally,
when no specific reference is made, the term ferrite refers to the a ferrite, since this is the
phase we encounter more often during the heat treatment of steels. Note that certain
ceramic materials used in magnetic applications are also known as ferrites but are not
related to the ferrite phase in the Fe-Fe
3
C system.
Because interstitial holes in the FCC crystal structure are somewhat larger than the
holes in the BCC crystal structure, a greater number of carbon atoms can be accom-
modated in FCC iron. Thus, the maximum solubility of carbon in austenite is 2.11% C,
whereas the maximum solubility of carbon in BCC iron is much lower (i.e., @0.0218% C
in a and 0.09% C in d). The solid solutions of carbon in iron are relatively soft and duc-
tile, but are stronger than pure iron due to solid-solution strengthening by the carbon.
Compounds A stoichiometric compound Fe
3
C, or cementite, forms when the solubility
of carbon in solid iron is exceeded. The Fe
3
C contains 6.67% C, is extremely hard and
brittle (like a ceramic material), and is present in all commercial steels. By properly
controlling the amount, size, and shape of Fe
3
C, we control the degree of dispersion
strengthening and the properties of the steel.
Figure 12-11 The Fe-Fe
3
C phase diagram (a portion of the Fe-C diagram). The vertical line at
6.67% C is the stoichiometric compound Fe
3
C.
12-9 The Eutectoid Reaction 371
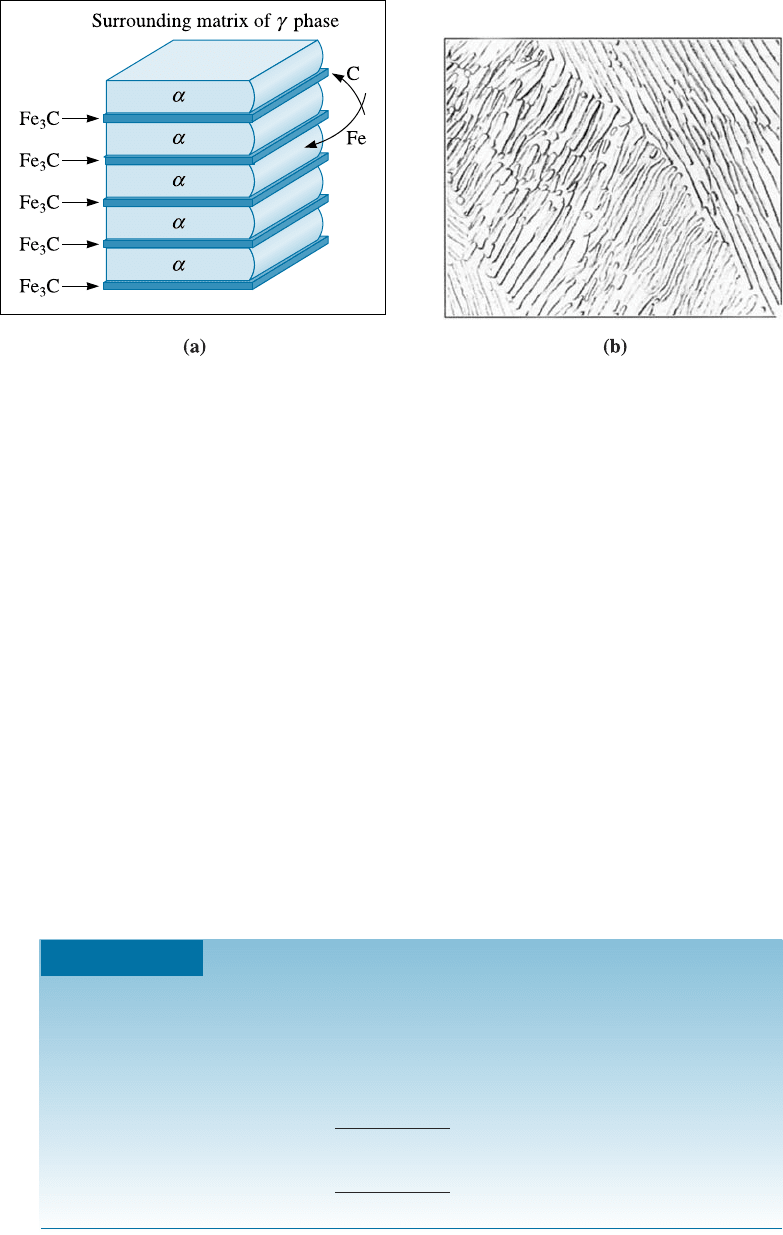
The Eutectoid Reaction If we heat a Fe-C steel containing the eutectoid composition
of 0.77% C above 727
C, we produce a structure containing only austenite grains.
When austenite cools to 727
C, the eutectoid reaction begins:
gð0:77% CÞ!að0:0218% CÞþFe
3
Cð6:67% CÞð12-6Þ
As in the eutectic reaction, the two phases that form have di¤erent compositions, so
atoms must di¤use during the reaction (Figure 12-12). Most of the carbon in the aus-
tenite di¤uses to the Fe
3
C, and most of the iron atoms di¤use to a. This redistribution
of atoms is easiest if the di¤usion distances are short, which is the case when the a and
Fe
3
C grow as thin lamellae, or plates.
Pearlite The lamellar structure of a and Fe
3
C that develops in the iron-carbon system
is called pearlite, which is a microconsti tuent in steel. This was so named because a
polished and etched pearlite shows the colorfuln ess of mother-of-pearl. The lamellae in
pearlite are much finer than the lamellae in the lead-tin eutectic because the iron and
carbon atoms must di¤use through solid austenite rather than through liquid. One way
to think about pearlite is to consider it as a metal-ceramic nano-composite. The fol-
lowing example shows the calculation of the amounts of the phases in the pearlite
microconstituent.
Figure 12-12 Growth and structure of pearlite: (a) redistribution of carbon and iron, and
(b) photomicrograph of the pearlite lamellae (2000). (From ASM Handbook, Vol. 7, (1972),
ASM International, Materials Park, OH 44073.)
EXAMPLE 12-5
Phases and Composition of Pearlite
Calculate the amounts of ferrite and cementite present in pearlite.
SOLUTION
Since pearlite must contain 0.77% C, using the lever rule:
% a ¼
6:67 0:77
6:67 0:0218
100 ¼ 88:7%
%Fe
3
C ¼
0:77 0:0218
6:67 0:0218
100 ¼ 11:3%
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment372

In Example 12-5, we saw that most of the pearlite is composed of ferrite. In fact, if
we examine the pearlite closely, we find that the Fe
3
C lamellae are surrounded by a.
The pearlite structure therefore provides dispersion strengthening—the continuous
ferrite phase is relatively soft and ductile and the hard, brittle cementite is dispersed.
The nex t example illustrates the similarity and di¤erences between composites and
pearlites.
EXAMPLE 12-6
Tungsten Carbide (WC)-Cobalt (Co) Composite and Pearlite
Tungsten carbide-cobalt composites, known as cemented carbides or carbides,
are used as bits for cutting tools and drills (Chapter 1). What features are sim-
ilar between these ‘‘cemented carbides’’ and pearlite, a microconstituent in
steels? What are some of the major di¤erences?
SOLUTION
Pearlite is very similar to the tungsten carbide-cobalt (WC-Co) composites
known in industry as carbides. You may recall from earlier chapters that WC-
Co are ceramic-metal composites (known as cermets) and used as cutting tools,
drill bits, etc. In both materials, we take advantage of the toughness of one
phase (ferrite or cobalt metal, in the case of pearlite in steel and WC-Co, respec-
tively) and the hard ceramic-like phase (WC and Fe
3
C, in WC-Co and steel,
respectively). The metallic phase helps with ductility and the hard phase helps
with strength. The di¤erence is, WC and Co are two separate compounds that
are sintered together using the powder metallurgy route. Pearlite is a micro-
constituent made up of two phases derived from same two elements (Fe-C).
Another di¤erence is in pearlite, the phases are formed via a eutectoid reaction.
No such reaction occurs in the formation of WC-Co composites. Typically,
WC-Co microstructure consists mainly of WC grains that are ‘‘glued’’ by co-
balt grains. In pearlite, the metal-like ferrite phase dominates.
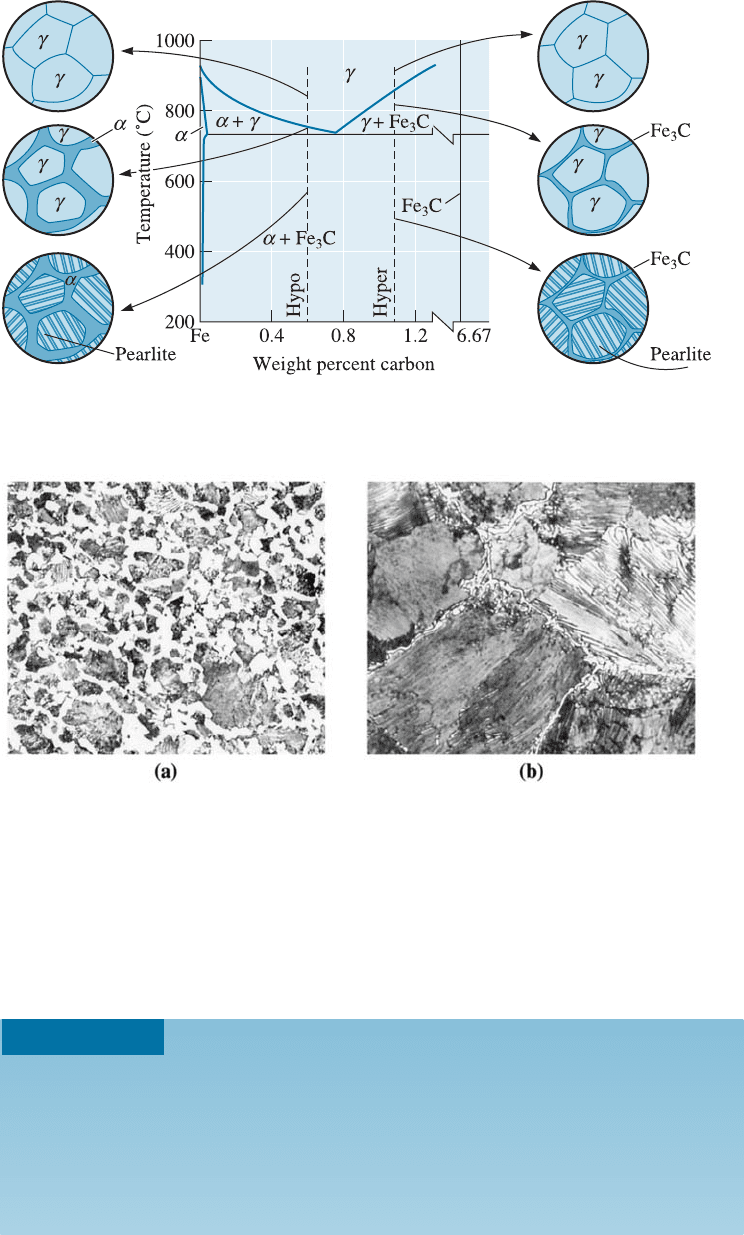
Primary Microconstituents Hypoeutectoid steels contain less than 0.77% C, and hy-
pereutectoid steels contain more than 0.77% C. Ferrite is the primary or proeutectoid
microconstituent in hypoeutectoid alloys, and cementite is the primary or proeutectoid
microconstituent in hypereutectoid alloys. If we heat a hypoeutectoid alloy containing
0.60% C above 750
C, only austenite remains in the microstructure. Figure 12-13 shows
what happens when the austenite cools. Just below 750
C, ferrite nucleates and grows,
usually at the austenite grain boundaries. Primary ferrite continues to grow until the
temperature falls to 727
C. The remaining austenite at that temperature is now sur-
rounded by ferrite and has changed in composition from 0.60% C to 0.77% C. Sub-
sequent cooling to below 727
C causes all of the remaining austenite to transform
to pearlite by the eutectoid reaction. The structure contains two phases—ferrite and
cementite—arranged as two microconstituents—primary ferrite and pearlite. The
final microstructure contains islands of pearlite surrounded by the primary ferrite [Figure
12-14(a)]. This structure permits the alloy to be strong, due to the dispersion-strengthened
pearlite, yet ductile, due to the continuous primary ferrite.
In hypereutectoid alloys, the primary phase is Fe
3
C, which forms at the austenite
grain boundaries. After the austenite cools through the eutectoid reaction, the steel
contains hard, brittle cementite surrounding islands of pearlite [Figure 12-14(b)].
Now, because the hard, brittle microconstituent is continuous, the steel is also brittle.
12-9 The Eutectoid Reaction 373
Fortunately, we can improve the microstructure and properties of the hypereutectoid
steels by heat treatment. The following example shows the calculation for the compo-
sition of phases and microconstituent in a plain carbon steel.
Figure 12-13 The evolution of the microstructure of hypoeutectoid and hypereutectoid steels
during cooling, in relationship to the Fe-Fe
3
C phase diagram.
Figure 12-14 (a) A hypoeutectoid steel showing primary a (white) and pearlite (400). (b) A
hypereutectoid steel showing primary Fe
3
C surrounding pearlite (800). (From ASM
Handbook, Vol. 7, (1972), ASM International, Materials Park, OH 44073.)
EXAMPLE 12-7
Phases in Hypoeutectoid Plain Carbon Steel
Calculate the amounts and compositions of phases and microconstituents in a
Fe-0.60% C alloy at 726
C.
SOLUTION
The phases are ferrite and cementite. Using a tie line and working the lever law
at 726
C, we find:
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment374

a ð0:0218% CÞ % a ¼
6:67 0:60
6:67 0:0218
100 ¼ 91:3%
Fe
3
C ð6:67% CÞ %Fe
3
C ¼
0:60 0:0218
6:67 0:0218
100 ¼ 8: 7%
The microconstituents are primary ferrite and pearlite. If we construct a tie line
just above 727
C, we can calculate the amounts and compositions of ferrite and
austenite just before the eutectoid reaction starts. All of the austenite at that
temperature will have the eutectoid composition (i.e., it will contain 0.77% C)
and will transform to pearlite; all of the proeutectoid ferrite will remain as
primary ferrite.
Primary a : 0:0218% C % Primary a ¼
0:77 0:60
0:77 0:0218
100 ¼ 22:7%
Austentite just above 727
C ¼ Pearlite : 0:77% C
% Pearlite ¼
0:60 0:0218
0:77 0:0218
100 ¼ 77:3%
12-10 Controlling the Eutectoid Reaction
We control dispersion strengthening in the eutectoid alloys in much the same way that
we did in eutectic alloys (Chapter 11).
Controlling the Amount of the Eutectoid By changing the composition of the alloy, we
change the amount of the hard second phase. As the carbon content of steel increases
towards the eutectoid composition of 0.77% C, the amounts of Fe
3
C and pea rlite
increase, thus increasing the strength. However, this strengthening e¤ect eventually
peaks and the properties level out or even decrease when the carbon content is too high
(Table 12-1).
Controlling the Austenite Grain Size Pearlite grows as grains or colonies. Within each
colony, the orientation of the lamellae is identical. The colonies nucleate most easily
TABLE 12-1 9 The effect of carbon on the strength of steels
Slow Cooling (Coarse Pearlite) Fast Cooling (Fine Pearlite)
Carbon
%
Yield
Strength
(MPa)
Tensile
Strength
(MPa)
%
Elongation
Yield
Strength
(MPa)
Tensile
Strength
(MPa)
%
Elongation
0.20 295 394 36.5 347 441 36.0
0.40 353 519 30.0 374 590 28.0
0.60 372 626 23.0 420 776 18.0
0.80 376 615 25.0 524 1010 11.0
0.95 379 657 13.0 500 1014 9.5
After Metals Progress Materials and Processing Databook, 1981.
12-10 Controlling the Eutectoid Reaction 375

at the grain boundaries of the original austenite grains. We can increase the number of
pearlite colonies by reducing the prior austenite grain size, usually by using low tem-
peratures to produce the austenite. Typically, we can increase the strength of the alloy
by reducing the initial austenite grain size, thus increasing the number of colonies.
Controlling the Cooling Rate By increasing the cooling rate during the eutectoid re-
action, we reduce the distance that the atoms are able to di¤use. Consequently, the
lamellae produced during the reaction are finer or more closely spaced. By producing
fine pearlite, we increase the strength of the alloy (Table 12-1 and Figure 12-15).
Controlling the Transformation Temperature The solid-s tate eutectoid reaction is
rather slow, and the steel may cool below the equilibrium eutectoid temperature before
the transformation begins (i.e., the austenite phase can be undercooled). Lower trans-
formation temperatures give a finer, stronger structure (Figure 12-16), influence the
time required for transformation, and even alter the arrangeme nt of the two phases.
This information is contained in the time-temperature-transformation (TTT) diagram
(Figure 12-17). This diagram, also called the isotherma l transformation (IT) diagram or
Figure 12-15
The effect of interlamellar spacing (l)
on the yield strength of pearlite.
Figure 12-16
The effect of the austenite trans-
formation temperature on the
interlamellar spacing in pearlite.
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment376
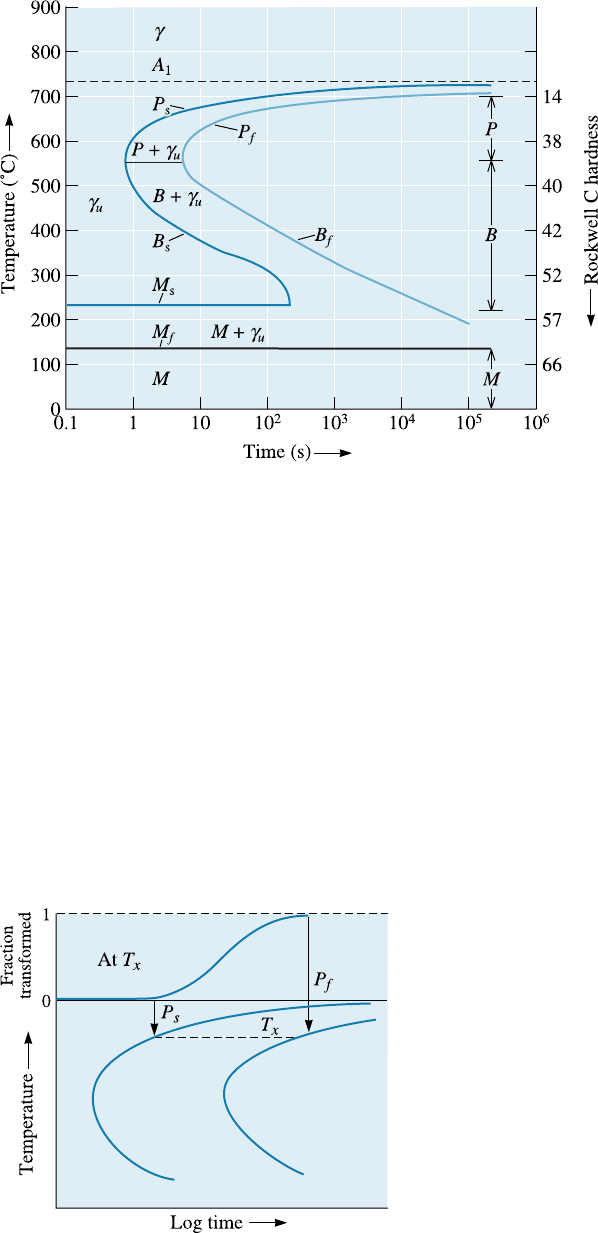
the C-curve, permits us to predict the structure, properties, and heat treatment required
in steels.
The shape of the TTT diagram is a consequence of the kinetics of the eutectoid re-
action and is similar to the diagram shown by the Avrami relationship (Figure 12-3). At
any particular temperature, a sigmoidal curve describes the rate at which the austenite
transforms to a mixture of ferrite and cementite (Figure 12-18). An incubation time is
required for nucleation. The P
s
(pearlite start) curve represents the time at which aus-
tenite starts to transform to ferrite and cementite via the eutectoid transformation. The
sigmoidal curve also gives the time at which the transformation is completed; this time
is given by the P
f
(pearlite finish) curve. When the temperature decreases from 727
C,
the rate of nucleation increases, while the rate of growth of the eutectoid decreases. As
in Figure 12-3, a maximum transformation rate, or minimum transformation time, is
found; the maximum rate of transformation occurs near 550
C for an eutectoid steel
(Figure 12-17).
Figure 12-17 The time-temperature-transformation (TTT) diagram for an eutectoid steel.
Figure 12-18
The sigmoidal curve is related to the start
and finish times on the TTT diagram for
steel. In this case, austenite is transform-
ing to pearlite.
12-10 Controlling the Eutectoid Reaction 377
Two types of microc onstituents are produced as a result of the transformation.
Pearlite (P) forms above 550
C, and bainite (B) forms at lower temperatures:
1. Nucleation and growth of phases in pearlite: If we quench to just below the eu-
tectoid temperature, the austenite is only slightly undercooled. Long times are required
before stable nuclei for ferrite and cementite form. After the phases that form pearlite
nucleate, atoms di¤use rapidly and coarse pearlite is produced; the transformation is
complete at the pearlite finish (P
f
) time. Austenite quenched to a lower temperature
is more highly undercooled. Consequently, nucleation occurs more rapidly and the P
s
is shorter. However, di¤usion is also slower, so atoms di¤use only short distances and
fine pearlite is produced. Even though growth rates are slower, the overall time required
for the transformation is reduced because of the shorter incubation time. Finer pearlite
forms in shorter times as we reduce the isothermal transformation temperature to about
550
C, which is the nose,orknee, of the TTT curve (Figure 12-17).
2. Nucleation and growth of phases in bainite: At a temperature just below the nose
of the TTT diagram, di¤usion is very slow and total transformation times increase. In
addition, we find a di¤erent microstructure! At low transformation temperatures, the
lamellae in pearlite would have to be extremely thin and, consequently, the boundary
area between the ferrite and Fe
3
C lamellae would be very large. Because of the energy
associated with the ferrite-cementite interface, the total energy of the steel would have
to be very high. Instead the steel reduces its internal energy by permitting the cemen-
tite to precipitate as discrete, rounded particles in a ferrite matrix. This new micro-
constituent, or arrangement of ferrite and cementite, is called bainite. Transformation
begins at a bainite start (B
s
) time and ends at a bainite finish (B
f
) time.
The times required for austenite to begin and finish its tran sformation to bainite in-
crease and the bainite becomes finer as the transformation temperature continues to de-
crease. The bainite that forms just below the nose of the curve is called coarse bainite,
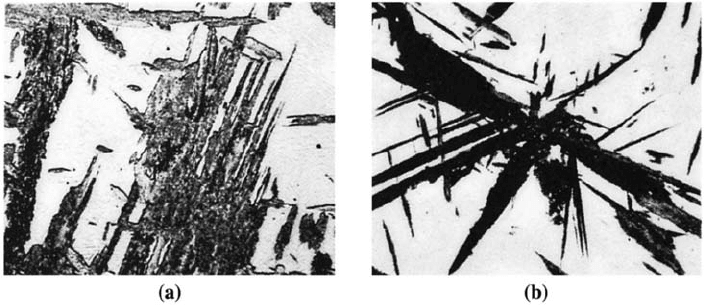
upper bainite, or feathery bainite. The bainite that forms at lower temperatures is called
fine bainite, lower bainite, or acicular bainite. Figure 12-19 shows typical microstructures
of bainite. Note that the morphology of bainite depends on the heat treatment used.
Figure 12-20 shows the e¤ect of transformation temperature on the properties of
eutectoid (0.77% C) steel. As the temperature decreases, there is a general trend toward
higher strength and lower ductility due to the finer microstructure that is produced. The
following two examples illustrate how we can design heat treatments of steels to pro-
duce desired microstructures and properties.
Figure 12-19 (a) Upper bainite (gray, feathery plates) (600). (b) Lower bainite (dark needles)
(400). (From ASM Handbook, Vol. 8, (1973), ASM International, Materials Park, OH 44073.)
C H A P T E R 12 Dispersion Stren gthening by Phase Transfor mations and Heat Treatment378
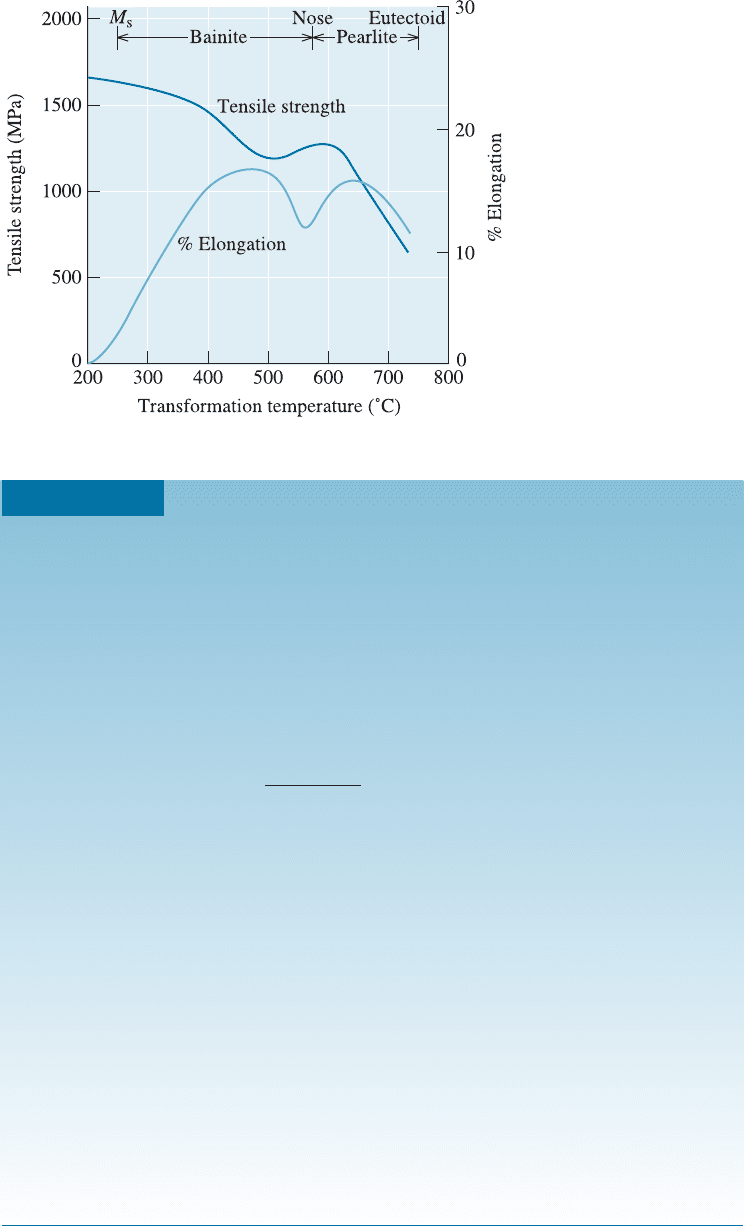
Figure 12-20
The effect of transforma-
tion temperature on
the properties of an
eutectoid steel
(0.77% C).
EXAMPLE 12-8
Design of a Heat Treatment to Generate Pearlite Microstructure
Design a heat treatment to produce the pearlite structure shown in Figure
12-12(b).
SOLUTION
First, we need to determine the interlamellar spacing of the pearlite. If we
count the number of lamellar spacings in the upper right of Figure 12-12(b),
remembering that the interlamellar spacing is measured from one a plate to
the next a plate, we find 14 spacings over a 2-cm distance. Due to the 2000
magnification, this 2-cm distance is actually 0.001 cm. Thus:
l ¼
0:001 cm
14 spacings
¼ 7:14 10
5
cm
If we assume that the pearlite is formed by an isothermal transformation, we
find from Figure 12-16 that the transformation temperature must have been
approximately 700
C. From the TTT diagram (Figure 12-17), our heat treat-
ment must have been:
1. Heat the steel to about 750
C and hold—per haps for 1 h—to produce all
austenite. A higher temperature may cause excessive growth of austenite
grains (Chapter 5).
2. Quench to 700
C and hold for at least 10
5
s (the P
f
time). We assume here
that the steel cools instantly to 700
C. In practice, this does not happen and
thus the transformation does not occur at one temperature. We may need to
use the continuous cooling transformation diagrams to be more precise
(Chapter 13).
3. Cool to room temperature.
The steel should have a hardness of HRC 14 (Figure 12-17) and a yield
strength of about 200 MPa, as shown in Figure 12-15.
12-10 Controlling the Eutectoid Reaction 379
